Cholera, the Current Status of Cholera Vaccines and Recommendations for Travellers
Abstract
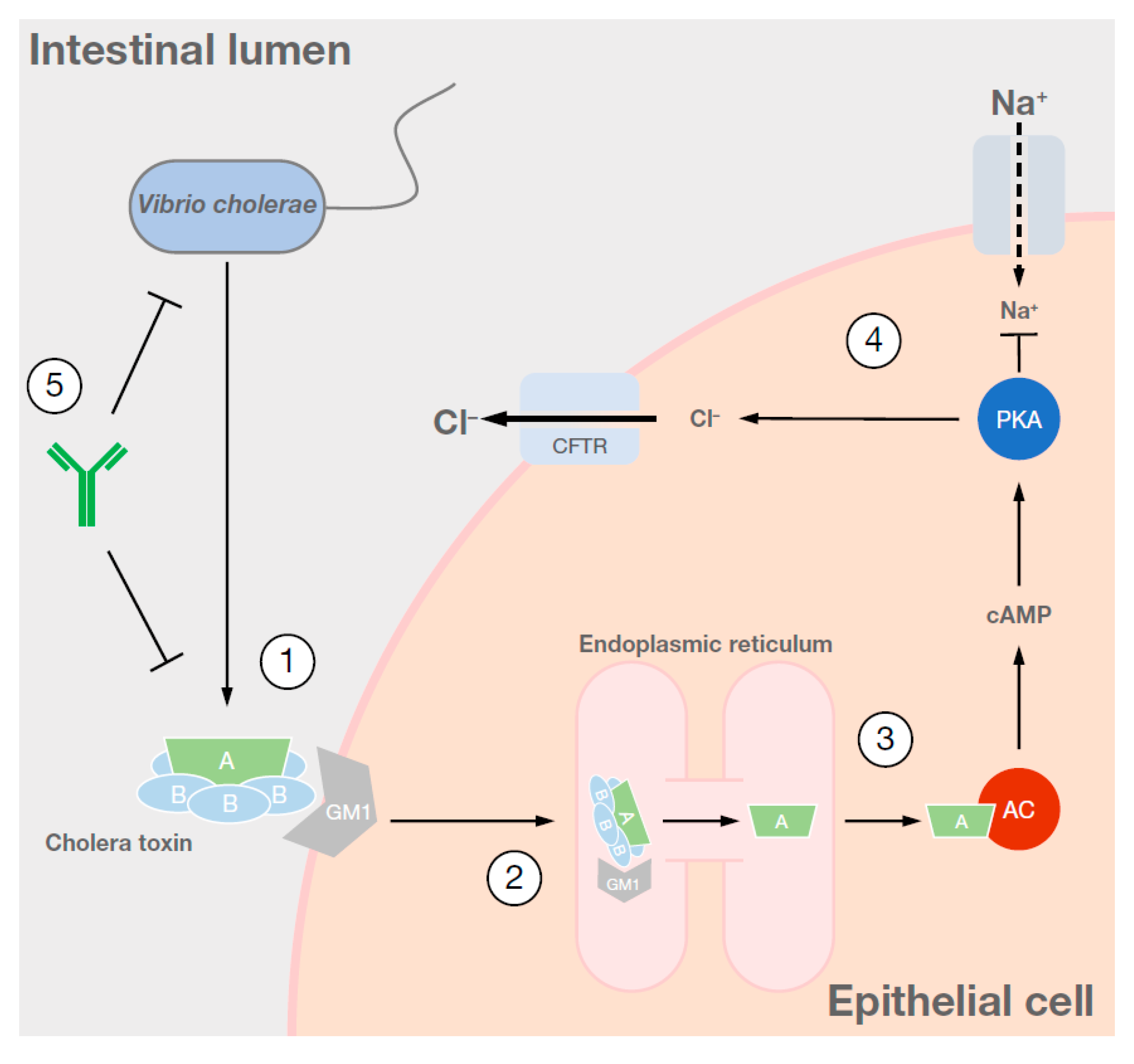
1. Cause and Symptoms of Cholera
2. Epidemiology of Cholera
2.1. Global Overview
2.2. Cholera in Developed Countries
2.3. Cholera in Travellers
2.4. Under-Reporting of Cholera Cases
2.5. Natural Immunity
3. Prevention of Cholera in Travellers
3.1. Preventive Hygiene Measures Including Washing Hands, Food Control, etc.
- Practice regular hygiene, especially hand hygiene with soap and water or, if not available, with an alcohol-based hand sanitiser solution;
- Practice hand hygiene especially before touching the nose, eyes, or mouth, and after using the toilet or touching objects at high risk of being contaminated;
- All precautions should be taken to avoid the ingestion of potentially contaminated food, drink, and drinking water by consuming food and water only from safe, known sources;
- Follow the five rules for food safety: wash hands often and always before handling and consuming food; make sure the food is cooked thoroughly; peel all vegetables and fruit if they are to be eaten raw; drink bottled water where available, or if the source of water is uncertain, bring it to a vigorous boil; separate cooked food from raw food, avoid uncooked food, and keep food at safe temperatures.
3.2. Chemoprophylaxis
3.3. Vaccination
4. Vaccines Available for Cholera
4.1. WC-rBS, Killed Whole-Cell Monovalent (O1) Vaccines with a Recombinant B Subunit of Cholera Toxin (Dukoral®)
4.2. BivWC, Killed Modified Whole-Cell Bivalent (O1 and O139) Vaccines without B Subunit
4.3. Recombinant Live OCV CVD 103-HgR (Vaxchora®)
| Vaccine and Manufacturer | Killed Whole-Cell Monovalent (O1) Vaccine with a Recombinant Cholera Toxin B Subunit WC-rBS (Dukoral®) Valneva (Sweden) [68] | Killed Whole-Cell Bivalent (O1 and O139) Vaccine without the Cholera Toxin B Subunit BivWC (Shanchol®) Shantha Biotechnics (India) [81] | Killed Whole-Cell Bivalent (O1 and O139 without Cholera Toxin B Subunit) BivWC (Euvichol™/Euvichol-Plus™) EuBiologics (S Korea) [85] | Live Attenuated Vaccine CVD 103-HgR (Vaxchora®) Emergent BioSolutions (USA) [65] |
|---|---|---|---|---|
| Composition | V. cholerae O1 Inaba, classical biotype (heat inactivated) 31.25 × 109 bacteria V. cholerae O1 Inaba, El Tor biotype (formalin inactivated) 31.25 × 109 bacteria V. cholerae O1 Ogawa, classical biotype (heat inactivated) 31.25 × 109 bacteria V. cholerae O1 Ogawa, classical biotype (formalin inactivated) 31.25 × 109 bacteria Recombinant cholera toxin B subunit 1 mg (produced in V. cholerae O1 Inaba, classical biotype strain 213) | V. cholerae O1 Inaba El Tor strain Phil 6973 (formaldehyde inactivated) 600 LEU V. cholerae O1 Ogawa classical strain Cairo 50 (heat inactivated) 300 EU LEU V. cholerae O1 Ogawa classical strain Cairo 50 (formaldehyde inactivated) 300 LEU V. cholerae O1 Inaba classical strain Cairo 48 (heat inactivated) 300 LEU V. cholerae O139 strain 4260B (formaldehyde killed) 600 LEU | V. cholerae O1 Inaba Cairo 48 classical biotype (heat inactivated) 300 LEU V. cholerae O1 Inaba Phil 6973 El Tor biotype (formalin inactivated) 600 LEU V. cholerae O1 Ogawa Cairo 50 classical biotype (formalin inactivated) 300 LEU V. cholerae O1 Ogawa Cairo 50 classical biotype (heat inactivated) 300 LEU V. cholerae O139 4260B (formalin inactivated) 600 LEU | V. cholerae strain CVD 103-HgR (live, attenuated) 4 × 108–2 × 109 CFU |
| Pharmaceutical form | Suspension and effervescent granules for suspension | Suspension | Suspension | Suspension |
| Route of administration | Oral | Oral | Oral | Oral |
| Recommended dose/regimen | 2 doses in adults and children ≥6 years of age, 3 doses for children aged <6 years; ≥1 week before potential exposure | 2 doses at an interval of 2 weeks; earliest onset of protection 7–10 days after completion | 2 doses at an interval of 2 weeks | Single dose; ≥10 days before potential exposure |
| Recommended age of vaccination | Adults and children ≥2 years of age | Adults and children ≥1 year of age | Adults and children ≥1 year of age | Adults and children aged ≥6 years |
| Availability | Worldwide (unavailable in the USA) | Worldwide (unavailable in the USA) | Worldwide (unavailable in the USA) | USA and Europe |
5. Development of Live Attenuated V. cholerae Vaccines
6. Unmet Needs in Cholera Vaccination
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Clemens, J.D.; Nair, G.B.; Ahmed, T.; Qadri, F.; Holmgren, J. Cholera. Lancet 2017, 390, 1539–1549. [Google Scholar] [CrossRef]
- Harris, J.B.; LaRocque, R.C.; Qadri, F.; Ryan, E.T.; Calderwood, S.B. Cholera. Lancet 2012, 379, 2466–2476. [Google Scholar] [CrossRef]
- Siddique, A.K.; Nair, G.B.; Alam, M.; Sack, D.A.; Huq, A.; Nizam, A.; Longini, I.M., Jr.; Qadri, F.; Faruque, S.M.; Colwell, R.R.; et al. El Tor cholera with severe disease: A new threat to Asia and beyond. Epidemiol. Infect. 2010, 138, 347–352. [Google Scholar] [CrossRef]
- World Health Organization. Cholera. Available online: https://www.who.int/health-topics/cholera (accessed on 1 May 2020).
- Childers, B.M.; Klose, K.E. Regulation of virulence in Vibrio cholerae: The ToxR regulon. Future Microbiol. 2007, 2, 335–344. [Google Scholar] [CrossRef]
- Taylor, R.K.; Miller, V.L.; Furlong, D.B.; Mekalanos, J.J. Use of phoA gene fusions to identify a pilus colonization factor coordinately regulated with cholera toxin. Proc. Natl. Acad. Sci. USA 1987, 84, 2833–2837. [Google Scholar] [CrossRef]
- Centers for Disease Contol and Prevention. Cholera—Vibrio Cholerae Infection. Available online: https://www.cdc.gov/cholera/general/index.html (accessed on 1 May 2020).
- Centers for Disease Contol and Prevention. Travellers’ Health. Available online: https://wwwnc.cdc.gov/travel/diseases/cholera (accessed on 1 May 2020).
- Centers for Disease Contol and Prevention. Cholera—Treatment. Available online: https://www.cdc.gov/cholera/treatment/index.html (accessed on 1 May 2020).
- Ali, M.; Nelson, A.R.; Lopez, A.L.; Sack, D.A. Updated global burden of cholera in endemic countries. PLoS Negl. Trop. Dis. 2015, 9, e0003832. [Google Scholar] [CrossRef]
- World Health Organization. Cholera 2017. Wkly. Epidemiol. Rec. 2018, 93, 489–500. [Google Scholar]
- World Health Organization. Cholera 2017. Wkly. Epidemiol. Rec. 2017, 92, 477–500. [Google Scholar]
- Zuckerman, J.N.; Rombo, L.; Fisch, A. The true burden and risk of cholera: Implications for prevention and control. Lancet Infect. Dis. 2007, 7, 521–530. [Google Scholar] [CrossRef]
- Scallan, E.; Hoekstra, R.M.; Angulo, F.J.; Tauxe, R.V.; Widdowson, M.A.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne illness acquired in the United States--Major pathogens. Emerg. Infect. Dis. 2011, 17, 7–15. [Google Scholar] [CrossRef]
- Steffen, R.; Acar, J.; Walker, E.; Zuckerman, J. Cholera: Assessing the risk to travellers and identifying methods of protection. Travel Med. Infect. Dis. 2003, 1, 80–88. [Google Scholar] [CrossRef]
- World Health Organization. Management of the Patient with Cholera. Available online: https://www.who.int/csr/resources/publications/cholera/whocddser9115rev1.pdf?ua=1 (accessed on 30 July 2020).
- Ley, B.; Khatib, A.M.; Thriemer, K.; von Seidlein, L.; Deen, J.; Mukhopadyay, A.; Chang, N.Y.; Hashim, R.; Schmied, W.; Busch, C.J.; et al. Evaluation of a rapid dipstick (Crystal VC) for the diagnosis of cholera in Zanzibar and a comparison with previous studies. PLoS ONE 2012, 7, e36930. [Google Scholar] [CrossRef] [PubMed]
- Learoyd, T.P.; Gaut, R.M. Cholera: Under diagnosis and differentiation from other diarrhoeal diseases. J. Travel Med. 2018, 25, S46–S51. [Google Scholar] [CrossRef] [PubMed]
- Legros, D.; Partners of the Global Task Force on Cholera Control. Global cholera epidemiology: Opportunities to reduce the burden of cholera by 2030. J. Infect. Dis. 2018, 218, S137–S140. [Google Scholar] [CrossRef]
- Harris, J.B. Cholera: Immunity and prospects in vaccine development. J. Infect. Dis. 2018, 218, S141–S146. [Google Scholar] [CrossRef]
- Holmgren, J.; Svennerholm, A.-M. Mechanisms of disease and immunity in cholera: A review. J. Infect. Dis. 1977, 136, S105–S112. [Google Scholar] [CrossRef]
- Saha, D.; LaRocque, R.C.; Khan, A.I.; Harris, J.B.; Begum, Y.A.; Akramuzzaman, S.M.; Faruque, E.T.; Ryan, E.T.; Qadri, F.; Calderwood, S.B. Incomplete correlation of serum vibriocidal antibody titer with protection from Vibrio cholerae infection in urban Bangladesh. J. Infect. Dis. 2004, 189, 2318–2322. [Google Scholar] [CrossRef][Green Version]
- Levinson, K.J.; Baranova, D.E.; Mantis, N.J. A monoclonal antibody that targets the conserved core/lipid A region of lipopolysaccharide affects motility and reduces intestinal colonization of both classical and El Tor Vibrio cholerae biotypes. Vaccine 2016, 34, 5833–5836. [Google Scholar] [CrossRef]
- Wang, Z.; Lazinski, D.W.; Camilli, A. Immunity provided by an outer membrane vesicle cholera vaccine is due to o-antigen-specific antibodies inhibiting bacterial motility. Infect. Immun. 2017, 85, e00626-16. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence. Diarrhoea—Prevention and Advice for Travellers. Available online: https://cks.nice.org.uk/diarrhoea-prevention-and-advice-for-travellers#!scenario (accessed on 1 May 2020).
- Wong, K.; Burdette, E.; Mahon, B.; Mintz, E.; Ryan, E.T.; Reingold, A. Recommendations of the Advisory Committee on Immunization Practices for Use of Cholera Vaccine. Morb. Mort. Wkly. Rep. 2017, 66, 482–485. [Google Scholar] [CrossRef]
- World Health Organization. International Travel and Health—Cholera. Available online: https://www.who.int/ith/2018-0925_travel-advice-cholera/en/ (accessed on 1 May 2020).
- Asociación Española de Vacunología. Cholera. Available online: https://www.vacunas.org/colera-2/ (accessed on 1 May 2020).
- Asociación Española de Vacunología. Vaccines for the Traveller. Available online: https://www.vacunas.org/vacunas-para-el-viajero/ (accessed on 1 May 2020).
- Fundacion IO. Cholera Vaccination. Available online: http://fundacionio.org/viajar/vacunas/vacunadelcolera.html. (accessed on 1 May 2020).
- Viajarseguro.org. Cholera. Available online: http://fundacionio.org/viajar/enfermedades/colera.html (accessed on 1 May 2020).
- Ministerio de Sanidad. Colera. Available online: https://www.mscbs.gob.es/profesionales/saludPublica/sanidadExterior/docs/COLERA.pdf (accessed on 30 July 2020).
- Viaggiaresicuri.it. Cholera. Available online: http://www.viaggiaresicuri.it/#/approfondimenti/saluteinviaggio (accessed on 1 May 2020).
- Società Italiana di Medicina dei Viaggi e delle Migrazioni. Health Precautions for International Travel Destinations. Available online: https://www.simvim.org/ (accessed on 1 May 2020).
- Ministero Della Salute. Cholera Prevention. Available online: http://www.salute.gov.it/portale/malattieInfettive/dettaglioSchedeMalattieInfettive.jsp?lingua=italiano&id=213&area=Malattie%20infettive&menu=indiceAZ&tab=6 (accessed on 1 May 2020).
- Fit for Travel. Fit for Travel—Germany. Available online: https://www.fit-for-travel.de/%C3%BCber-300-reiseziele/ (accessed on 22 September 2020).
- Centrum für Reisemedizin. Available online: https://www.crm.de/ (accessed on 22 September 2020).
- Robert Koch Institute. Available online: https://www.rki.de/EN/Home/homepage_node.html (accessed on 22 September 2020).
- Safetravel. Cholera. Available online: http://www.safetravel.ch/safetravel2/servlet/ch.ofac.wv.wv204j.pages.Wv204ConseilsSanteListeCtrl?action=afficheDetail&elementCourant=3 (accessed on 1 May 2020).
- Bundesamt fur Gesundheit. BAG-Bulletin. Available online: https://www.bag.admin.ch/bag/de/home/das-bag/publikationen/periodika/bag-bulletin.html (accessed on 1 May 2020).
- Tropimed. Cholera Factsheet. Available online: https://www.tropimed.com/tropimed/?locale=fr_CH#ch.ofac.td.client.command.DisplayMenuCommand?menu=Risques (accessed on 1 May 2020).
- Federal Office of Public Health. Vaccinations et Mesures Antipaludiques. Recommandations état Novembre 2018. Available online: https://www.infovac.ch/fr/?option=com_gd&view=listing&fid=1353&task=ofile (accessed on 1 May 2020).
- 1177.se. Vaccination against Cholera. Available online: https://www.1177.se/liv--halsa/reserad-och-vaccinationer/vaccinationer/vaccination-mot-kolera/ (accessed on 1 May 2020).
- Vaccin.se. Cholera. Available online: https://vaccin.se/sjukdomar/kolera/ (accessed on 1 May 2020).
- Vaccinationsguiden.se. Cholera. Available online: https://vaccinationsguiden.se/vaccinationsguiden/oversikt.html#sjukdom_kolera (accessed on 1 May 2020).
- Folkhälsomyndigheten. Cholera Vaccine. Available online: https://www.folkhalsomyndigheten.se/smittskydd-beredskap/vaccinationer/vacciner-a-o/kolera/ (accessed on 1 May 2020).
- Haut Conseil de la Santé Publique. Vaccins et Vaccinations du Voyageur. Available online: https://www.pasteur-lille.fr/vaccinations-voyages/fiches_recommandations/VACCINS.pdf (accessed on 1 May 2020).
- Institut Pasteur de Lille. The Métis app (Medical Advice to Travellers). Available online: https://www.pasteur-lille.fr/vaccinations-voyages/ (accessed on 1 May 2020).
- Institut Pasteur. Vibrios and Cholera. Available online: https://www.pasteur.fr/fr/sante-publique/cnr/les-cnr/vibrions-cholera/la-maladie-recommandations (accessed on 1 May 2020).
- MesVaccins.net. Cholera. Available online: https://www.mesvaccins.net/web/diseases/36-cholera (accessed on 1 May 2020).
- NHS Fit for Travel. Cholera. Available online: https://www.fitfortravel.nhs.uk/advice/disease-prevention-advice/cholera (accessed on 30 July 2020).
- Institut National de Santé Publique du Québec. Cholera Immunization. Available online: https://www.inspq.qc.ca/sante-voyage/guide/immunisation/cholera/immunisation (accessed on 1 May 2020).
- Centers for Disease Contol and Prevention. Cholera Vaccine for Travelers. Available online: https://wwwnc.cdc.gov/travel/news-announcements/cholera-vaccine-for-travelers (accessed on 1 May 2020).
- Australian Govenment Department of Health. Immunisation for Travel. Available online: https://www.health.gov.au/health-topics/immunisation/immunisation-throughout-life/immunisation-for-travel (accessed on 1 May 2020).
- Lopez, A.L.; Gonzales, M.L.; Aldaba, J.G.; Nair, G.B. Killed oral cholera vaccines: History, development and implementation challenges. Adv. Vacc. 2014, 2, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Bornside, G.H. Jaime Ferran and preventive inoculation against cholera. Bull. Hist. Med. 1981, 55, 516–532. [Google Scholar] [PubMed]
- Bornside, G.H. Waldemar Haffkine’s cholera vaccines and the Ferran-Haffkine priority dispute. J. Hist. Med. Allied Sci. 1982, 37, 399–422. [Google Scholar] [CrossRef]
- Pollitzer, R.; Burrows, W. Cholera studies. IV. Problems in immunology. Bull. World Health Organ. 1955, 12, 945–1107. [Google Scholar]
- Shin, S.; Desai, S.N.; Sah, B.K.; Clemens, J.D. Oral vaccines against cholera. Clin. Infect. Dis. 2011, 52, 1343–1349. [Google Scholar] [CrossRef] [PubMed][Green Version]
- World Health Organization. Prequalified Vaccines. Available online: https://extranet.who.int/gavi/PQ_Web/ (accessed on 1 May 2020).
- World Health Organization. WHO Prequalified Vaccines. Dukoral. Available online: https://extranet.who.int/gavi/PQ_Web/PreviewVaccine.aspx?nav=0&ID=116 (accessed on 1 May 2020).
- World Health Organization. WHO Prequalified Vaccines. Shanchol. Available online: https://extranet.who.int/gavi/PQ_Web/PreviewVaccine.aspx?nav=0&ID=249 (accessed on 1 April 2020).
- World Health Organization. Euvichol (Oral Cholera Vaccine). Available online: https://www.who.int/immunization_standards/vaccine_quality/pq_298_euvichol_1dose_eubiologics_PI.pdf?ua=1 (accessed on 1 April 2020).
- World Health Organization. Oral Cholera Vaccines. Available online: https://www.who.int/cholera/vaccines/en/ (accessed on 22 September 2020).
- PaxVax Bermuda Ltd. VAXCHORA (Cholera Vaccine, Live, Oral). Suspension for Oral Administration. Available online: https://www.paxvaxconnect.com/PDF/Vaxchora_Prescribing_Information.pdf (accessed on 1 May 2020).
- European Medicines Agency. Vaxchora. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/vaxchora#authorisation-details-section (accessed on 1 May 2020).
- European Medicines Agency. V. cholerae Live, Attenuated Strain CVD 103-HgR (Vaxchora). Available online: https://www.ema.europa.eu/en/documents/product-information/vaxchora-epar-product-information_en.pdf (accessed on 1 May 2020).
- Valneva UK Limited. Dukoral Suspension and Effervescent Granules—Summary of Product Characteristics. Available online: https://www.medicines.org.uk/emc/product/5087/smpc/print (accessed on 1 May 2020).
- Concha, A.; Giraldo, A.; Castaneda, E.; Martinez, M.; de la Hoz, F.; Rivas, F.; Depetris, A.; Svennerholm, A.M.; Sack, D.A. Safety and immunogenicity of oral killed whole cell recombinant B subunit cholera vaccine in Barranquilla, Colombia. Bull. Pan Am. Health Organ. 1995, 29, 312–321. [Google Scholar] [PubMed]
- Jertborn, M.; Svennerholm, A.M.; Holmgren, J. Safety and immunogenicity of an oral recombinant cholera B subunit-whole cell vaccine in Swedish volunteers. Vaccine 1992, 10, 130–132. [Google Scholar] [CrossRef]
- Sanchez, J.; Holmgren, J. Recombinant system for overexpression of cholera toxin B subunit in Vibrio cholerae as a basis for vaccine development. Proc. Natl Acad. Sci. USA 1989, 86, 481–485. [Google Scholar] [CrossRef]
- Shaikh, H.; Lynch, J.; Kim, J.; Excler, J.L. Current and future cholera vaccines. Vaccine 2020, 38 (Suppl. 1), A118–A126. [Google Scholar] [CrossRef]
- Clemens, J.D.; Sack, D.A.; Harris, J.R.; Chakraborty, J.; Khan, M.R.; Stanton, B.F.; Kay, B.A.; Khan, M.U.; Yunus, M.; Atkinson, W.; et al. Field trial of oral cholera vaccines in Bangladesh. Lancet 1986, 2, 124–127. [Google Scholar] [CrossRef]
- Van Loon, F.; Clemens, J.; Chakraborty, J.; Rao, M.; Kay, B.; Sack, D.A.; Yunus, M.; Ali, M.; Svennerholm, A.; Holmgren, J. Field trial of inactivated oral cholera vaccines in Bangladesh: Results from 5 years of follow-up. Vaccine 1996, 14, 162–166. [Google Scholar] [CrossRef]
- Sanchez, J.L.; Vasquez, B.; Begue, R.E.; Meza, R.; Castellares, G.; Cabezas, C.; Watts, D.M.; Svennerholm, A.M.; Sadoff, J.C.; Taylor, D.N. Protective efficacy of oral whole-cell/recombinant-B-subunit cholera vaccine in Peruvian military recruits. Lancet 1994, 344, 1273–1276. [Google Scholar] [CrossRef]
- Taylor, D.N.; Cardenas, V.; Sanchez, J.L.; Begue, R.E.; Gilman, R.; Bautista, C.; Perez, J.; Puga, R.; Gaillour, A.; Meza, R.; et al. Two-year study of the protective efficacy of the oral whole cell plus recombinant B subunit cholera vaccine in Peru. J. Infect. Dis. 2000, 181, 1667–1673. [Google Scholar] [CrossRef] [PubMed]
- Clemens, J.D.; Sack, D.A.; Ivanoff, B. Misleading negative findings in a field trial of killed, oral cholera vaccine in Peru. J. Infect. Dis. 2001, 183, 1306–1309. [Google Scholar] [CrossRef]
- Lucas, M.E.; Deen, J.L.; von Seidlein, L.; Wang, X.Y.; Ampuero, J.; Puri, M.; Ali, M.; Ansaruzzaman, M.; Amos, J.; Macuamule, A.; et al. Effectiveness of mass oral cholera vaccination in Beira, Mozambique. N. Engl. J. Med. 2005, 352, 757–767. [Google Scholar] [CrossRef]
- Khatib, A.M.; Ali, M.; von Seidlein, L.; Kim, D.R.; Hashim, R.; Reyburn, R.; Ley, B.; Thriemer, K.; Enwere, G.; Hutubessy, R.; et al. Effectiveness of an oral cholera vaccine in Zanzibar: Findings from a mass vaccination campaign and observational cohort study. Lancet Infect. Dis. 2012, 12, 837–844. [Google Scholar] [CrossRef]
- Trach, D.D.; Clemens, J.D.; Ke, N.T.; Thuy, H.T.; Son, N.D.; Canh, D.G.; Hang, P.V.; Rao, M.R. Field trial of a locally produced, killed, oral cholera vaccine in Vietnam. Lancet 1997, 349, 231–235. [Google Scholar] [CrossRef]
- Shantha. Killed Bivalent (O1 and O139) Whole Cell Oral Cholera Vaccine (Shanchol). Available online: http://shanthabiotech.com/wp-content/uploads/2015/05/shanchol-pack-insert.pdf (accessed on 1 May 2020).
- Sur, D.; Lopez, A.L.; Kanungo, S.; Paisley, A.; Manna, B.; Ali, M.; Niyogi, S.K.; Park, J.K.; Sarkar, B.; Puri, M.K.; et al. Efficacy and safety of a modified killed-whole-cell oral cholera vaccine in India: An interim analysis of a cluster-randomised, double-blind, placebo-controlled trial. Lancet 2009, 374, 1694–1702. [Google Scholar] [CrossRef]
- Wierzba, T.F.; Kar, S.K.; Mogasale, V.V.; Kerketta, A.S.; You, Y.A.; Baral, P.; Khuntia, H.K.; Ali, M.; Kim, Y.H.; Rath, S.B.; et al. Effectiveness of an oral cholera vaccine campaign to prevent clinically-significant cholera in Odisha State, India. Vaccine 2015, 33, 2463–2469. [Google Scholar] [CrossRef]
- Sur, D.; Kanungo, S.; Sah, B.; Manna, B.; Ali, M.; Paisley, A.M.; Niyogi, S.K.; Park, J.K.; Sarkar, B.; Puri, M.K.; et al. Efficacy of a low-cost, inactivated whole-cell oral cholera vaccine: Results from 3 years of follow-up of a randomized, controlled trial. PLoS Negl. Trop. Dis. 2011, 5, e1289. [Google Scholar] [CrossRef]
- World Health Organization. Use of Antibiotics for the Treatment of Cholera. Available online: https://www.who.int/cholera/task_force/use-of-antibiotics-for-the-treatment-of-cholera.pdf?ua=1 (accessed on 1 April 2020).
- Baik, Y.O.; Choi, S.K.; Olveda, R.M.; Espos, R.A.; Ligsay, A.D.; Montellano, M.B.; Yeam, J.S.; Yang, J.S.; Park, J.Y.; Kim, D.R.; et al. A randomized, non-inferiority trial comparing two bivalent killed, whole cell, oral cholera vaccines (Euvichol vs Shanchol) in the Philippines. Vaccine 2015, 33, 6360–6365. [Google Scholar] [CrossRef] [PubMed]
- Bi, Q.; Ferreras, E.; Pezzoli, L.; Legros, D.; Ivers, L.C.; Date, K.; Qadri, F.; Digilio, L.; Sack, D.A.; Ali, M.; et al. Protection against cholera from killed whole-cell oral cholera vaccines: A systematic review and meta-analysis. Lancet Infect. Dis 2017, 17, 1080–1088. [Google Scholar] [CrossRef]
- ClinicalTrials.gov National Library of Medicine (US). VAXCHORA Paediatric Trial NCT03220737. Available online: https://clinicaltrials.gov/ct2/show/NCT03220737 (accessed on 1 May 2020).
- Chen, W.H.; Cohen, M.B.; Kirkpatrick, B.D.; Brady, R.C.; Galloway, D.; Gurwith, M.; Hall, R.H.; Kessler, R.A.; Lock, M.; Haney, D.; et al. Single-dose Live Oral Cholera Vaccine CVD 103-HgR Protects Against Human Experimental Infection with Vibrio cholerae O1 El Tor. Clin. Infect. Dis. 2016, 62, 1329–1335. [Google Scholar] [CrossRef] [PubMed]
- McCarty, J.M.; Lock, M.D.; Bennett, S.; Hunt, K.M.; Simon, J.K.; Gurwith, M. Age-related immunogenicity and reactogenicity of live oral cholera vaccine CVD 103-HgR in a randomized, controlled clinical trial. Vaccine 2019, 37, 1389–1397. [Google Scholar] [CrossRef] [PubMed]
- McCarty, J.M.; Lock, M.D.; Hunt, K.M.; Simon, J.K.; Gurwith, M. Safety and immunogenicity of single-dose live oral cholera vaccine strain CVD 103-HgR in healthy adults age 18–45. Vaccine 2018, 36, 833–840. [Google Scholar] [CrossRef]
- McCarty, J.M.; Gierman, E.C.; Bedell, L.; Lock, M.D.; Bennett, S. Safety and immunogenicity of live oral cholera vaccine cvd 103-hgr in children and adolescents aged 6–17 years. Am. J. Trop. Med. Hyg. 2020, 102, 48–57. [Google Scholar] [CrossRef]
- Chen, W.H.; Greenberg, R.N.; Pasetti, M.F.; Livio, S.; Lock, M.; Gurwith, M.; Levine, M.M. Safety and immunogenicity of single-dose live oral cholera vaccine strain CVD 103-HgR, prepared from new master and working cell banks. Clin. Vaccine Immunol. CVI 2014, 21, 66–73. [Google Scholar] [CrossRef]
- Hubbard, T.P.; Billings, G.; Dorr, T.; Sit, B.; Warr, A.R.; Kuehl, C.J.; Kim, M.; Delgado, F.; Mekalanos, J.J.; Lewnard, J.A.; et al. A live vaccine rapidly protects against cholera in an infant rabbit model. Sci. Transl. Med. 2018, 10, eaap8423. [Google Scholar] [CrossRef]
- Sit, B.; Zhang, T.; Fakoya, B.; Akter, A.; Biswas, R.; Ryan, E.T.; Waldor, M.K. Oral immunization with a probiotic cholera vaccine induces broad protective immunity against Vibrio cholerae colonization and disease in mice. PLoS Negl. Trop. Dis. 2019, 13, e0007417. [Google Scholar] [CrossRef]
- Ismail, S.; Ahmad, S.; Azam, S.S. Vaccinomics to design a novel single chimeric subunit vaccine for broad-spectrum immunological applications targeting nosocomial Enterobacteriaceae pathogens. Eur. J. Pharm. Sci. 2020, 146, 105258. [Google Scholar] [CrossRef]
- Das, S.; Angsantikul, P.; Le, C.; Bao, D.; Miyamoto, Y.; Gao, W.; Zhang, L.; Eckmann, L. Neutralization of cholera toxin with nanoparticle decoys for treatment of cholera. PLoS Negl. Trop. Dis. 2018, 12, e0006266. [Google Scholar] [CrossRef] [PubMed]
- Luquero, F.J.; Azman, A.A. Protection of young children with cholera vaccine. Lancet Infect. Dis. 2018, 18, 947–948. [Google Scholar] [CrossRef]
- Qadri, F.; Ali, M.; Lynch, J.; Chowdhury, F.; Khan, A.I.; Wierzba, T.F.; Excler, J.L.; Saha, A.; Islam, M.T.; Begum, Y.A.; et al. Efficacy of a single-dose regimen of inactivated whole-cell oral cholera vaccine: Results from 2 years of follow-up of a randomised trial. Lancet Infect. Dis. 2018, 18, 666–674. [Google Scholar] [CrossRef]
- Ciglenecki, I.; Bichet, M.; Tena, J.; Mondesir, E.; Bastard, M.; Tran, N.T.; Antierens, A.; Staderini, N. Cholera in pregnancy: Outcomes from a specialized cholera treatment unit for pregnant women in Leogane, Haiti. PLoS Negl. Trop. Dis. 2013, 7, e2368. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, H.; Wang, B.; Song, G.; Hayden, J.C.; Amirthalingam, P.; Rahmani, J.; Bhagavathula, A.S.; Li, Z. Pregnancy outcomes after a mass vaccination campaign with an oral cholera vaccine: A systematic review and meta-analysis. BJOG Int. J. Obstet. Gynaecol. 2020, 17, 538–544. [Google Scholar] [CrossRef]
- World Health Organization. Ending Cholera—A Global Roadmap to 2030. Available online: https://www.who.int/cholera/publications/global-roadmap-summary.pdf (accessed on 1 May 2020).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
| Country | Recommending Bodies | Cholera Vaccination a Consideration for These Populations and under These Conditions |
|---|---|---|
Spain | Asociación Española de Vacunología (AEV) [28,29] | ALL |
| Viajarseguro.org [30,31] | NGO HCPs VFR | |
| Ministerio de Sanidad [32] | NGO HCPs VFR | |
Italy | Viaggiare Sicuri [33] | NGO |
| Società Italiana di Medicina dei Viaggi e delle Migrazioni (SIMVIM) [34] | No general advice—check website for specific recommendations according to type of trip | |
| Ministero della Salute [35] | ALL in endemic areas or during epidemic | |
Germany | Fit for Travel Germany [36] | NGO HCPs VFR during epidemic or prolonged stay |
| Centrum für Reisemedizin (CRM) [37] | NGO HCPs | |
| Robert Koch Institute (RKI) [38] | NGO HCPs VFR | |
Switzerland | Safetravel [39] | NGO HCPs VFR |
| Bundesamt für gesundheit (BAG) [40] | NGO SEA | |
| Tropimed Suisse [41] | NGO HCPs | |
| Infovac [42] | NGO HCPs SEA | |
Sweden | 1177 Vardguiden [43] | VFR ALL during epidemic |
| Vaccin.se [44] | NGO VFR | |
| VaccinationsGuiden.se [45] | ILL ALL in endemic areas or during epidemic | |
| Public Health Agency (PHA) [46] | NGO VFR ILL | |
France | Institut Pasteur de Lille [47,48] | NGO |
| Institut Pasteur (Paris) [49] | NGO HCPs | |
| MesVaccins.net [50] | NGO and HCPs only during an epidemic | |
United Kingdom | NHS Fit for Travel [51] | NGO HCPs VFR during epidemic |
Canada | Institut National de Santé Publique du Québec (INSPQ) [52] | NGO HCPs VFR during epidemic ILL |
USA | Centers for Disease Control and Prevention (CDC); Advisory Committee on Immunization Practices [53] | ALL adults (aged 18–64 years) during epidemic |
Australia | Healthdirect.gov.au [54] | NGO HCPs VFR ILL |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gabutti, G.; Rossanese, A.; Tomasi, A.; Giuffrida, S.; Nicosia, V.; Barriga, J.; Florescu, C.; Sandri, F.; Stefanati, A. Cholera, the Current Status of Cholera Vaccines and Recommendations for Travellers. Vaccines 2020, 8, 606. https://doi.org/10.3390/vaccines8040606
Gabutti G, Rossanese A, Tomasi A, Giuffrida S, Nicosia V, Barriga J, Florescu C, Sandri F, Stefanati A. Cholera, the Current Status of Cholera Vaccines and Recommendations for Travellers. Vaccines. 2020; 8(4):606. https://doi.org/10.3390/vaccines8040606
Chicago/Turabian StyleGabutti, Giovanni, Andrea Rossanese, Alberto Tomasi, Sandro Giuffrida, Vincenzo Nicosia, Juan Barriga, Caterina Florescu, Federica Sandri, and Armando Stefanati. 2020. "Cholera, the Current Status of Cholera Vaccines and Recommendations for Travellers" Vaccines 8, no. 4: 606. https://doi.org/10.3390/vaccines8040606
APA StyleGabutti, G., Rossanese, A., Tomasi, A., Giuffrida, S., Nicosia, V., Barriga, J., Florescu, C., Sandri, F., & Stefanati, A. (2020). Cholera, the Current Status of Cholera Vaccines and Recommendations for Travellers. Vaccines, 8(4), 606. https://doi.org/10.3390/vaccines8040606







