Impact of Pre-Existing Immunity on Live Attenuated Influenza Vaccine-Induced Cross-Protective Immunity
Abstract
1. Introduction
2. Materials and Methods
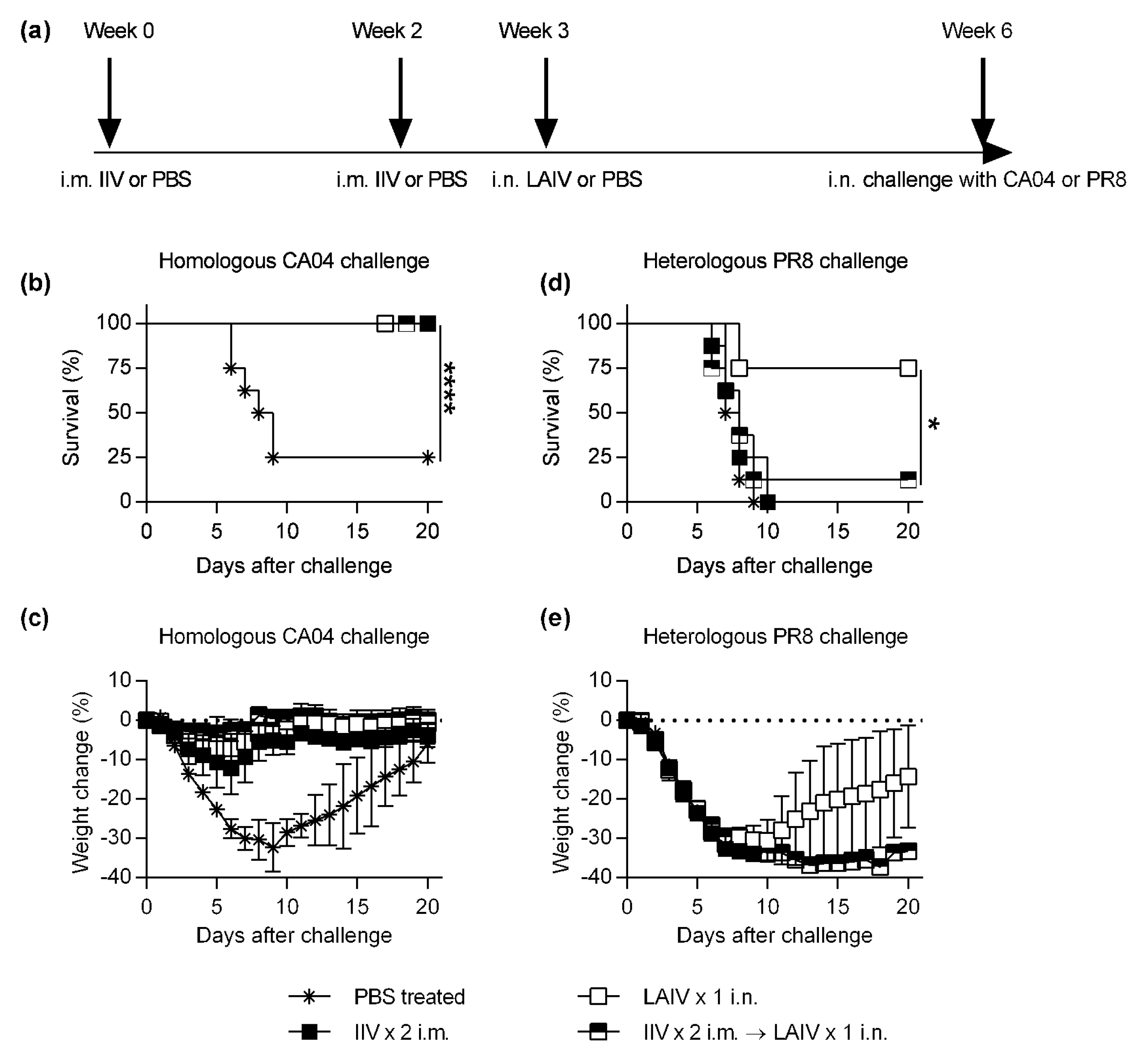
2.1. Immunisation and Challenge
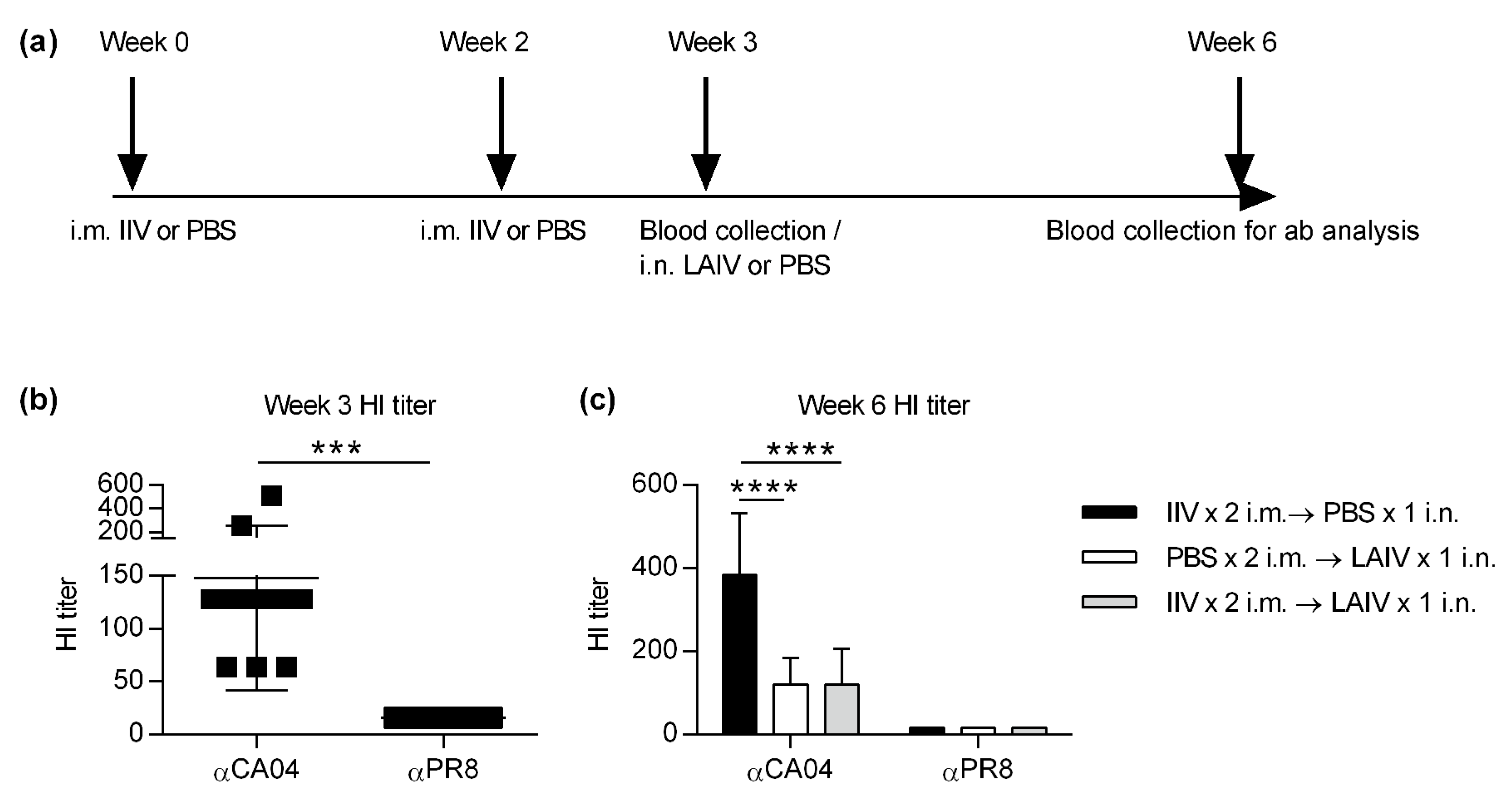
2.2. Hemagglutination Inhibition (HI) Assay
2.3. Statistical Analysis
3. Results
3.1. IIV-Derived Pre-Existing Immunity Inhibits LAIV-Induced Cross-Protection
3.2. IIV Does Not Impact Subsequent LAIV-Elicited Cross-Protective Hemagglutinin Inhibition (HI) Titers
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chen, G.L.; Lamirande, E.W.; Jin, H.; Kemble, G.; Subbarao, K. Safety, immunogencity, and efficacy of a cold-adapted A/Ann Arbor/6/60 (H2N2) vaccine in mice and ferrets. Virology 2010, 398, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Cox, A.; Baker, S.F.; Nogales, A.; Martinez-Sobrido, L.; Dewhurst, S. Development of a mouse-adapted live attenuated influenza virus that permits in vivo analysis of enhancements to the safety of live attenuated influenza virus vaccine. J. Virol. 2015, 89, 3421–3426. [Google Scholar] [CrossRef] [PubMed]
- Suguitan, A.L., Jr.; McAuliffe, J.; Mills, K.L.; Jin, H.; Duke, G.; Lu, B.; Luke, C.J.; Murphy, B.; Swayne, D.E.; Kemble, G.; et al. Live, attenuated influenza A H5N1 candidate vaccines provide broad cross-protection in mice and ferrets. PLoS Med. 2006, 3, e360. [Google Scholar] [CrossRef] [PubMed]
- Belshe, R.B.; Ambrose, C.S.; Yi, T. Safety and efficacy of live attenuated influenza vaccine in children 2–7 years of age. Vaccine 2008, 26 (Suppl. 4), D10–D16. [Google Scholar] [CrossRef]
- Chung, J.R.; Flannery, B.; Ambrose, C.S.; Begue, R.E.; Caspard, H.; DeMarcus, L.; Fowlkes, A.L.; Kersellius, G.; Steffens, A.; Fry, A.M.; et al. Live Attenuated and Inactivated Influenza Vaccine Effectiveness. Pediatrics 2019, 143. [Google Scholar] [CrossRef] [PubMed]
- Dowell, D.; Haegerich, T.M.; Chou, R. CDC Guideline for Prescribing Opioids for Chronic Pain—United States, 2016. MMWR Recomm. Rep. 2016, 65, 1–49. [Google Scholar] [CrossRef] [PubMed]
- Grohskopf, L.A.; Sokolow, L.Z.; Broder, K.R.; Walter, E.B.; Bresee, J.S.; Fry, A.M.; Jernigan, D.B. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices—United States, 2017–2018 Influenza Season. MMWR Recomm. Rep. 2017, 66, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Brickley, E.B.; Wright, P.F.; Khalenkov, A.; Neuzil, K.M.; Ortiz, J.R.; Rudenko, L.; Levine, M.Z.; Katz, J.M.; Brooks, W.A. The Effect of Preexisting Immunity on Virus Detection and Immune Responses in a Phase II, Randomized Trial of a Russian-Backbone, Live, Attenuated Influenza Vaccine in Bangladeshi Children. Clin. Infect. Dis. 2019, 69, 786–794. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Liepkalns, J.; Reber, A.J.; Lu, X.; Music, N.; Jacob, J.; Sambhara, S. Prior infection with influenza virus but not vaccination leaves a long-term immunological imprint that intensifies the protective efficacy of antigenically drifted vaccine strains. Vaccine 2016, 34, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Bodewes, R.; Kreijtz, J.H.; Baas, C.; Geelhoed-Mieras, M.M.; de Mutsert, G.; van Amerongen, G.; van den Brand, J.M.; Fouchier, R.A.; Osterhaus, A.D.; Rimmelzwaan, G.F. Vaccination against human influenza A/H3N2 virus prevents the induction of heterosubtypic immunity against lethal infection with avian influenza A/H5N1 virus. PLoS ONE 2009, 4, e5538. [Google Scholar] [CrossRef] [PubMed]
- Staeheli, P.; Grob, R.; Meier, E.; Sutcliffe, J.G.; Haller, O. Influenza virus-susceptible mice carry Mx genes with a large deletion or a nonsense mutation. Mol. Cell Biol. 1988, 8, 4518–4523. [Google Scholar] [CrossRef] [PubMed]
- Furuya, Y.; Chan, J.; Regner, M.; Lobigs, M.; Koskinen, A.; Kok, T.; Manavis, J.; Li, P.; Mullbacher, A.; Alsharifi, M. Cytotoxic T cells are the predominant players providing cross-protective immunity induced by {gamma}-irradiated influenza A viruses. J. Virol. 2010, 84, 4212–4221. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, J.; Arevalo, M.T.; Chen, Y.; Chen, S.; Zeng, M. T-cell-mediated cross-strain protective immunity elicited by prime-boost vaccination with a live attenuated influenza vaccine. Int. J. Infect. Dis. 2014, 27, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Zens, K.D.; Chen, J.K.; Farber, D.L. Vaccine-generated lung tissue-resident memory T cells provide heterosubtypic protection to influenza infection. JCI Insight 2016, 1. [Google Scholar] [CrossRef] [PubMed]
- Grohskopf, L.A.; Sokolow, L.Z.; Fry, A.M.; Walter, E.B.; Jernigan, D.B. Update: ACIP Recommendations for the Use of Quadrivalent Live Attenuated Influenza Vaccine (LAIV4)—United States, 2018–2019 Influenza Season. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 643–645. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roy, S.; Williams, C.M.; Pardo, J.; Wijesundara, D.K.; Furuya, Y. Impact of Pre-Existing Immunity on Live Attenuated Influenza Vaccine-Induced Cross-Protective Immunity. Vaccines 2020, 8, 459. https://doi.org/10.3390/vaccines8030459
Roy S, Williams CM, Pardo J, Wijesundara DK, Furuya Y. Impact of Pre-Existing Immunity on Live Attenuated Influenza Vaccine-Induced Cross-Protective Immunity. Vaccines. 2020; 8(3):459. https://doi.org/10.3390/vaccines8030459
Chicago/Turabian StyleRoy, Sreeja, Clare M Williams, Julian Pardo, Danushka K Wijesundara, and Yoichi Furuya. 2020. "Impact of Pre-Existing Immunity on Live Attenuated Influenza Vaccine-Induced Cross-Protective Immunity" Vaccines 8, no. 3: 459. https://doi.org/10.3390/vaccines8030459
APA StyleRoy, S., Williams, C. M., Pardo, J., Wijesundara, D. K., & Furuya, Y. (2020). Impact of Pre-Existing Immunity on Live Attenuated Influenza Vaccine-Induced Cross-Protective Immunity. Vaccines, 8(3), 459. https://doi.org/10.3390/vaccines8030459



