Evaluating the Relative Vaccine Effectiveness of Adjuvanted Trivalent Influenza Vaccine Compared to High-Dose Trivalent and Other Egg-Based Influenza Vaccines among Older Adults in the US during the 2017–2018 Influenza Season
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Overview
2.2. Data Sources
2.3. Study Population
2.4. Study Measures
2.5. Analyses
2.6. Economic Analysis
3. Results
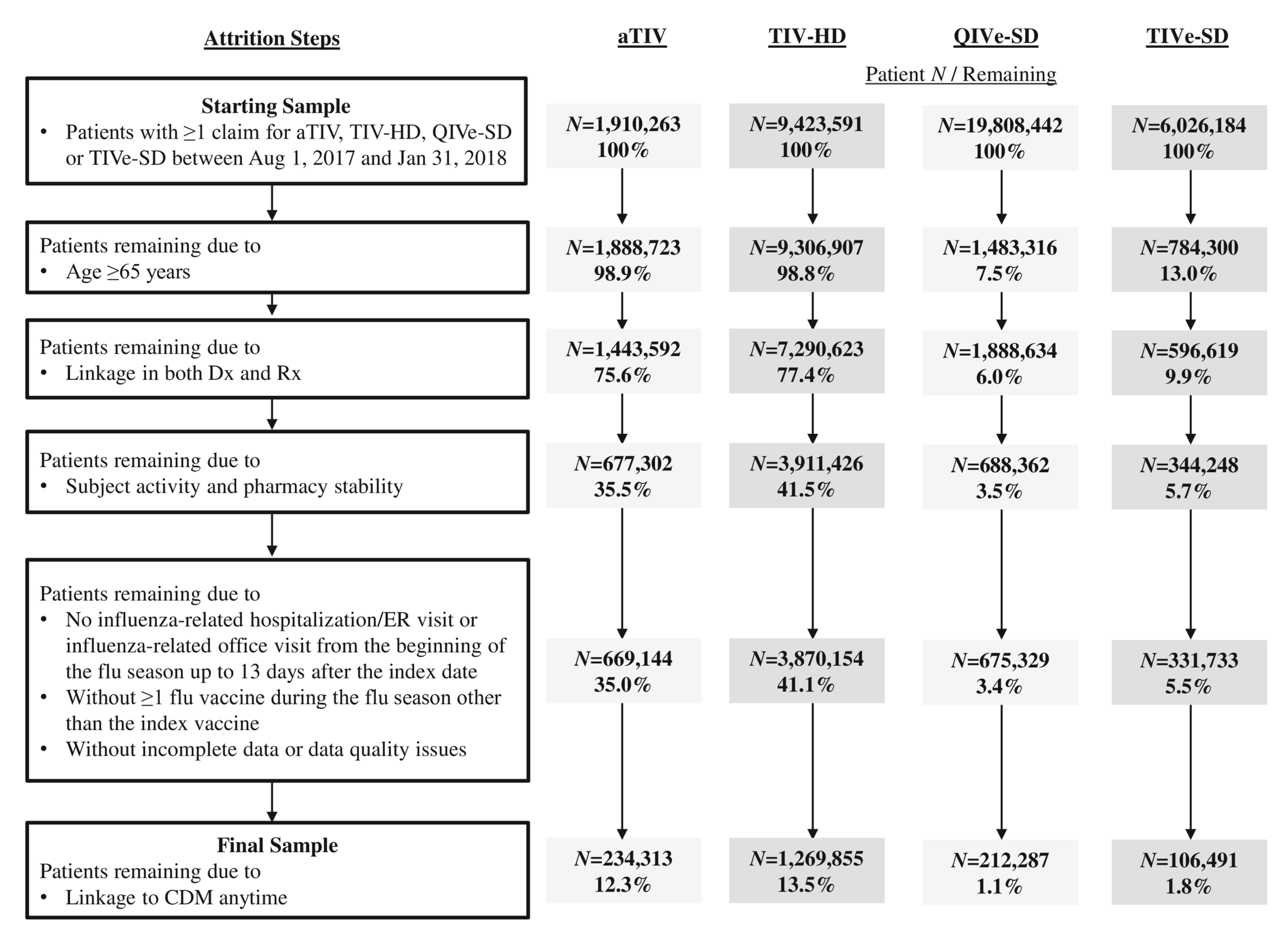
3.1. Study Sample
3.2. Patient Characteristics
3.3. Clinical Outcomes
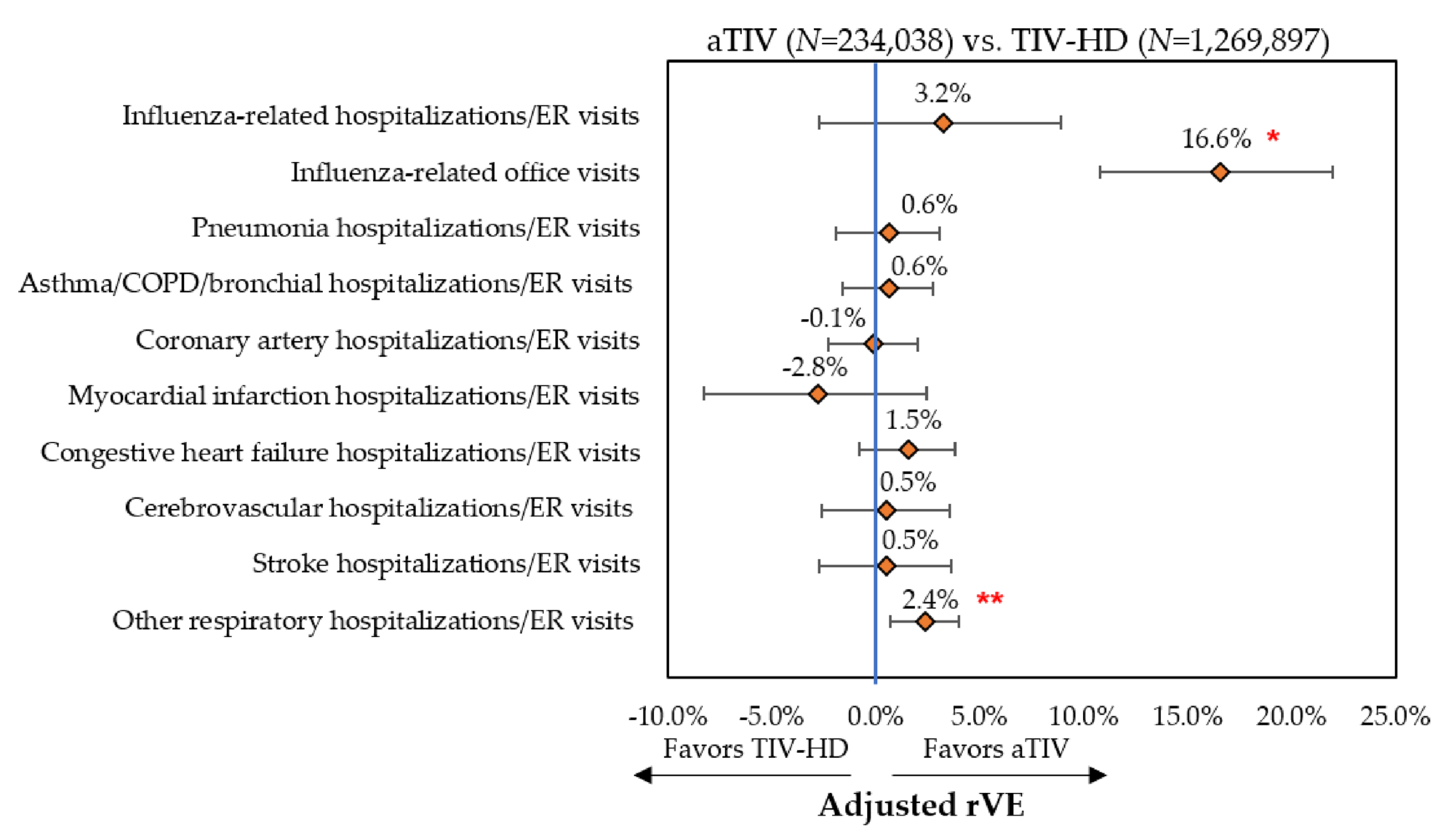
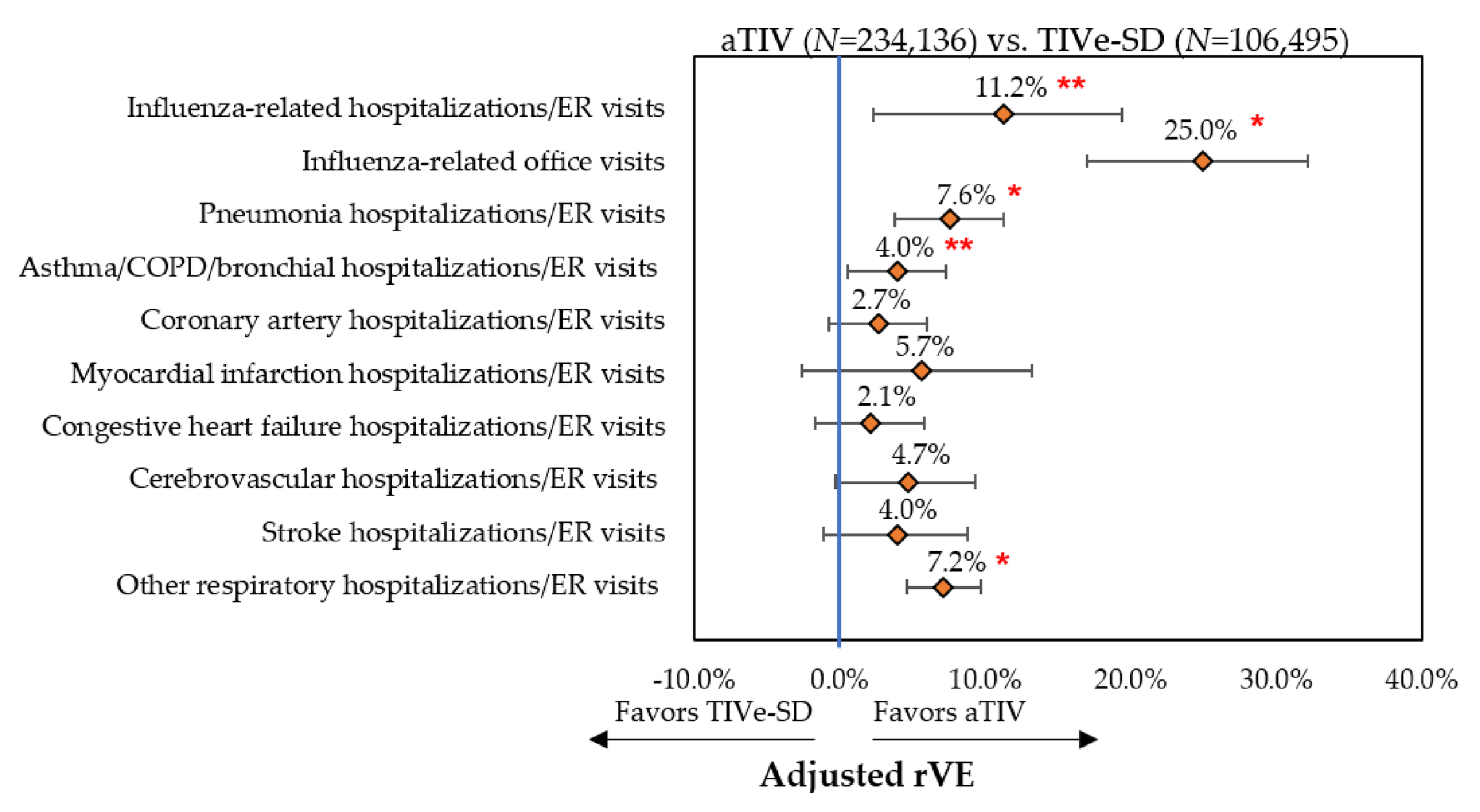
3.3.1. Influenza-Related Hospitalizations/ER Visits and Office Visits
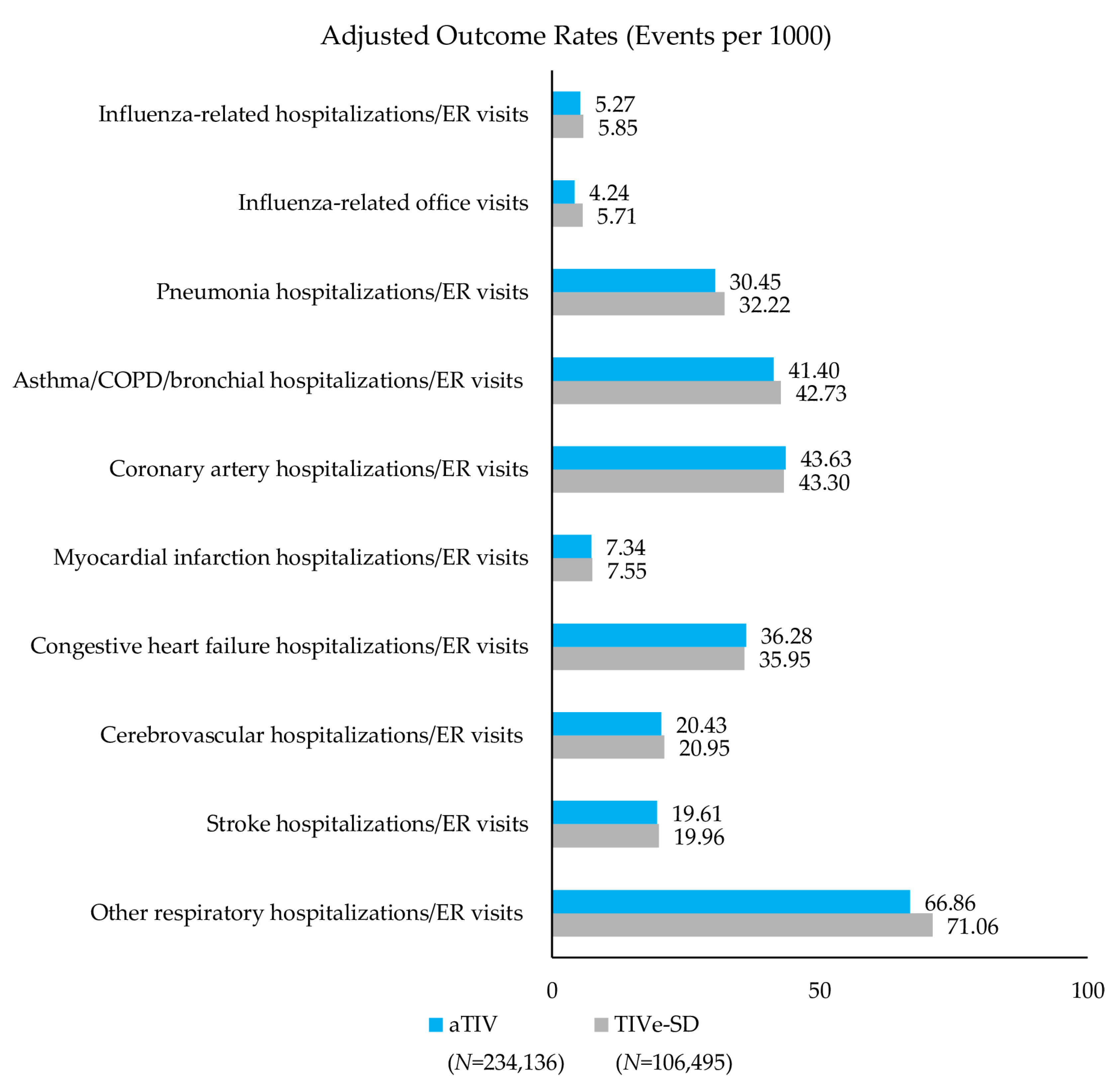
3.3.2. Cardio-Respiratory Hospitalizations/ER Visits
3.4. Economic Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Centers for Disease Control and Prevention. Estimated Influenza Illnesses, Medical visits, Hospitalizations, and Deaths in the United States—2017–2018 Influenza Season. 22 November 2019. Available online: https://www.cdc.gov/flu/about/burden/2017-2018.htm (accessed on 3 December 2019).
- Wilhelm, M. Influenza in older patients: A call to action and recent updates for vaccinations. Am. J. Manag. Care 2018, 24, S15–S24. [Google Scholar] [PubMed]
- Sullivan, S.G.; Price, O.H.; Regan, A.K. Burden, effectiveness and safety of influenza vaccines in elderly, paediatric and pregnant populations. Ther. Adv. Vaccines Immunother. 2019, 7, 2515135519826481. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. People 65 Years and Older & Influenza. 12 November 2019. Available online: https://www.cdc.gov/flu/highrisk/65over.htm (accessed on 3 December 2019).
- Ng, T.W.Y.; Cowling, B.J.; Gao, H.Z.; Thompson, M.G. Comparative Immunogenicity of Enhanced Seasonal Influenza Vaccines in Older Adults: A Systematic Review and Meta-analysis. J. Infect. Dis. 2019, 219, 1525–1535. [Google Scholar] [CrossRef] [PubMed]
- Domnich, A.; Arata, L.; Amicizia, D. Effectiveness of MF59-adjuvanted seasonal influenza vaccine in the elderly: A systematic review and meta-analysis. Vaccine 2017, 35, 513–520. [Google Scholar] [CrossRef]
- Baxter, D. Evaluating the case for trivalent or quadrivalent influenza vaccines. Hum. Vaccines Immunother. 2016, 12, 2712–2717. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. 2017–18 Summary of Recommendations. 2 April 2018. Available online: https://www.cdc.gov/flu/professionals/acip/2017-18summary.htm (accessed on 3 December 2019).
- Centers for Disease Control and Prevention. Summary of the 2017–2018 Influenza Season. 5 September 2019. Available online: https://www.cdc.gov/flu/about/season/flu-season-2017-2018.htm (accessed on 3 December 2019).
- Iob, A.; Brianti, G.; Zamparo, E.; Gallo, T. Evidence of increased clinical protection of an MF59-adjuvant influenza vaccine compared to a non-adjuvant vaccine among elderly residents of long-term care facilities in Italy. Epidemiol. Infect. 2005, 133, 687–693. [Google Scholar] [CrossRef]
- Mannino, S.; Villa, M.; Apolone, G.; Weiss, N.S.; Groth, N.; Aquino, I.; Boldori, L.; Caramaschi, F.; Gattinoni, A.; Malchiodi, G.; et al. Effectiveness of adjuvanted influenza vaccination in elderly subjects in northern Italy. Am. J. Epidemiol. 2012, 176, 527–533. [Google Scholar] [CrossRef]
- Lapi, F.; Marconi, E.; Simonetti, M.; Baldo, V.; Rossi, A.; Sessa, A.; Cricelli, C. Adjuvanted versus nonadjuvanted influenza vaccines and risk of hospitalizations for pneumonia and cerebro/cardiovascular events in the elderly. Expert Rev. Vaccines 2019, 18, 663–670. [Google Scholar] [CrossRef]
- NHS England. The National Flu Immunisation Programme 2018/19. 26 March 2019. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/694779/Annual_national_flu_programme_2018-2019.pdf (accessed on 7 November 2019).
- Public Health England. Surveillance of Influenza and Other Respiratory Viruses in the UK: Winter 2017 to 2018. May 2019. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/740606/Surveillance_of_influenza_and_other_respiratory_viruses_in_the_UK_2017_to_2018.pdf (accessed on 3 December 2019).
- Izurieta, H.S.; Chillarige, Y.; Kelman, J.; Wei, Y.; Lu, Y.; Xu, W.; Sung, H.M.; Lindaas, A.; Wernecke, M.; Macurdy, T.; et al. Relative effectiveness of cell-cultured and egg-based influenza vaccines among the U.S. elderly, 2017–2018. J. Infect. Dis. 2019, 220, 1255–1264. [Google Scholar] [CrossRef]
- Van Aalst, R.; Gravenstein, S.; Mor, V.; Mahmud, S.M.; Wilschut, J.; Postma, M.; Chit, A. Comparative effectiveness of high-dose versus adjuvanted influenza vaccine: A retrospective cohort study. Vaccine 2020, 38, 372–379. [Google Scholar] [CrossRef]
- Lee, J.K.H.; Lam, G.K.L.; Shin, T.; Kim, J.; Krishnan, A.; Greenberg, D.P.; Chit, A. Efficacy and effectiveness of high-dose versus standard-dose influenza vaccination for older adults: A systematic review and meta-analysis. Expert Rev. Vaccines 2018, 17, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Young-Xu, Y.; Van Aalst, R.; Mahmud, S.M.; Rothman, K.J.; Snider, J.T.; Westreich, D.; Mor, V.; Gravenstein, S.; Lee, J.K.H.; Thommes, E.W.; et al. Relative Vaccine Effectiveness of High-Dose Versus Standard-Dose Influenza Vaccines Among Veterans Health Administration Patients. J. Infect. Dis. 2018, 217, 1718–1727. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Chillarige, Y.; Izurieta, H.S.; Wei, Y.; Xu, W.; Lu, M.; Sung, H.M.; Lindaas, A.; Wernecke, M.; Macurdy, T.; et al. Effect of age on relative effectiveness of high-dose versus standard-dose influenza vaccines among US Medicare beneficiaries ages 65 years and older. J. Infect. Dis. 2019, 220, 1511–1520. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.C.; Dekoven, M.; Bouchard, J.; Massoudi, M.; Langer, J. Improved real-world glycaemic outcomes with liraglutide versus other incretin-based therapies in type 2 diabetes. Diabetes Obes. Metab. 2014, 16, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Sepassi, A.; Chingcuanco, F.; Gordon, R.; Meier, A.; Divino, V.; DeKoven, M.; Ben-Joseph, R. Resource utilization and charges of patients with and without diagnosed venous thromboembolism during primary hospitalization and after elective inpatient surgery: A retrospective study. J. Med. Econ. 2018, 21, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Agency for Healthcare Research and Quality. HCUP Cost-to-Charge Ratio Files. September 2018. Available online: https://www.hcup-us.ahrq.gov/db/state/costtocharge.jsp (accessed on 15 June 2019).
- Centers for Medicare & Medicaid Services. Hospital Outpatient Prospective Payment. Annual Policy Files; 2019. Available online: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/HospitalOutpatientPPS/Annual-Policy-Files.html (accessed on 3 December 2019).
- DiazGranados, C.A.; Robertson, C.A.; Talbot, H.K.; Landolfi, V.; Dunning, A.J.; Greenberg, D.P. Prevention of serious events in adults 65 years of age or older: A comparison between high-dose and standard-dose inactivated influenza vaccines. Vaccine 2015, 33, 4988–4993. [Google Scholar] [CrossRef] [PubMed]
- Peasah, S.K.; Meltzer, M.I.; Vu, M.; Moulia, D.L.; Bridges, C.B. Cost-effectiveness of increased influenza vaccination uptake against readmissions of major adverse cardiac events in the US. PLoS ONE 2019, 14, e0213499. [Google Scholar] [CrossRef]
- Austin, P.C. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat. Med. 2009, 28, 3083–3107. [Google Scholar] [CrossRef]
- Thoemmes, F.; Ong, A.D. A Primer on Inverse Probability of Treatment Weighting and Marginal Structural Models. Emerg. Adulthood 2016, 4, 40–59. [Google Scholar] [CrossRef]
- Austin, P.C.; Stuart, E.A. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat. Med. 2015, 34, 3661–3679. [Google Scholar] [CrossRef]
- Rothman, K.J. No adjustments are needed for multiple comparisons. Epidemiology 1990, 1, 43–46. [Google Scholar] [CrossRef]
- Saville, D.J. Multiple Comparison Procedures: The Practical Solution. Am. Stat. 1990, 44, 174–180. [Google Scholar]
- Bender, R.; Lange, S. Adjusting for multiple testing—When and how? J. Clin. Epidemiol. 2001, 54, 343–349. [Google Scholar] [CrossRef]
- Crown, W.H. Propensity-score matching in economic analyses: Comparison with regression models, instrumental variables, residual inclusion, differences-in-differences, and decomposition methods. Appl. Health Econ. Health Policy 2014, 12, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Rathouz, P.J. Estimating marginal and incremental effects on health outcomes using flexible link and variance function models. Biostatistics 2005, 6, 93–109. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.; Vogt, A. Outliers: An evaluation of methodologies. In Proceedings of the Survey Research Methods, Joint Statistical Meeting 2012, San Diego, CA, USA, 28 July–2 August 2012. [Google Scholar]
- Harding, A.T.; Heaton, N.S. Efforts to Improve the Seasonal Influenza Vaccine. Vaccines 2018, 6, 19. [Google Scholar] [CrossRef]
- Fingar, K.R.; Liang, L.; Stocks, C. Inpatient Hospital Stays and Emergency Department Visits Involving Influenza, 2006–2016: Statistical Brief #253. In Healthcare Cost and Utilization Project (HCUP) Statistical Briefs; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2006. [Google Scholar]
- Mylona, E.K.; Benitez, G.; Shehadeh, F.; Fleury, E.; Mylonakis, S.C.; Kalligeros, M.; Mylonakis, E. The association of obesity with health insurance coverage and demographic characteristics: A statewide cross-sectional study. Medicine 2020, 99, e21026. [Google Scholar] [CrossRef]
- Basu, J.; Hanchate, A.; Bierman, A. Racial/ethnic disparities in readmissions in US hospitals: The role of insurance coverage. INQUIRY J. Health Care Organ. Provis. Financ. 2018, 55, 0046958018774180. [Google Scholar] [CrossRef]
- Uddin, M.J.; Groenwold, R.H.; van Staa, T.P.; de Boer, A.; Belitser, S.V.; Hoes, A.W.; Roes, K.C.; Klungel, O.H. Performance of prior event rate ratio adjustment method in pharmacoepidemiology: A simulation study. Pharmacoepidemiol. Drug Saf. 2015, 24, 468–477. [Google Scholar] [CrossRef]
- Joint Committee on Vaccination and Immunisation. Minute 2019 10 Draft. 13 November 2019. Available online: https://app.box.com/s/iddfb4ppwkmtjusir2tc (accessed on 28 December 2019).
- Sanofi. Press Release. UK Marketing Authorisation Granted for Sanofi Pasteur’s Trivalent Influenza Vaccine (Split Virion, Inactivated) High Dose (TIV High Dose). 18 June 2019. Available online: https://www.sanofi.co.uk/-/media/Project/One-Sanofi-Web/Websites/Europe/Sanofi-UK/Home/media/2019/Sanofi-TIV-HD-MHRA-Approval-Press-Release-FINAL.pdf?la=encdc.gov/flu/about/season/flu-season-2017-2018.htm (accessed on 7 November 2019).
- Johnson, E.K.; Nelson, C.P. Values and pitfalls of the use of administrative databases for outcomes assessment. J. Urol. 2013, 190, 17–18. [Google Scholar] [CrossRef] [PubMed]
- Izurieta, H.S.; Thadani, N.; Shay, D.K.; Lu, Y.; Maurer, A.; Foppa, I.M.; Franks, R.; Pratt, D.; Forshee, R.A.; MaCurdy, T.; et al. Comparative effectiveness of high-dose versus standard-dose influenza vaccines in US residents aged 65 years and older from 2012 to 2013 using Medicare data: A retrospective cohort analysis. Lancet Infect. Dis. 2015, 15, 293–300. [Google Scholar] [CrossRef]



| Vaccine Cohort | aTIV | TIV-HD | SMD 1 | aTIV | QIVe-SD | SMD 1 | aTIV | TIVe-SD | SMD 1 |
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | N = 234,038 | N = 1,269,897 | - | N = 234,351 | N = 212,120 | - | N = 234,136 | N = 106,495 | - |
| Mean Age | 75.0 | 75.0 | 0.01 | 74.8 | 74.4 | −0.06 | 75.1 | 74.6 | −0.09 |
| SD | 6.3 | 6.3 | - | 6.3 | 6.7 | - | 6.3 | 6.5 | - |
| Median | 74 | 74 | - | 74 | 74 | - | 74 | 74 | - |
| Age Group (%) | |||||||||
| 65–74 years | 51.0% | 50.7% | −0.01 | 53.2% | 53.2% | 0.00 | 50.3% | 53.3% | 0.06 |
| 75–84 years | 33.4% | 34.0% | 0.01 | 31.7% | 31.7% | 0.00 | 33.8% | 31.6% | −0.05 |
| ≥85 years | 15.6% | 15.3% | −0.01 | 15.1% | 15.1% | 0.00 | 15.9% | 15.1% | −0.02 |
| Gender (%) | |||||||||
| Female | 59.6% | 59.9% | 0.01 | 59.3% | 60.6% | 0.03 | 59.5% | 61.7% | 0.04 |
| Geographic Region (%) | |||||||||
| Northeast | 17.3% | 16.9% | −0.01 | 15.9% | 15.9% | 0.00 | 14.8% | 15.1% | 0.01 |
| Midwest | 17.4% | 17.7% | 0.01 | 14.7% | 14.7% | 0.00 | 11.3% | 11.8% | 0.02 |
| South | 44.9% | 44.8% | 0.00 | 47.1% | 46.8% | −0.01 | 50.3% | 49.7% | −0.01 |
| West | 20.4% | 20.5% | 0.00 | 22.3% | 22.6% | 0.01 | 23.6% | 23.4% | −0.01 |
| Payer Type (%) | |||||||||
| Third party | 32.2% | 32.1% | 0.00 | 34.2% | 34.3% | 0.00 | 28.5% | 28.6% | 0.00 |
| Medicare Part D | 29.6% | 29.5% | 0.00 | 24.9% | 25.0% | 0.00 | 28.0% | 28.8% | 0.02 |
| Medicare | 37.6% | 37.7% | 0.00 | 39.7% | 39.6% | 0.00 | 42.4% | 41.5% | −0.02 |
| Other/Unknown | 0.7% | 0.7% | 0.00 | 1.2% | 1.1% | 0.00 | 1.2% | 1.1% | −0.01 |
| Vaccine Cohort | aTIV | TIV-HD | SMD 1 | aTIV | QIVe-SD | SMD 1 | aTIV | TIVe-SD | SMD 1 |
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | N = 234,038 | N = 1,269,897 | N = 234,351 | N = 212,120 | N = 234,136 | N = 106,495 | |||
| Month of Flu Vaccination (%) | |||||||||
| August | 6.0% | 6.1% | 0.00 | 6.1% | 6.0% | −0.01 | 6.3% | 5.7% | −0.03 |
| September | 30.4% | 30.9% | 0.01 | 26.6% | 26.6% | 0.00 | 25.5% | 25.2% | −0.01 |
| October | 41.1% | 41.1% | 0.00 | 40.9% | 41.0% | 0.00 | 41.2% | 41.6% | 0.01 |
| November | 14.3% | 14.0% | −0.01 | 15.4% | 15.3% | 0.00 | 15.5% | 15.6% | 0.00 |
| December | 4.7% | 4.5% | −0.01 | 5.9% | 5.9% | 0.00 | 5.9% | 6.0% | 0.01 |
| January | 3.5% | 3.4% | −0.01 | 5.1% | 5.2% | 0.00 | 5.7% | 5.8% | 0.01 |
| CCI Score (%) | |||||||||
| 0 | 56.9% | 55.1% | −0.04 | 53.3% | 53.5% | 0.00 | 53.6% | 53.8% | 0.00 |
| 1 | 20.8% | 21.4% | 0.01 | 21.6% | 21.5% | 0.00 | 21.4% | 21.3% | 0.00 |
| 2 | 11.9% | 12.3% | 0.01 | 12.8% | 12.8% | 0.00 | 12.8% | 12.7% | 0.00 |
| 3+ | 10.4% | 11.3% | 0.03 | 12.4% | 12.2% | 0.00 | 12.2% | 12.2% | 0.00 |
| Mean CCI Score | 0.9 | 0.9 | 0.04 | 1.0 | 1.0 | 0.00 | 0.9 | 1.0 | 0.01 |
| SD | 1.3 | 1.3 | - | 1.4 | 1.4 | - | 1.4 | 1.4 | - |
| Median | 0 | 0 | - | 0 | 0 | - | 0 | 0 | - |
| Pre-index Comorbidities (%) | |||||||||
| Asthma | 3.6% | 3.8% | 0.01 | 4.0% | 3.9% | −0.01 | 3.9% | 4.0% | 0.00 |
| Blood Disorders | 0.3% | 0.3% | 0.00 | 0.3% | 0.3% | 0.00 | 0.3% | 0.3% | 0.00 |
| Chronic Lung Disease | 8.4% | 8.8% | 0.01 | 9.1% | 9.5% | 0.01 | 9.0% | 9.8% | 0.03 |
| Diabetes | 20.0% | 21.6% | 0.04 | 22.1% | 23.6% | 0.03 | 21.8% | 24.1% | 0.06 |
| Heart Disease | 12.1% | 12.5% | 0.01 | 13.3% | 13.2% | 0.00 | 13.3% | 13.0% | −0.01 |
| Kidney Disorders | 8.2% | 8.8% | 0.02 | 9.5% | 8.8% | −0.02 | 9.5% | 9.0% | −0.02 |
| Liver Disorders | 2.1% | 2.1% | 0.00 | 2.4% | 2.4% | 0.00 | 2.3% | 2.4% | 0.00 |
| Neurological or Neurodevelopmental Conditions | 4.8% | 4.8% | 0.00 | 5.0% | 5.1% | 0.01 | 5.0% | 4.8% | −0.01 |
| Weakened Immune system 2 | 9.7% | 9.9% | 0.01 | 9.8% | 9.8% | 0.00 | 9.8% | 9.4% | −0.01 |
| IBD | 0.6% | 0.6% | 0.00 | 0.6% | 0.6% | 0.00 | 0.6% | 0.6% | 0.00 |
| Indicators of Frail Health Status (%) | |||||||||
| Home oxygen use | 4.1% | 4.0% | 0.00 | 4.3% | 4.4% | 0.00 | 4.3% | 4.4% | 0.00 |
| Wheelchair use | 2.2% | 2.3% | 0.01 | 2.3% | 2.7% | 0.03 | 2.3% | 2.5% | 0.02 |
| Walker use | 3.3% | 3.3% | 0.00 | 3.4% | 3.5% | 0.01 | 3.4% | 3.5% | 0.01 |
| Dementia | 1.3% | 1.3% | 0.00 | 1.4% | 1.2% | −0.01 | 1.4% | 1.3% | 0.00 |
| Urinary catheter use | 0.4% | 0.4% | 0.00 | 0.5% | 0.4% | −0.01 | 0.5% | 0.4% | −0.02 |
| Falls | 0.9% | 0.9% | 0.00 | 0.9% | 0.8% | −0.01 | 0.9% | 0.9% | 0.00 |
| Fractures | 0.6% | 0.6% | 0.00 | 0.6% | 0.5% | −0.01 | 0.6% | 0.6% | 0.00 |
| Pre-index hospitalization (%) | 7.8% | 7.9% | 0.00 | 8.2% | 7.8% | −0.01 | 8.2% | 7.7% | −0.02 |
| Mean pre-index outpatient pharmacy costs | $2398 | $2430 | 0.01 | $2473 | $2323 | −0.03 | $2449 | $2479 | 0.01 |
| SD | $5924 | $5488 | $6010 | $5918 | $6011 | $5244 | |||
| Median | $852 | $889 | $885 | $786 | $879 | $959 | |||
| Mean pre-index inpatient costs | $827 | $825 | 0.00 | $927 | $839 | −0.01 | $925 | $846 | −0.01 |
| SD | $9156 | $7774 | $9997 | $9983 | $10,191 | $8009 | |||
| Median | $0 | $0 | $0 | $0 | $0 | $0 | |||
| Mean pre-index outpatient medical costs | $1734 | $1696 | −0.01 | $1822 | $1633 | −0.03 | $1812 | $1624 | −0.03 |
| SD | $6347 | $6621 | $6523 | $6409 | $6463 | $6493 | |||
| Median | $400 | $397 | $419 | $371 | $416 | $361 | |||
| Mean TOTAL pre-index costs 3 | $4958 | $4951 | 0.01 | $5221 | $4795 | −0.08 | $5186 | $4950 | −0.02 |
| SD | $13,353 | $12,259 | $14,116 | $13,852 | $14,214 | $12,259 | |||
| Median | $2028 | $2051 | $2112 | $1856 | $2100 | $2039 | |||
| Predicted Mean Annualized Cost | aTIV N = 234,313 | TIV-HD N = 234,313 | Incremental Mean | ||
|---|---|---|---|---|---|
| Mean | 95% CIs | Mean | 95% CIs | ||
| All-cause total | $9999 | $9925–$10,069 | $10,022 | $9963–$10,088 | $23.24 |
| Influenza-related total | $28.21 | $24.60–$32.39 | $31.77 | $27.73–$36.26 | $3.56 |
| Influenza-related hospitalizations | $27.59 | $23.00–$32.85 | $26.29 | $22.41–$30.81 | −$1.30 |
| Influenza-related ER | $3.97 | $3.52–$4.43 | $4.49 | $3.99–$4.97 | $0.52 |
| Influenza-related office visit + oseltamivir | $1.10 | $1.04–$1.17 | $1.36 | $1.29–$1.45 | $0.26 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pelton, S.I.; Divino, V.; Shah, D.; Mould-Quevedo, J.; DeKoven, M.; Krishnarajah, G.; Postma, M.J. Evaluating the Relative Vaccine Effectiveness of Adjuvanted Trivalent Influenza Vaccine Compared to High-Dose Trivalent and Other Egg-Based Influenza Vaccines among Older Adults in the US during the 2017–2018 Influenza Season. Vaccines 2020, 8, 446. https://doi.org/10.3390/vaccines8030446
Pelton SI, Divino V, Shah D, Mould-Quevedo J, DeKoven M, Krishnarajah G, Postma MJ. Evaluating the Relative Vaccine Effectiveness of Adjuvanted Trivalent Influenza Vaccine Compared to High-Dose Trivalent and Other Egg-Based Influenza Vaccines among Older Adults in the US during the 2017–2018 Influenza Season. Vaccines. 2020; 8(3):446. https://doi.org/10.3390/vaccines8030446
Chicago/Turabian StylePelton, Stephen I., Victoria Divino, Drishti Shah, Joaquin Mould-Quevedo, Mitch DeKoven, Girishanthy Krishnarajah, and Maarten J. Postma. 2020. "Evaluating the Relative Vaccine Effectiveness of Adjuvanted Trivalent Influenza Vaccine Compared to High-Dose Trivalent and Other Egg-Based Influenza Vaccines among Older Adults in the US during the 2017–2018 Influenza Season" Vaccines 8, no. 3: 446. https://doi.org/10.3390/vaccines8030446
APA StylePelton, S. I., Divino, V., Shah, D., Mould-Quevedo, J., DeKoven, M., Krishnarajah, G., & Postma, M. J. (2020). Evaluating the Relative Vaccine Effectiveness of Adjuvanted Trivalent Influenza Vaccine Compared to High-Dose Trivalent and Other Egg-Based Influenza Vaccines among Older Adults in the US during the 2017–2018 Influenza Season. Vaccines, 8(3), 446. https://doi.org/10.3390/vaccines8030446




