1. Introduction
Marek’s disease (MD), which is caused by the MD virus (MDV), causes major economic losses to the poultry industry [
1]. MDV is divided into three serotypes: type I (MDV-1), type II (MDV-2), and type III (MDV-3) [
2], which have a strong ability to spread [
3]. The feather follicle epithelium of sick and infected chickens contains large quantities of MDV virions, which could cause environmental pollution [
4,
5]. Therefore, MDV-infected chickens continuously release infectious virions that, when mixed with dust, spread through the air, increasing the risk of spreading MDV through flocks [
5].
There are three types of variations in a virus: one caused by mutation of the nucleic acid sequence in the virus replication; one caused by an exchange of the genome segments between different viruses; and one involving gene recombination between viruses in different genera [
6]. Recombination can be an essential evolutionary driving force in herpesvirus, and the presence of inverted repeats in the alphaherpesvirus genome allows segment inversion as a consequence of specific recombination between repeated sequences during DNA replication [
7]. In the 1990s, Isfort et al. discovered that MDV and reticuloendotheliosis virus (REV) can facilitate natural gene recombination when MDV and REV are serially passaged in chicken embryo fibroblasts (CEFs) [
8,
9]. Davidson et al. amplified an MDV fragment containing the REV-long terminal repeat (LTR) from tumor samples using the hotspot-combined PCR (HS-cPCR) method, which confirmed that genetic recombination between MDV and REV can also occur in chickens [
10]. In China, a large number of epidemiological investigations have found tumors in vaccine-immunized chickens. In 2001, Zhang et al. isolated a natural recombinant MDV wild-type strain, GX0101, integrated with REV-LTR [
11]. Because of the low probability of natural recombination between MDV and REV, the recombinant virus can be continuously isolated, indicating that the restructuring process has a competitive advantage [
12].
MD can be successfully controlled by vaccination, and CVI988/Rispens is widely used in the intensive production of the poultry industry [
13]. With the use of MDV vaccines, the virulence of MDV isolates tends to improve [
14,
15]. In recent years, tumors have been on the rise even in vaccinated flocks, which means that MD cannot be fully protected by conventional vaccines, especially from supervirulent strains. Thus, a better vaccine is needed. Genetic recombinant vaccines have become a widely researched topic. Using an infectious clone of GX0101, Aijun et al. knocked out REV-LTR through homologous recombination and found that the horizontal transmission ability of GX0101∆LTR was greatly reduced while its pathogenicity was increased, which meant that LTR accelerated the virus’s replication rate and decreased the virulence of the virus [
16]. A comparative analysis between a recombinant LTR-inserted MDV and a virulent virus suggested that the horizontal transmission capacity was not necessarily consistent with the pathogenicity of MDV, that is LTR accelerated the virus’s replication but did not change the virulence of the parental virus [
17]. Mays et al. found that the insertion of REV-LTR into vvMDV Md5 was fully attenuated in maternal antibody-positive chickens after 40 passages. However, although the vaccination of chicks at hatching with this recombinant virus could protect chickens against MDV-induced bursa and thymic atrophy, it would not provide the same level of protection against MD tumors as Rispens [
18]. Previous studies have laid the theoretical foundation to introduce LTR into vaccine strains to construct a new MD vaccine strain. In this study, a recombinant LTR-integrated MDV CVI988/Rispens (rMDV) was constructed, and its safety and efficacy were evaluated to develop a new, effective vaccine against MDV.
2. Materials and Methods
2.1. Animals
One-day-old SPF chickens were incubated and kept under controlled temperatures (28 °C–30 °C). The chickens were housed in an isolator with a 12 h light/dark cycle and were given free access to food and water during the study. The care and maintenance of all the animals was done in accordance with the Institutional Animal Care and Use Committee guidelines set by the Shanghai Veterinary Research Institute, Chinese Academy of Agricultural Science (Approval No. SHVRI(SH2015-0118)).
2.2. Viruses
CVI988/Rispens was preserved in a laboratory, and the vvMDV Md5 strain was provided by Professor Cui of Shandong Agriculture University. Viral titers were determined by a viral plaque assay [
19]. Viruses were stored in liquid nitrogen until use.
2.3. rMDV and Rispens Growth Rate Curves
Chicken embryo fibroblasts (CEF) cells were prepared and cultured for 24 h. Rispens and rMDV were separately diluted at 50–60 PFU/mL and then inoculated onto four plates of CEF cultures. Two plates for each group were harvested at each time point. The virus titer from each culture was determined by a plaque assay. The data used to generate the virus growth rate curves were expressed as the PFU per day/PFU by day 6.
2.4. DNA Extraction and PCR
Total DNA was extracted from the tissues of the immunized or control chickens. Briefly, we prepared the pretreatment buffer first, containing 40 μL Tween-20, 40 μL NP40, and 920 μL PBS. Then, we thoroughly mixed 60 μL PBS with 30 μL trypsin K. Next, we took 85 μL tissue grinding fluid in a PCR tube and added 15 μL pretreatment buffer to perform the following procedure: 55 °C for 45 min and 95 °C for 15 min. The DNA extraction was conserved at 4 °C before use.
PCR was carried out in a total volume of 50 μL containing 25 μL of 2× PCR mix (TaKaRa, Japan), 10 μmol/L each of the primers (
Table 1), and 2 μL DNA templates. The PCR conditions and cycling parameters were the same for all primer pairs used: denaturation at 94 °C for 2 min, 30 cycles of amplification at 94 °C for 30 s, 55 °C for 30 s, and 68 °C for 3 min, followed by 68 °C for 5 min.
2.5. Safety of rMDV
One hundred one-day-old SPF chickens were studied to analyze the safety of rMDV. The chickens were separated into a control group and three virus-inoculated groups. Forty chickens were subcutaneously inoculated with an overdose (10 times the dose) of rMDV containing 30,000 PFU of the virus. The two other groups, with 20 chickens each, were subcutaneously inoculated with one-dose rMDV containing 3000 PFU of the virus and CVI988/Rispens, respectively. These chickens were inoculated 14 days later. The 20 control chickens were subcutaneously injected with phosphate-buffered saline (PBS) (
Table 2). All chickens were kept under the same conditions and observed for 60 days.
2.6. Horizontal Transmission Capacity of rMDV
Ninety chickens were divided into three large groups (G1, G2, and G3), and each group was divided into two groups (i.e., G1a and G1b; G2a and G2b; and G3a and G3b). Fifteen chickens were then immunized with rMDV, Rispens, or PBS using an overdose (30,000 PFU/dose), and 15 non-immunized chickens cohabited with the immunized chickens (
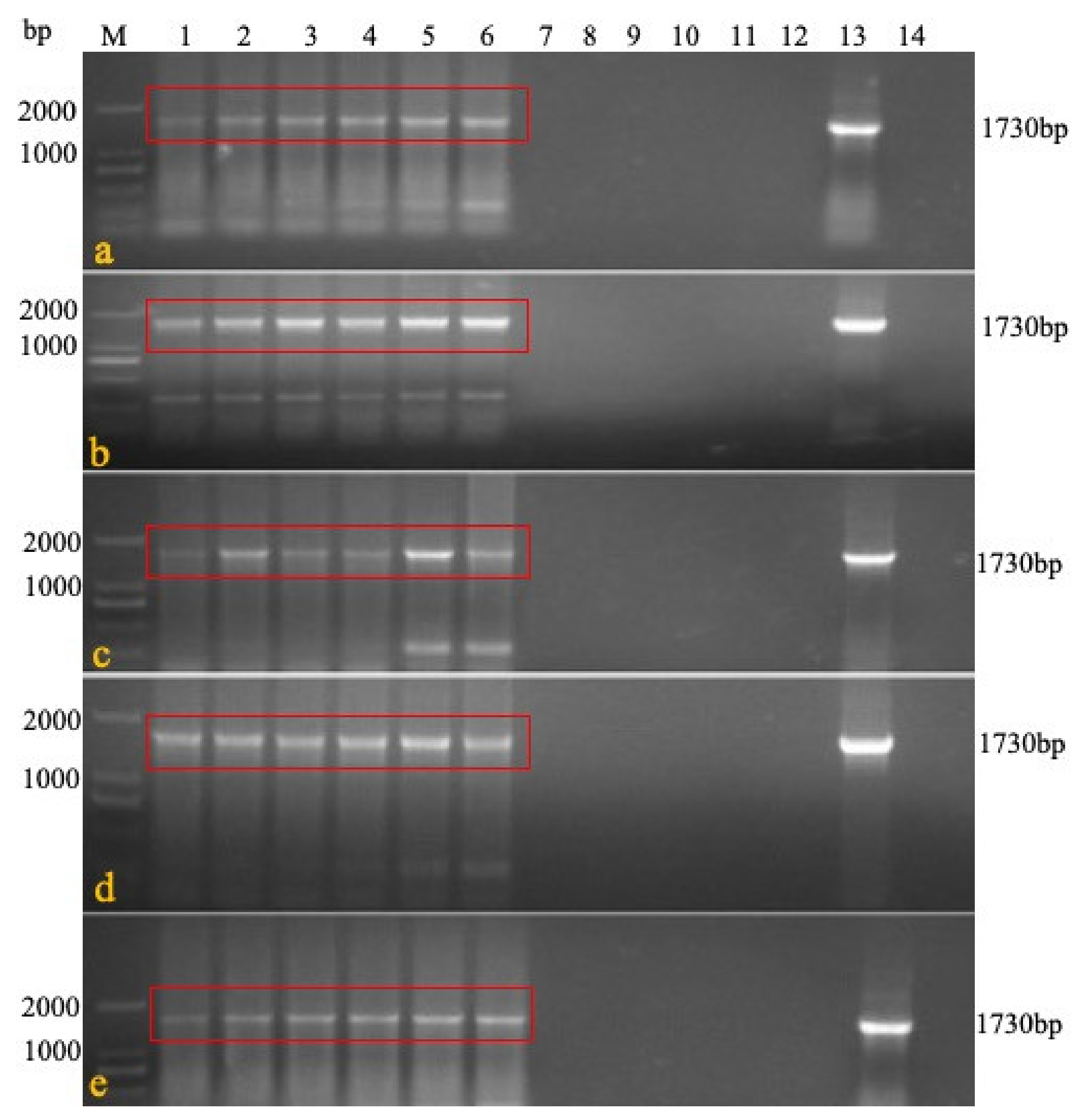
Table 3). On days 7, 14, and 21 after immunization, three chickens were randomly selected from each subgroup. Their blood, feathers, tracheal and rectal swabs, livers, spleens, kidneys, and lungs were collected to detect the specific genes by PCR and to isolate rMDV to investigate the horizontal transmission ability of rMDV. Primers 1 + 4 (
Table 1) were used to detect the presence of LTR in IRS. In rMDV, the specific gene was 1730 bp, while that in Rispens was 1088 bp. The CEF cells were inoculated with the treated blood, organs, swabs, feed, water, litter feces, and litter to observe the cytopathic effect (CPE).
2.7. Evaluation of the In Vivo Stability of rMDV
We confirmed the stability of the REV LTR fragment in the recombinant virus during its passage in vivo. Six one-day-old SPF chickens were immunized with rMDV at a 3000 PFU/dose. Seven days later, blood was collected to immunize the six other one-day-old SPF chickens one-by-one for five generations. The last generation was isolated and reared for 60 days. On the 60th day, the feathers were collected, and genomic DNA was extracted. Primers 1 + 2 were used to confirm that the virus was a recombinant virus, and primers 1 + 3 and 1 + 4 were used to identify the presence of LTR in terminal repeat sequence (TRS) and intrinsic repeat sequence (IRS) (
Table 1). On the 60th day, visceral tissue, such as that from the liver, spleen, and bursa, were collected for histological examination.
2.8. Distribution and Detoxification of rMDV
A safety experiment was conducted to detect the distribution and detoxification of the recombinant virus in the organs of the chickens. Five chickens were culled on days 3, 7, 21, 42, and 60 after inoculation in the overdose groups. Their blood, feathers, tracheal and rectal swabs, livers, spleens, kidneys, and lungs were collected to detect rMDV by PCR and isolate rMDV. Meanwhile, the air, feed, water, feces, and litter of the breeding environment were also obtained.
2.9. Comparison of the Protective Effects between rMDV and Rispens
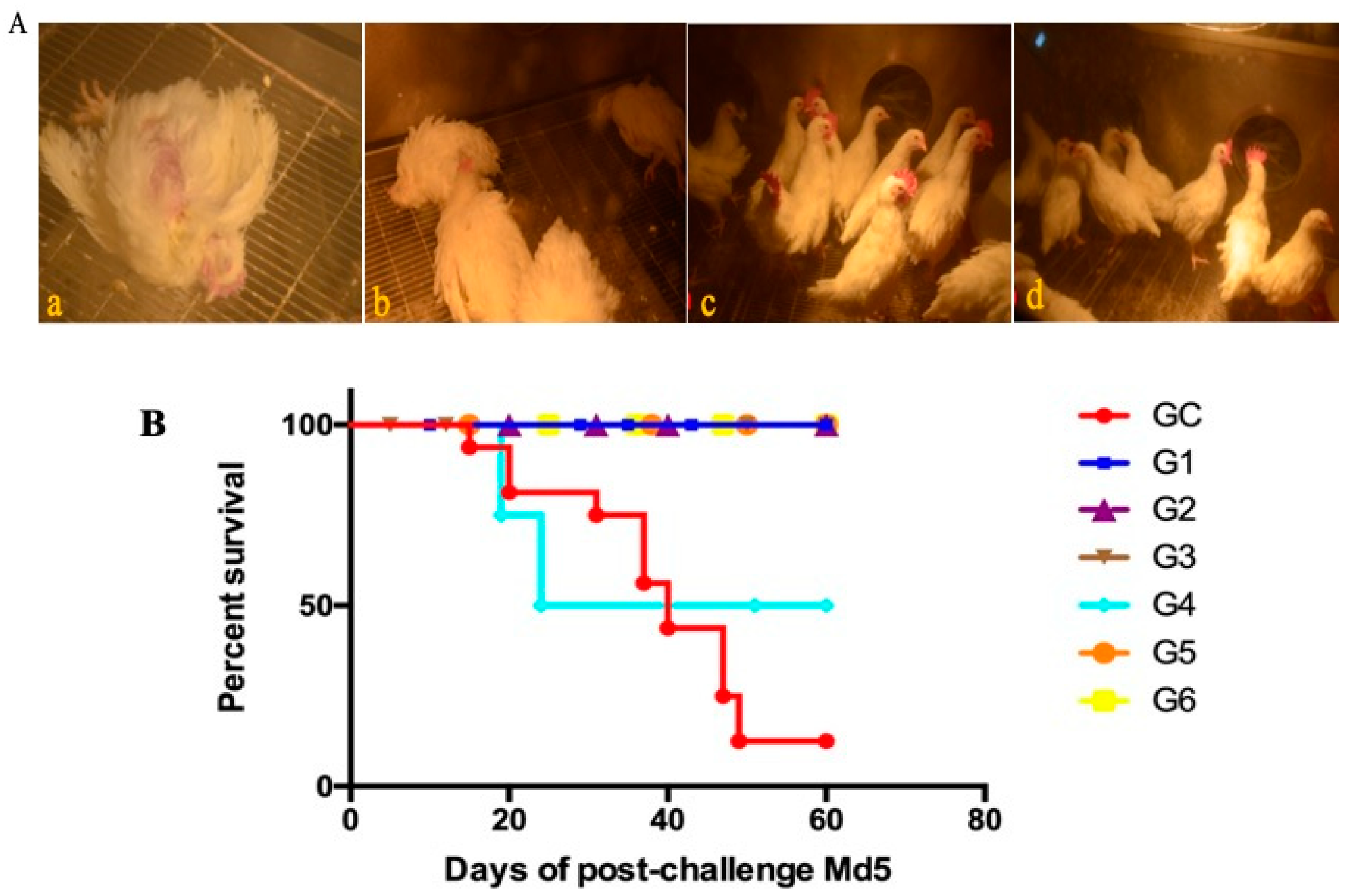
SPF chickens that were 61 days old were randomly divided into six experimental groups (G1–G6). Chickens in G1–G3 were immunized with rMDV, and those in G4, G5, and G6 were immunized with Rispens containing 125 PFU/0.2 mL, 250 PFU/0.2 mL, and 500 PFU/0.2 mL, respectively. Twenty chickens were used as controls (control group [GC]) (
Table 4). Seven days after vaccination, the chickens were challenged with 500 PFU vvMDV Md5 to observe the protective effects of rMDV and Rispens. All chickens were kept under the same conditions and observed for 60 days.
2.10. Pathological Identification and Histological Examination
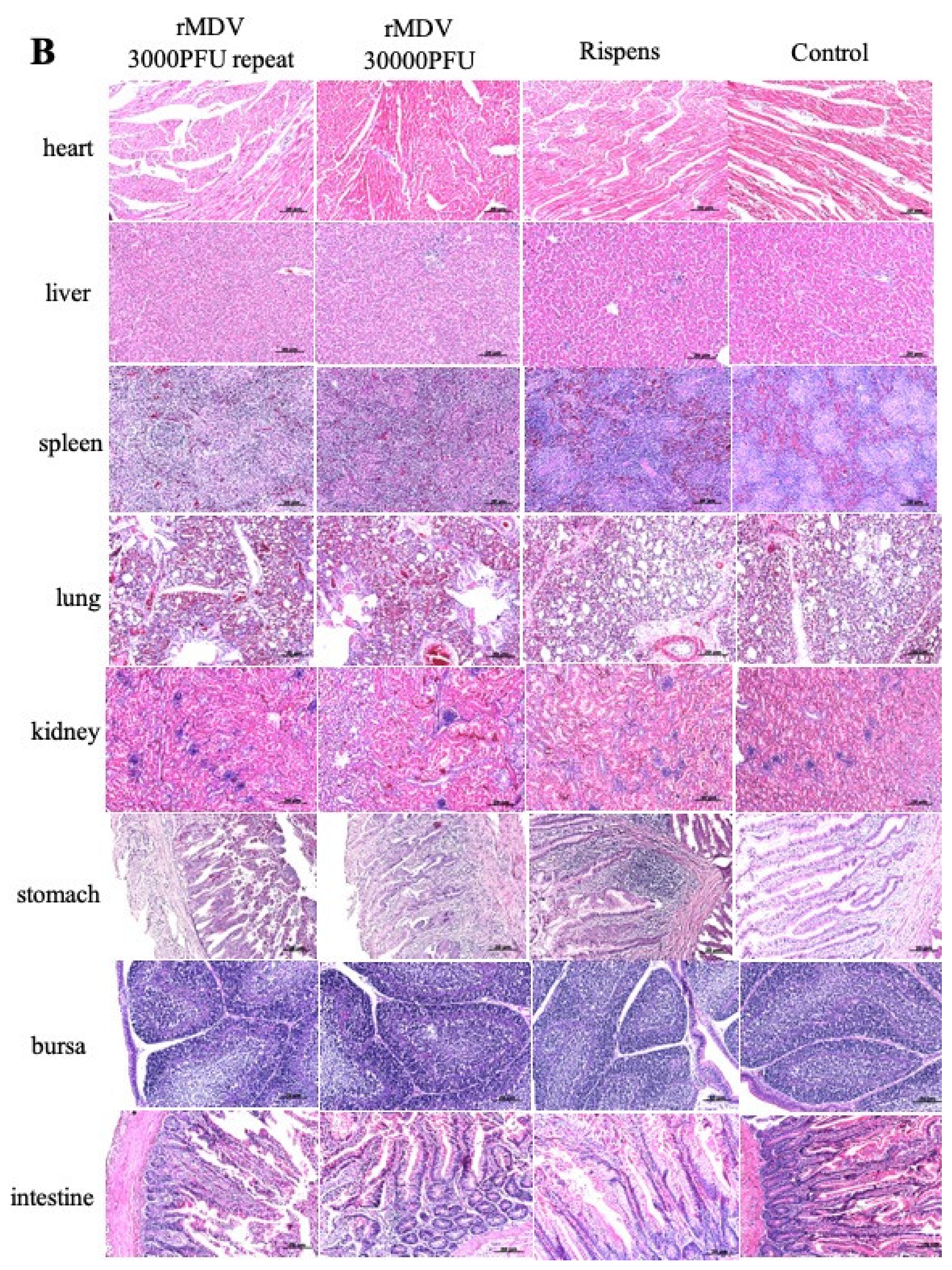
In the safety experiment, the clinical signs of each group were recorded after MDV infection. All experimental animals were euthanized and their hearts, livers, spleens, lungs, kidneys, glandular stomach, bursa, and intestines were removed for histological analysis, which was performed as described elsewhere. After 24 h of fixation in 4% formalin, the samples were dehydrated using graded alcohol, embedded in paraffin, and sliced into 4 μm-thick sections. These sections were stained with hematoxylin and eosin and observed under a light microscope (Eclipse TS100; Nikon, Tokyo, Japan).
2.11. Evaluation Standard
Chickens with MD clinical symptoms, those with ocular lesions, and those that had no observable lesions but presented pathological changes during the experiment were deemed positive. Chickens that had no clinical observations, no gross lesions, and no pathological changes were considered negative. The suspected samples were subject to confirmation by laboratory tests.
MD incidence criteria was as follows: experimental chicken death (excluding non-specific death), severe weight loss, paralysis syndrome, thymus and bursa Fabricius atrophy, diffuse enlargement of internal organs (including liver, kidneys, heart, spleen, ovaries, etc.) or tumors, peripheral nerve stripes, and swelling signified the onset of MD.
2.12. Statistical Analyses
The mean initial weights and mean percentage body weight gains for each group were compared using a one-way ANOVA and Tukey’s multiple comparison test (Prism version 8.0.2). A p value ≤ 0.05 was regarded as significant.
4. Discussion
MD is an infectious disease characterized by lymphoid hyperplasia, peripheral nerve paralysis, and tumors in the organs, muscles, and skin of chickens.
Currently, the vaccines for MD are the attenuated serotype I virus vaccine, non-virulent serotype II virus vaccine, and serotype III virus strain HVT. Serotype I MDV vaccines, such as Md11/75 [
22] and CVI988/Rispens [
23], are mostly produced by virulent strains or medium virulent strains purified by plaque and passaged to reduce their virulence and retain immunogenicity [
14]. Serotype II MDV vaccines, such as SB1 [
24] and Z4, are isolated from chickens. They are non-pathogenic and tumorigenic but have good immunogenicity [
25]. Serotype III MDV vaccines, such as the turkey herpes virus (HVT)-FC126 strain [
26,
27], were the earliest introduced commercial vaccines for the prevention of MD in China. They are also among the most widely used vaccines for the prevention and control of MD around the world. Multivalent vaccines for MDV currently represent the main promotion and application of bivalent vaccines in the international market, such as the bivalent live vaccine CVI988/Rispens strain + HVT FC126 strain produced by Merria [
28]. In addition, trivalent vaccines [
29] such as Md11/75 + SB1 + HVT, which is more effective than single-valent vaccines, have been developed internationally. In recent years, conventional vaccines could not produce sufficient protection against MDV because of coinfection with other viruses, including the avian leukemia virus (ALV) [
30] and REV [
31]. Various methods have been used to construct candidate vaccines for MDV. Genetically engineering vaccines has since become a widely researched topic and breakthrough point.
REV and MDV are avian diseases that can cause tumors in chickens, and co-infection with MDV and REV is very common in China [
32,
33]. More than ten years ago, researchers discovered that REV-LTR can be integrated into the genome of MDV when MDV is continuously passaged on cells contaminated with REV [
31]. The probability of natural recombination between MDV and REV is very low. Nevertheless, in recent years, several strains of MDVs that have naturally integrated LTR in REV have been isolated [
10,
11,
34], suggesting that a recombinant virus containing this sequence may have a certain survival advantage. This finding provides ideas for the development of a new vaccine for MDV [
35]. In this study, two LTR sequences were introduced into the classical MDV vaccine strain Rispens to construct a novel rMDV [
20]. The insertion of REV-LTR in MDV offered a faster in vitro replication capacity than Rispens. Although the LTR insertion of rMDV was beneficial for virus replication, there were no clinical symptoms of MD, no difference in body weight, and organ weight, and no obvious MD pathological lesions in the immunized chickens. rMDV could also not be detected in the surrounding environment by PCR and virus isolation. Moreover, in the cohabitation test, the virus could be detected from the feathers and tissues of the non-immunized chickens, indicating that the recombinant virus can spread horizontally. In addition, there was no in vivo deletion or mutation in the insertion gene of the fifth generation, indicating the stability of the recombinant virus. This further illustrates that the novel rMDV was as safe as the parent virus (Rispens) for the target chickens and was harmless to the environment. Together, these results suggest that LTR could enhance virus replication but not virus virulence. This provides a guarantee for the use of LTR to improve the development of MDV.
In the efficacy comparison experiment, on the seventh day after immunization, all chickens were challenged with Md5. The results showed that, for 125 PFU immunity, the rMDV and Rispens protection indexes against Md5 were 77.78% and 66.67%, respectively. For a single vaccine, CVI988/Rispens is currently the best option and can provide good clinical protection against MDV. MDV is a strictly cell-associated virus which makes it difficult to protect against. At low doses, the insertion of REV-LTR into the Rispens virus has an advantage over using Rispens against Md5, meaning that the insertion of LTR could be a good direction for improvement. In the future, multivalent vaccines or combined vaccines could be developed, which will have a major impact on the development of MDV vaccines.
Therefore, rMDV with integrated REV-LTR is safe for chickens and could assist against vvMDV, which makes it an ideal candidate vaccine strain.