A Canine-Directed Chimeric Multi-Epitope Vaccine Induced Protective Immune Responses in BALB/c Mice Infected with Leishmania infantum
Abstract
1. Introduction
2. Materials and Methods
2.1. In Silico Prediction of Helper T Lymphocyte (HTL) and Cytotoxic T Lymphocyte (CTL) Epitopes
2.2. Design and In Silico Evaluation of the Multi-Epitope Vaccine
2.3. Computational Structural and Docking Analyses of Heparin-Binding Hemagglutinin (HBHA)
2.4. Expression and Purification of the Chimeric Multi-Epitope Vaccine
2.5. Canine Peripheral Blood Mononuclear Cells (PBMCs) Lymphoproliferation
2.6. Mice
2.7. Parasites and Soluble Leishmania Antigen Production
2.8. Immunization and Challenge of Mice
2.9. Determination of Live Parasite Burden by Limited Dilution Analysis (LDA)
2.10. Measurement of Delayed-Type Hypersensitivity (DTH)
2.11. Detection of LiChimera- and Parasite-Specific IgG, IgG1, and IgG2a Antibodies
2.12. LiChimera- and Parasite-Specific Proliferation Assay
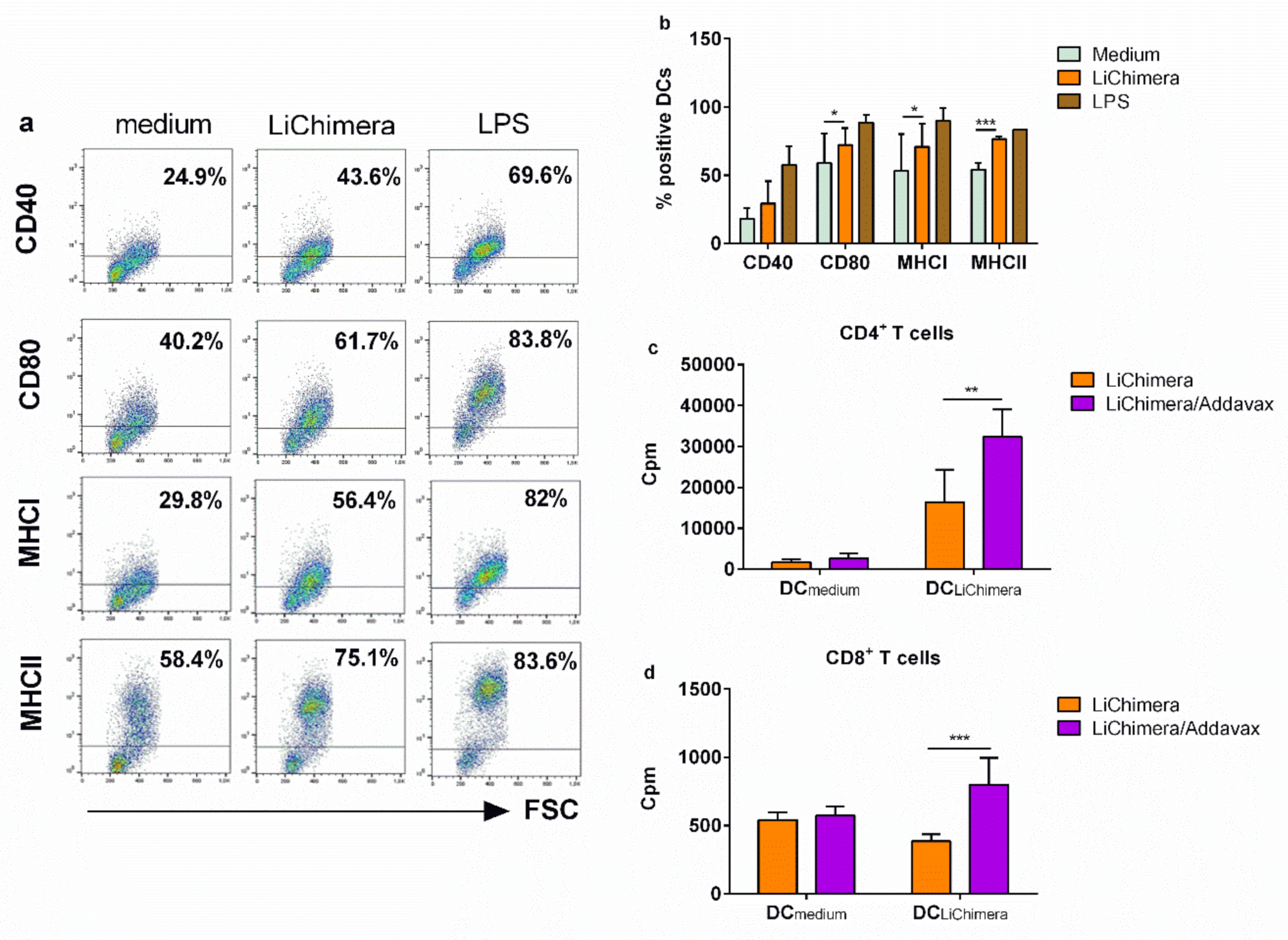
2.13. Bone Marrow-Derived Dendritic Cell (BMDC) Differentiation and Evaluation of Their Maturation after Sensitization with Multi-Epitope Vaccine
2.14. Antigen-Presenting Capacity of LiChimera-Pulsed BMDCs
2.15. Multiparametric Flow Cytometric Analysis
2.16. Bone Marrow-Derived Macrophages (BMMs) Generation and In Vitro Infection with L. infantum
2.17. Measurement of NO Production
2.18. Statistical Analysis
3. Results
3.1. In Silico Prediction and Selection of Candidate CTL and HTL Epitopes for the Design of the Multi-Epitope Vaccine
3.2. Design and Characterization of the Multi-Epitope Vaccine Construct
3.3. Effects of the Multi-Epitope Domain of the Chimeric Protein on Interactions with the TLR4/MD2 Complex
3.4. Recombinant LiChimera Recognized by Canine PBMCs
3.5. Detection of Vaccine Safety
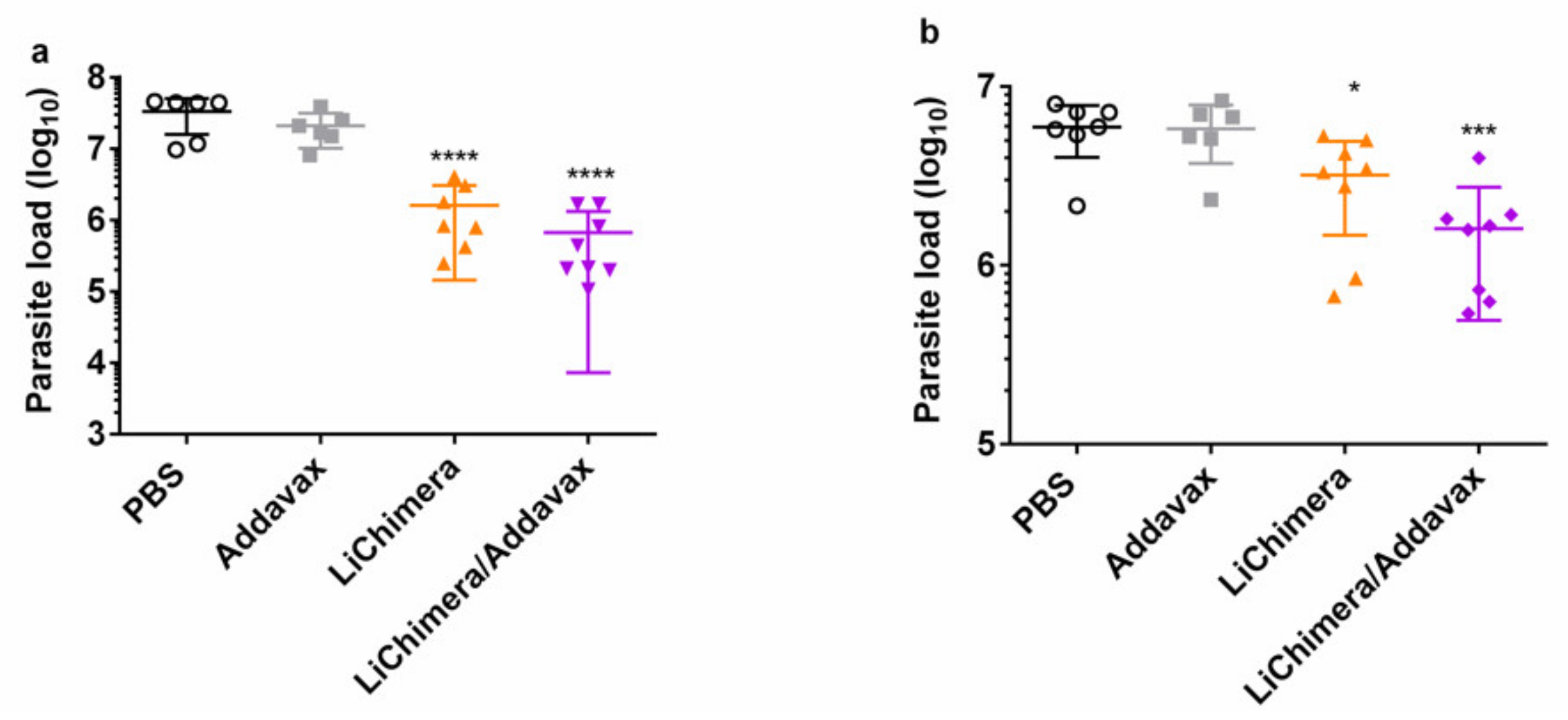
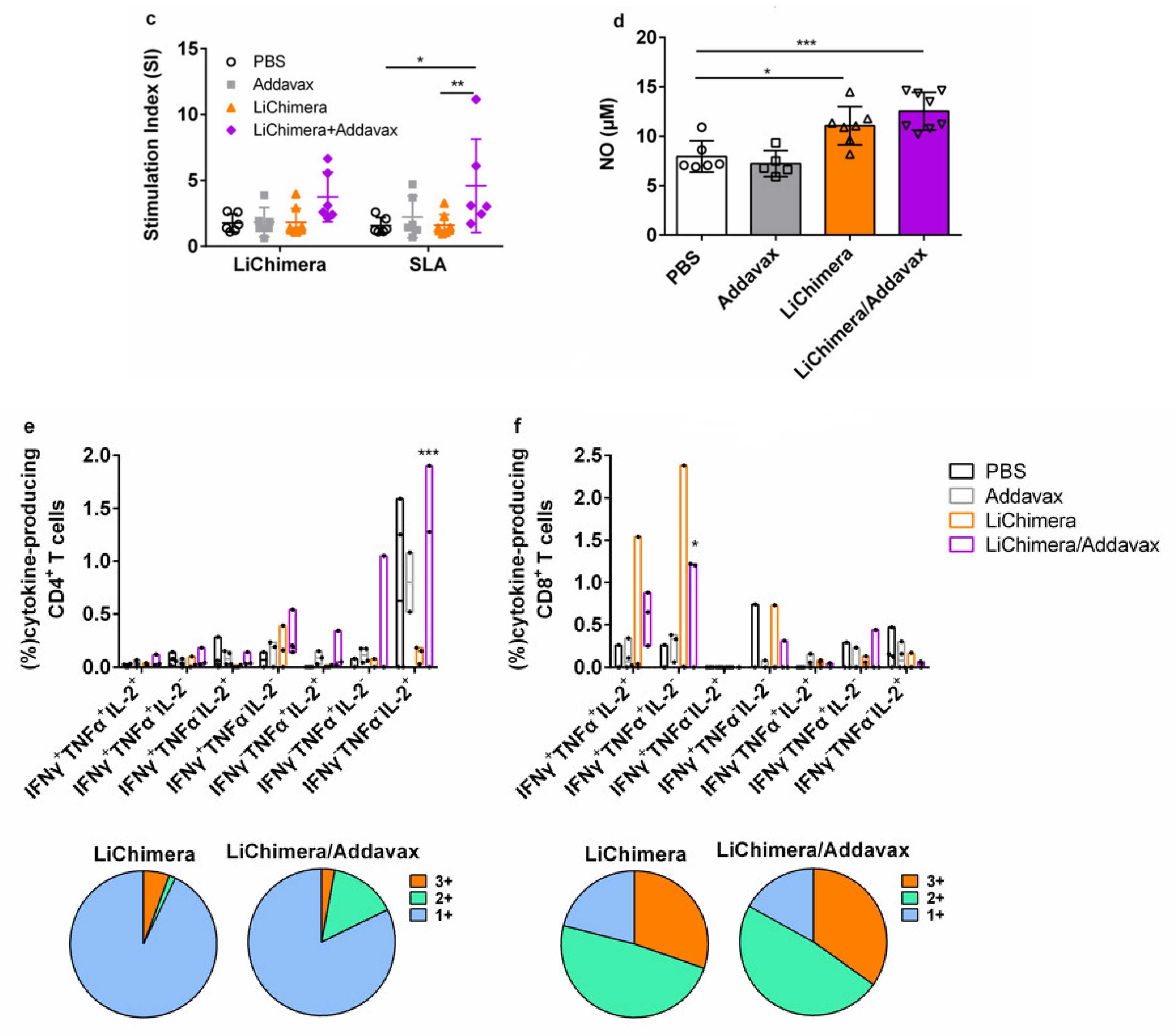
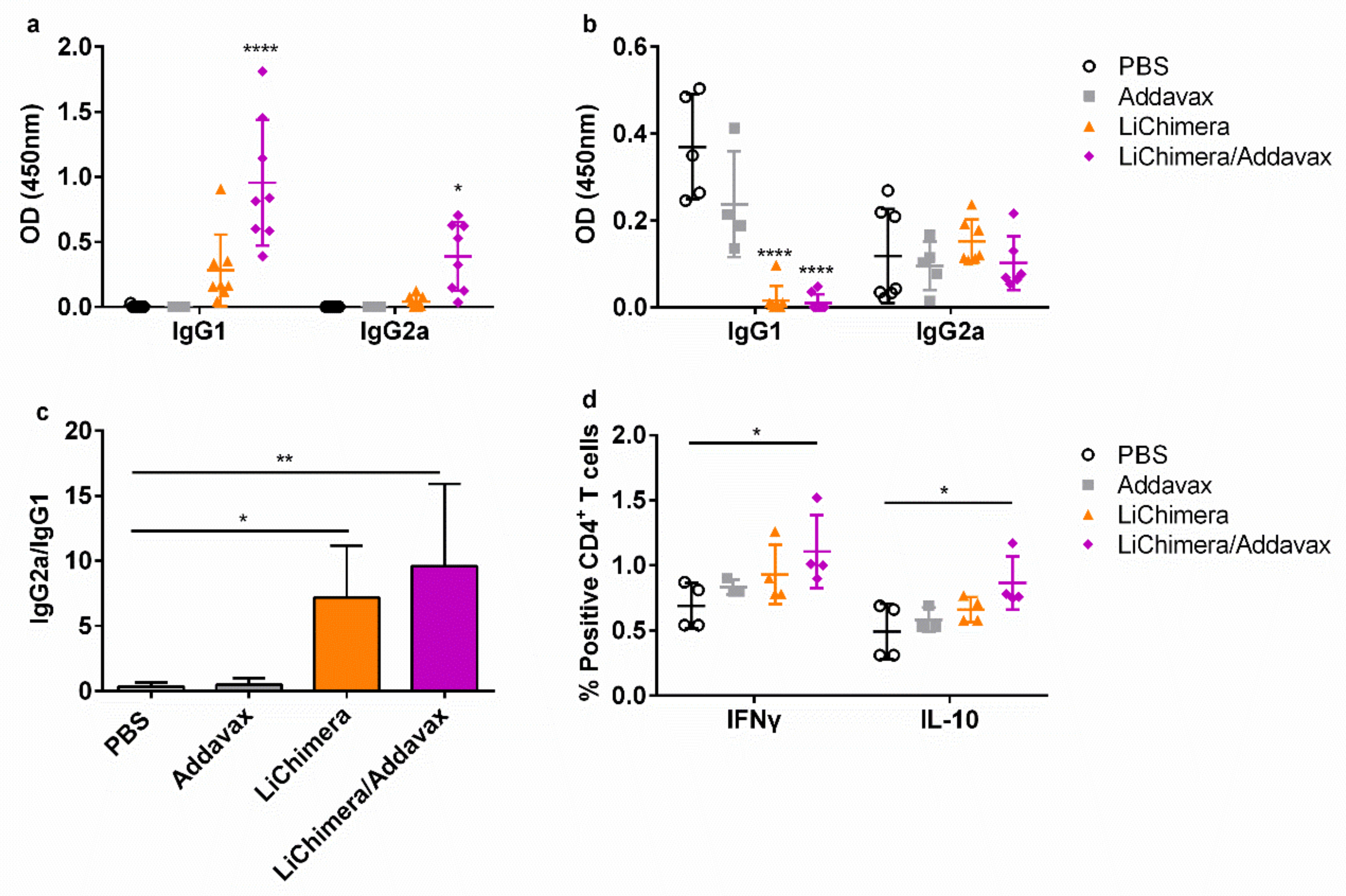
3.6. Characterization of Humoral and Cellular Immune Responses Induced by LiChimera Vaccination in a Murine Experimental Model of Visceral Leishmaniasis
3.7. BMDCs Differentially Pulsed with LiChimera Elicit Strong Antigen-Specific CD4+ T Cell Responses
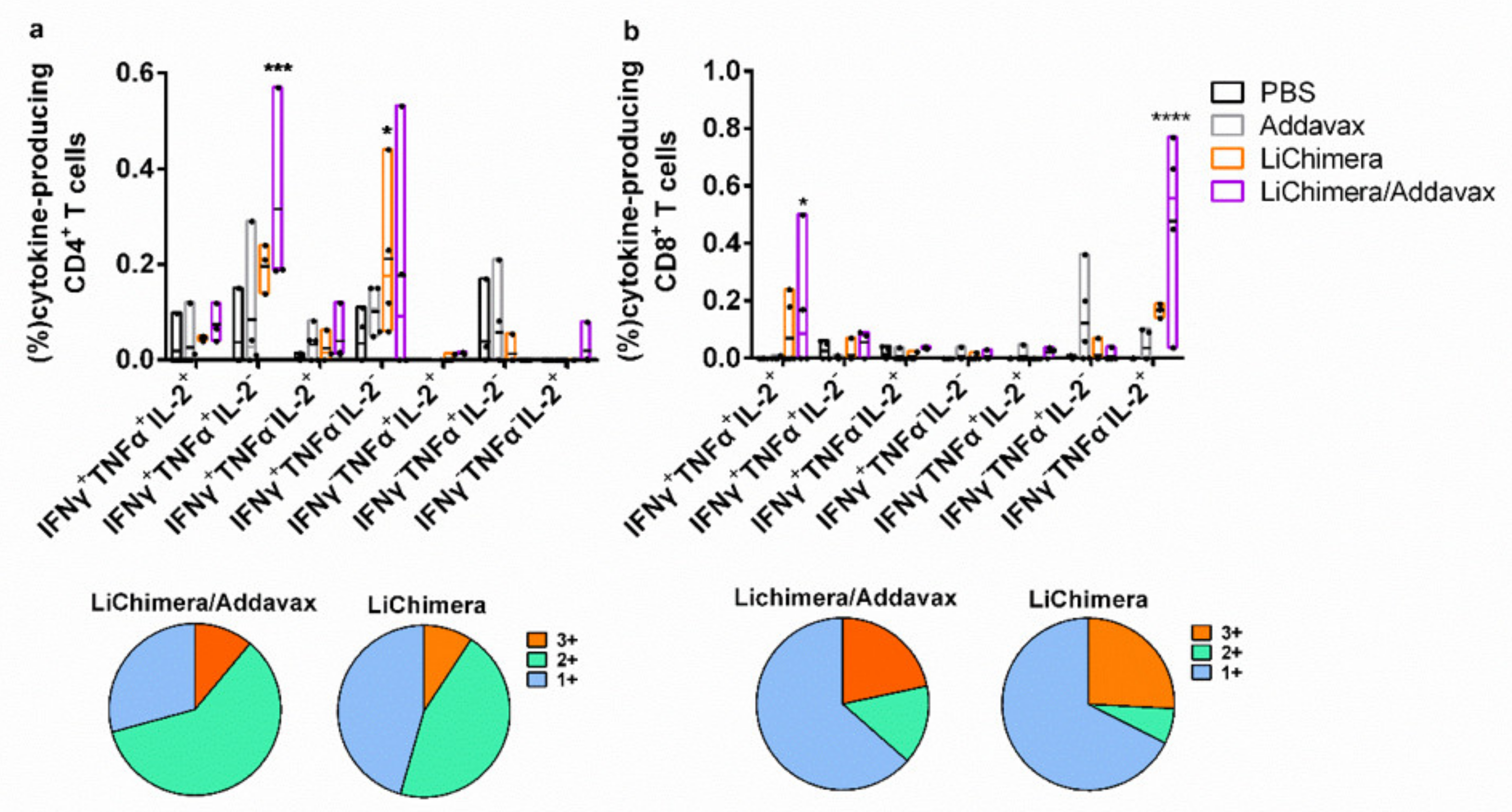
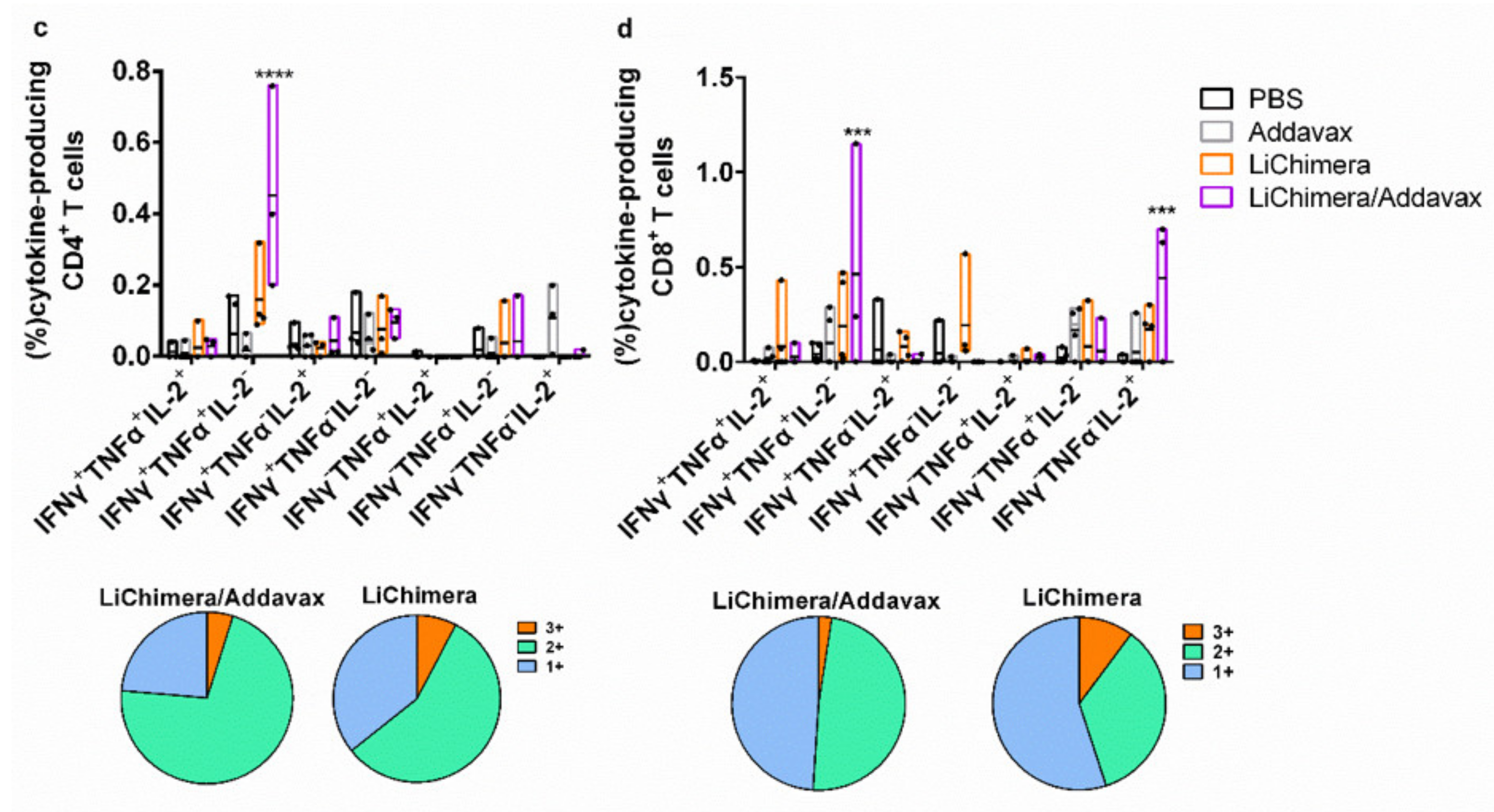
3.8. LiChimera Adjuvanted with Addavax Induced the Differentiation of Multifunctional T Cells
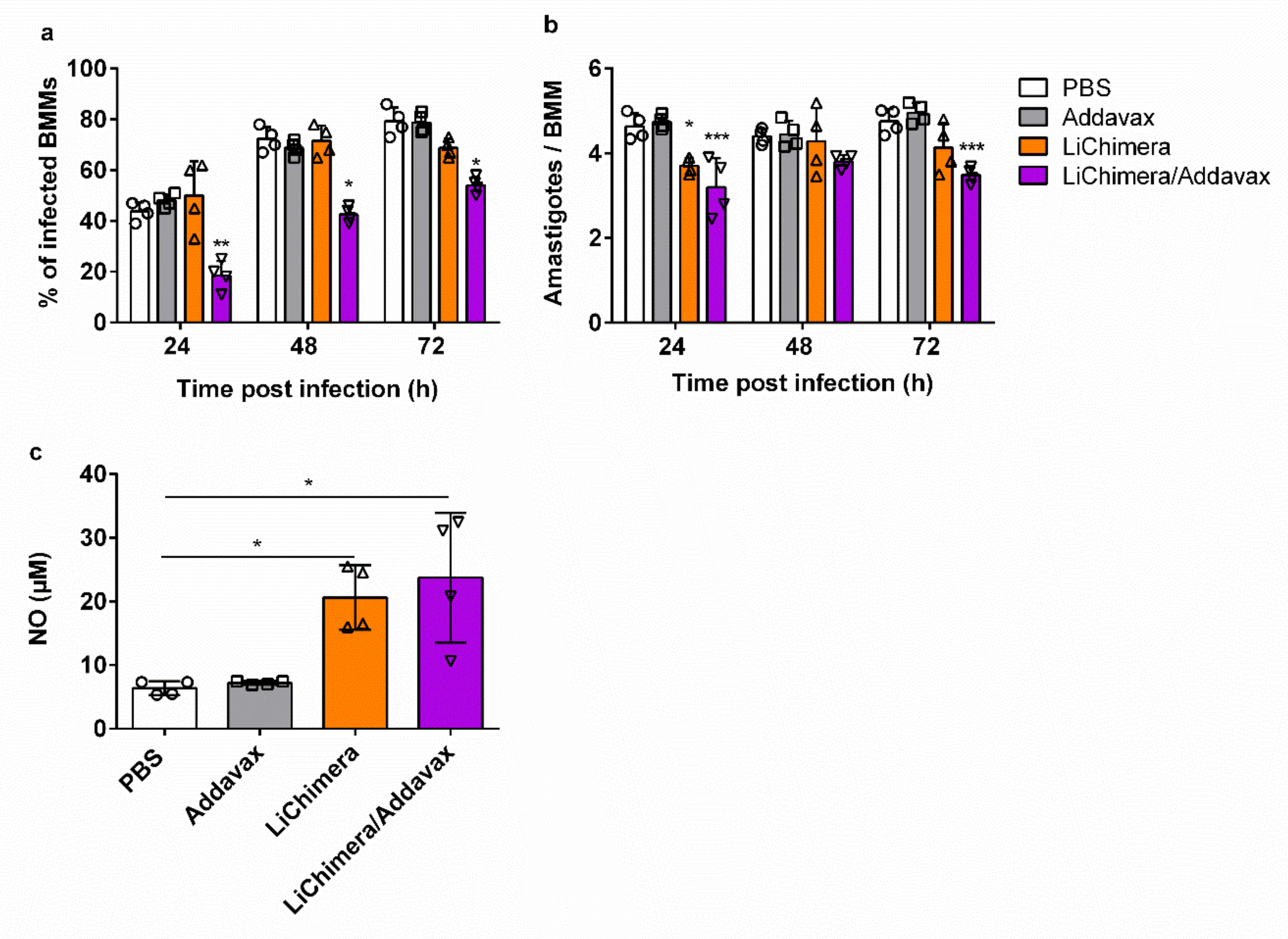
3.9. Effects of LiChimera on Activation of Macrophages Leishmanicidal Efficacy
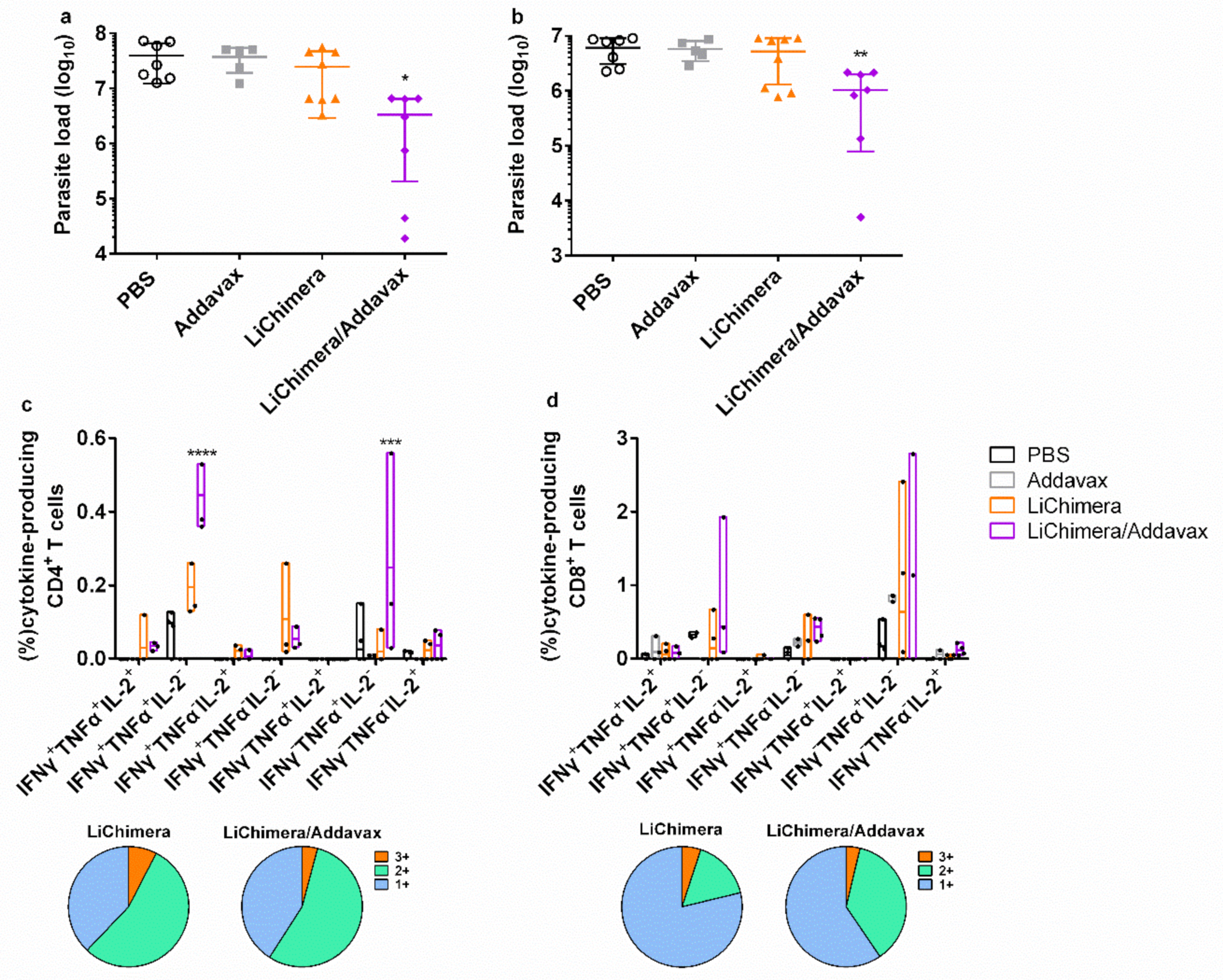
3.10. LiChimera/Addavax Vaccination Protects Against L. infantum Challenge
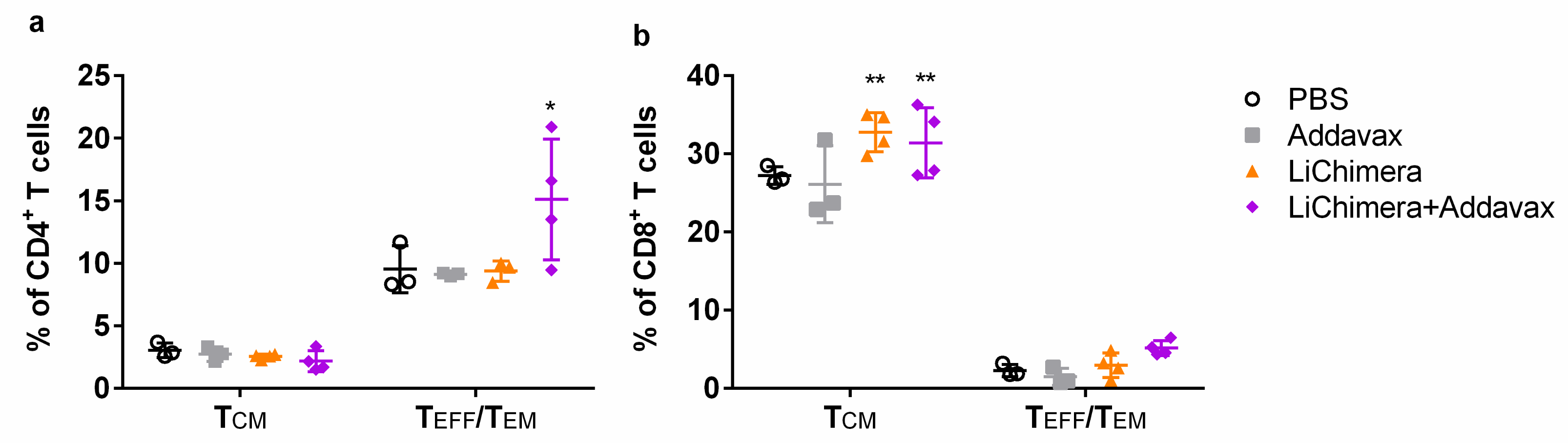
3.11. LiChimera/Addavax Vaccination Induced Persisting Immunity and Long-Term Protection against L. infantum
3.12. Effect Of CD8+ T Cell Depletion on Vaccine Efficacy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hohman, L.S.; Peters, N.C. CD4(+) T Cell-Mediated Immunity against the Phagosomal Pathogen Leishmania: Implications for Vaccination. Trends Parasitol. 2019, 35, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Dantas-Torres, F.; Otranto, D. Best Practices for Preventing Vector-Borne Diseases in Dogs and Humans. Trends Parasitol. 2016, 32, 43–55. [Google Scholar] [CrossRef]
- Burza, S.; Croft, S.L.; Boelaert, M. Leishmaniasis. Lancet 2018, 392, 951–970. [Google Scholar] [CrossRef]
- Colmenares, M.; Kar, S.; Goldsmith-Pestana, K.; McMahon-Pratt, D. Mechanisms of pathogenesis: Differences amongst Leishmania species. Trans. R. Soc. Trop. Med. Hyg. 2002, 96 (Suppl. 1), S3–S7. [Google Scholar] [CrossRef]
- Alvar, J.; Velez, I.D.; Bern, C.; Herrero, M.; Desjeux, P.; Cano, J.; Jannin, J.; den Boer, M.; WHO Leishmaniasis Control Team. Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE 2012, 7, e35671. [Google Scholar] [CrossRef] [PubMed]
- Reis, A.B.; Giunchetti, R.C.; Carrillo, E.; Martins-Filho, O.A.; Moreno, J. Immunity to Leishmania and the rational search for vaccines against canine leishmaniasis. Trends Parasitol. 2010, 26, 341–349. [Google Scholar] [CrossRef]
- Sacks, D.L. Vaccines against tropical parasitic diseases: A persisting answer to a persisting problem. Nat. Immunol. 2014, 15, 403–405. [Google Scholar] [CrossRef]
- Kedzierski, L.; Sakthianandeswaren, A.; Curtis, J.M.; Andrews, P.C.; Junk, P.C.; Kedzierska, K. Leishmaniasis: Current treatment and prospects for new drugs and vaccines. Curr. Med. Chem. 2009, 16, 599–614. [Google Scholar] [CrossRef]
- Khalil, E.A.; El Hassan, A.M.; Zijlstra, E.E.; Mukhtar, M.M.; Ghalib, H.W.; Musa, B.; Ibrahim, M.E.; Kamil, A.A.; Elsheikh, M.; Babiker, A.; et al. Autoclaved Leishmania major vaccine for prevention of visceral leishmaniasis: A randomised, double-blind, BCG-controlled trial in Sudan. Lancet 2000, 356, 1565–1569. [Google Scholar] [CrossRef]
- Velez, I.D.; del Pilar Agudelo, S.; Arbelaez, M.P.; Gilchrist, K.; Robledo, S.M.; Puerta, J.A.; Zicker, F.; Berman, J.; Modabber, F. Safety and immunogenicity of a killed Leishmania (L.) amazonensis vaccine against cutaneous leishmaniasis in Colombia: A randomized controlled trial. Trans. R. Soc. Trop. Med. Hyg. 2000, 94, 698–703. [Google Scholar] [CrossRef]
- Thomaz-Soccol, V.; Ferreira da Costa, E.S.; Karp, S.G.; Junior Letti, L.A.; Soccol, F.T.; Soccol, C.R. Recent Advances in Vaccines Against Leishmania Based on Patent Applications. Recent Pat. Biotechnol. 2018, 12, 21–32. [Google Scholar] [CrossRef]
- Iborra, S.; Solana, J.C.; Requena, J.M.; Soto, M. Vaccine candidates against leishmania under current research. Expert Rev. Vaccines 2018, 17, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Singh, O.P.; Hasker, E.; Sacks, D.; Boelaert, M.; Sundar, S. Asymptomatic Leishmania infection: A new challenge for Leishmania control. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2014, 58, 1424–1429. [Google Scholar] [CrossRef] [PubMed]
- Baneth, G.; Koutinas, A.F.; Solano-Gallego, L.; Bourdeau, P.; Ferrer, L. Canine leishmaniosis-new concepts and insights on an expanding zoonosis: Part one. Trends Parasitol. 2008, 24, 324–330. [Google Scholar] [CrossRef]
- Agallou, M.; Athanasiou, E.; Samiotaki, M.; Panayotou, G.; Karagouni, E. Identification of Immunoreactive Leishmania infantum Protein Antigens to Asymptomatic Dog Sera through Combined Immunoproteomics and Bioinformatics Analysis. PLoS ONE 2016, 11, e0149894. [Google Scholar] [CrossRef] [PubMed]
- Requena, J.M.; Alonso, C.; Soto, M. Evolutionarily conserved proteins as prominent immunogens during Leishmania infections. Parasitol. Today 2000, 16, 246–250. [Google Scholar] [CrossRef]
- Kumar, A.; Sisodia, B.; Misra, P.; Sundar, S.; Shasany, A.K.; Dube, A. Proteome mapping of overexpressed membrane-enriched and cytosolic proteins in sodium antimony gluconate (SAG) resistant clinical isolate of Leishmania donovani. Br. J. Clin. Pharmacol. 2010, 70, 609–617. [Google Scholar] [CrossRef]
- Matrangolo, F.S.; Liarte, D.B.; Andrade, L.C.; de Melo, M.F.; Andrade, J.M.; Ferreira, R.F.; Santiago, A.S.; Pirovani, C.P.; Silva-Pereira, R.A.; Murta, S.M. Comparative proteomic analysis of antimony-resistant and -susceptible Leishmania braziliensis and Leishmania infantum chagasi lines. Mol. Biochem. Parasitol. 2013, 190, 63–75. [Google Scholar] [CrossRef]
- Yau, W.L.; Pescher, P.; MacDonald, A.; Hem, S.; Zander, D.; Retzlaff, S.; Blisnick, T.; Rotureau, B.; Rosenqvist, H.; Wiese, M.; et al. The Leishmania donovani chaperone cyclophilin 40 is essential for intracellular infection independent of its stage-specific phosphorylation status. Mol. Microbiol. 2014, 93, 80–97. [Google Scholar] [CrossRef]
- Brotherton, M.C.; Bourassa, S.; Legare, D.; Poirier, G.G.; Droit, A.; Ouellette, M. Quantitative proteomic analysis of amphotericin B resistance in Leishmania infantum. Int. J. Parasitol. Drugs Drug Resist. 2014, 4, 126–132. [Google Scholar] [CrossRef]
- Singh, A.K.; Roberts, S.; Ullman, B.; Madhubala, R. A quantitative proteomic screen to identify potential drug resistance mechanism in alpha-difluoromethylornithine (DFMO) resistant Leishmania donovani. J. Proteom. 2014, 102, 44–59. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K.; Sisodia, B.S.; Sinha, S.; Hajela, K.; Naik, S.; Shasany, A.K.; Dube, A. Proteomic approach for identification and characterization of novel immunostimulatory proteins from soluble antigens of Leishmania donovani promastigotes. Proteomics 2007, 7, 816–823. [Google Scholar] [CrossRef]
- Kumari, S.; Samant, M.; Misra, P.; Khare, P.; Sisodia, B.; Shasany, A.K.; Dube, A. Th1-stimulatory polyproteins of soluble Leishmania donovani promastigotes ranging from 89.9 to 97.1 kDa offers long-lasting protection against experimental visceral leishmaniasis. Vaccine 2008, 26, 5700–5711. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Kumar, V.; Kushawaha, P.K.; Tripathi, C.P.; Joshi, S.; Sahasrabuddhe, A.A.; Mitra, K.; Sundar, S.; Siddiqi, M.I.; Dube, A. Characterization of glycolytic enzymes--rAldolase and rEnolase of Leishmania donovani, identified as Th1 stimulatory proteins, for their immunogenicity and immunoprophylactic efficacies against experimental visceral leishmaniasis. PLoS ONE 2014, 9, e86073. [Google Scholar] [CrossRef]
- De Brito, R.C.F.; Cardoso, J.M.O.; Reis, L.E.S.; Vieira, J.F.; Mathias, F.A.S.; Roatt, B.M.; Aguiar-Soares, R.; Ruiz, J.C.; Resende, D.M.; Reis, A.B. Peptide Vaccines for Leishmaniasis. Front. Immunol. 2018, 9, 1043. [Google Scholar] [CrossRef] [PubMed]
- Panina-Bordignon, P.; Tan, A.; Termijtelen, A.; Demotz, S.; Corradin, G.; Lanzavecchia, A. Universally immunogenic T cell epitopes: Promiscuous binding to human MHC class II and promiscuous recognition by T cells. Eur. J. Immunol. 1989, 19, 2237–2242. [Google Scholar] [CrossRef]
- van der Burg, S.H.; Bijker, M.S.; Welters, M.J.; Offringa, R.; Melief, C.J. Improved peptide vaccine strategies, creating synthetic artificial infections to maximize immune efficacy. Adv. Drug Deliv. Rev. 2006, 58, 916–930. [Google Scholar] [CrossRef]
- Jung, I.D.; Jeong, S.K.; Lee, C.M.; Noh, K.T.; Heo, D.R.; Shin, Y.K.; Yun, C.H.; Koh, W.J.; Akira, S.; Whang, J.; et al. Enhanced efficacy of therapeutic cancer vaccines produced by co-treatment with Mycobacterium tuberculosis heparin-binding hemagglutinin, a novel TLR4 agonist. Cancer Res. 2011, 71, 2858–2870. [Google Scholar] [CrossRef]
- Guerrero, G.G.; Feunou, F.P.; Locht, C. The coiled-coil N-terminal domain of the heparin-binding haemagglutinin is required for the humoral and cellular immune responses in mice. Mol. Immunol. 2008, 46, 116–124. [Google Scholar] [CrossRef]
- Nezafat, N.; Ghasemi, Y.; Javadi, G.; Khoshnoud, M.J.; Omidinia, E. A novel multi-epitope peptide vaccine against cancer: An in silico approach. J. Theor. Biol. 2014, 349, 121–134. [Google Scholar] [CrossRef]
- Nezafat, N.; Sadraeian, M.; Rahbar, M.R.; Khoshnoud, M.J.; Mohkam, M.; Gholami, A.; Banihashemi, M.; Ghasemi, Y. Production of a novel multi-epitope peptide vaccine for cancer immunotherapy in TC-1 tumor-bearing mice. Biol. J. Int. Assoc. Biol. Stand. 2015, 43, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Webb, B.; Sali, A. Protein Structure Modeling with MODELLER. Methods Mol. Biol. 2017, 1654, 39–54. [Google Scholar] [CrossRef]
- Soding, J.; Biegert, A.; Lupas, A.N. The HHpred interactive server for protein homology detection and structure prediction. Nucleic Acids Res. 2005, 33, W244–W248. [Google Scholar] [CrossRef]
- Van Der Spoel, D.; Lindahl, E.; Hess, B.; Groenhof, G.; Mark, A.E.; Berendsen, H.J. GROMACS: Fast, flexible, and free. J. Comput. Chem. 2005, 26, 1701–1718. [Google Scholar] [CrossRef] [PubMed]
- Van Zundert, G.C.P.; Rodrigues, J.; Trellet, M.; Schmitz, C.; Kastritis, P.L.; Karaca, E.; Melquiond, A.S.J.; van Dijk, M.; de Vries, S.J.; Bonvin, A. The HADDOCK2.2 Web Server: User-Friendly Integrative Modeling of Biomolecular Complexes. J. Mol. Biol. 2016, 428, 720–725. [Google Scholar] [CrossRef] [PubMed]
- Ohto, U.; Fukase, K.; Miyake, K.; Shimizu, T. Structural basis of species-specific endotoxin sensing by innate immune receptor TLR4/MD-2. Proc. Natl. Acad. Sci. USA 2012, 109, 7421–7426. [Google Scholar] [CrossRef]
- Kumari, R.; Kumar, R.; Open Source Drug Discovery Consortium; Lynn, A. g_mmpbsa--a GROMACS tool for high-throughput MM-PBSA calculations. J. Chem. Inf. Model. 2014, 54, 1951–1962. [Google Scholar] [CrossRef]
- Agallou, M.; Margaroni, M.; Athanasiou, E.; Toubanaki, D.K.; Kontonikola, K.; Karidi, K.; Kammona, O.; Kiparissides, C.; Karagouni, E. Identification of BALB/c Immune Markers Correlated with a Partial Protection to Leishmania infantum after Vaccination with a Rationally Designed Multi-epitope Cysteine Protease A Peptide-Based Nanovaccine. PLoS Negl. Trop. Dis. 2017, 11, e0005311. [Google Scholar] [CrossRef]
- Agallou, M.; Pantazi, E.; Tsiftsaki, E.; Toubanaki, D.K.; Gaitanaki, C.; Smirlis, D.; Karagouni, E. Induction of protective cellular immune responses against experimental visceral leishmaniasis mediated by dendritic cells pulsed with the N-terminal domain of Leishmania infantum elongation factor-2 and CpG oligodeoxynucleotides. Mol. Immunol. 2018, 103, 7–20. [Google Scholar] [CrossRef]
- Lutz, M.B.; Kukutsch, N.; Ogilvie, A.L.; Rossner, S.; Koch, F.; Romani, N.; Schuler, G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J. Immunol. Methods 1999, 223, 77–92. [Google Scholar] [CrossRef]
- Livingston, B.; Crimi, C.; Newman, M.; Higashimoto, Y.; Appella, E.; Sidney, J.; Sette, A. A rational strategy to design multiepitope immunogens based on multiple Th lymphocyte epitopes. J. Immunol. 2002, 168, 5499–5506. [Google Scholar] [CrossRef]
- de Oliveira, L.M.; Morale, M.G.; Chaves, A.A.; Cavalher, A.M.; Lopes, A.S.; Diniz Mde, O.; Schanoski, A.S.; de Melo, R.L.; Ferreira, L.C.; de Oliveira, M.L.; et al. Design, Immune Responses and Anti-Tumor Potential of an HPV16 E6E7 Multi-Epitope Vaccine. PLoS ONE 2015, 10, e0138686. [Google Scholar] [CrossRef] [PubMed]
- Arai, R.; Wriggers, W.; Nishikawa, Y.; Nagamune, T.; Fujisawa, T. Conformations of variably linked chimeric proteins evaluated by synchrotron X-ray small-angle scattering. Proteins 2004, 57, 829–838. [Google Scholar] [CrossRef] [PubMed]
- George, R.A.; Heringa, J. An analysis of protein domain linkers: Their classification and role in protein folding. Protein Eng. 2002, 15, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Campos-Neto, A.; Webb, J.R.; Greeson, K.; Coler, R.N.; Skeiky, Y.A.; Reed, S.G. Vaccination with plasmid DNA encoding TSA/LmSTI1 leishmanial fusion proteins confers protection against Leishmania major infection in susceptible BALB/c mice. Infect. Immun. 2002, 70, 2828–2836. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ghosh, A.; Zhang, W.W.; Matlashewski, G. Immunization with A2 protein results in a mixed Th1/Th2 and a humoral response which protects mice against Leishmania donovani infections. Vaccine 2001, 20, 59–66. [Google Scholar] [CrossRef]
- Basu, R.; Bhaumik, S.; Basu, J.M.; Naskar, K.; De, T.; Roy, S. Kinetoplastid membrane protein-11 DNA vaccination induces complete protection against both pentavalent antimonial-sensitive and -resistant strains of Leishmania donovani that correlates with inducible nitric oxide synthase activity and IL-4 generation: Evidence for mixed Th1- and Th2-like responses in visceral leishmaniasis. J. Immunol. 2005, 174, 7160–7171. [Google Scholar] [CrossRef]
- Stager, S.; Smith, D.F.; Kaye, P.M. Immunization with a recombinant stage-regulated surface protein from Leishmania donovani induces protection against visceral leishmaniasis. J. Immunol. 2000, 165, 7064–7071. [Google Scholar] [CrossRef]
- Rafati, S.; Zahedifard, F.; Nazgouee, F. Prime-boost vaccination using cysteine proteinases type I and II of Leishmania infantum confers protective immunity in murine visceral leishmaniasis. Vaccine 2006, 24, 2169–2175. [Google Scholar] [CrossRef]
- Melby, P.C.; Yang, J.; Zhao, W.; Perez, L.E.; Cheng, J. Leishmania donovani p36(LACK) DNA vaccine is highly immunogenic but not protective against experimental visceral leishmaniasis. Infect. Immun. 2001, 69, 4719–4725. [Google Scholar] [CrossRef]
- Gupta, R.; Kushawaha, P.K.; Tripathi, C.D.; Sundar, S.; Dube, A. A novel recombinant Leishmania donovani p45, a partial coding region of methionine aminopeptidase, generates protective immunity by inducing a Th1 stimulatory response against experimental visceral leishmaniasis. Int. J. Parasitol. 2012, 42, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Parody, N.; Soto, M.; Requena, J.M.; Alonso, C. Adjuvant guided polarization of the immune humoral response against a protective multicomponent antigenic protein (Q) from Leishmania infantum. A CpG + Q mix protects Balb/c mice from infection. Parasite Immunol. 2004, 26, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Goto, Y.; Bhatia, A.; Raman, V.S.; Liang, H.; Mohamath, R.; Picone, A.F.; Vidal, S.E.; Vedvick, T.S.; Howard, R.F.; Reed, S.G. KSAC, the first defined polyprotein vaccine candidate for visceral leishmaniasis. Clin. Vaccine Immunol. Cvi 2011, 18, 1118–1124. [Google Scholar] [CrossRef] [PubMed]
- Trigo, J.; Abbehusen, M.; Netto, E.M.; Nakatani, M.; Pedral-Sampaio, G.; de Jesus, R.S.; Goto, Y.; Guderian, J.; Howard, R.F.; Reed, S.G. Treatment of canine visceral leishmaniasis by the vaccine Leish-111f+MPL-SE. Vaccine 2010, 28, 3333–3340. [Google Scholar] [CrossRef] [PubMed]
- Molano, I.; Alonso, M.G.; Miron, C.; Redondo, E.; Requena, J.M.; Soto, M.; Nieto, C.G.; Alonso, C. A Leishmania infantum multi-component antigenic protein mixed with live BCG confers protection to dogs experimentally infected with L. infantum. Vet. Immunol. Immunopathol. 2003, 92, 1–13. [Google Scholar] [CrossRef]
- Coler, R.N.; Goto, Y.; Bogatzki, L.; Raman, V.; Reed, S.G. Leish-111f, a recombinant polyprotein vaccine that protects against visceral Leishmaniasis by elicitation of CD4+ T cells. Infect. Immun. 2007, 75, 4648–4654. [Google Scholar] [CrossRef]
- Duthie, M.S.; Raman, V.S.; Piazza, F.M.; Reed, S.G. The development and clinical evaluation of second-generation leishmaniasis vaccines. Vaccine 2012, 30, 134–141. [Google Scholar] [CrossRef]
- Llanos-Cuentas, A.; Calderon, W.; Cruz, M.; Ashman, J.A.; Alves, F.P.; Coler, R.N.; Bogatzki, L.Y.; Bertholet, S.; Laughlin, E.M.; Kahn, S.J.; et al. A clinical trial to evaluate the safety and immunogenicity of the LEISH-F1+MPL-SE vaccine when used in combination with sodium stibogluconate for the treatment of mucosal leishmaniasis. Vaccine 2010, 28, 7427–7435. [Google Scholar] [CrossRef]
- Nascimento, E.; Fernandes, D.F.; Vieira, E.P.; Campos-Neto, A.; Ashman, J.A.; Alves, F.P.; Coler, R.N.; Bogatzki, L.Y.; Kahn, S.J.; Beckmann, A.M.; et al. A clinical trial to evaluate the safety and immunogenicity of the LEISH-F1+MPL-SE vaccine when used in combination with meglumine antimoniate for the treatment of cutaneous leishmaniasis. Vaccine 2010, 28, 6581–6587. [Google Scholar] [CrossRef]
- Malito, E.; Carfi, A.; Bottomley, M.J. Protein Crystallography in Vaccine Research and Development. Int. J. Mol. Sci. 2015, 16, 13106–13140. [Google Scholar] [CrossRef]
- McLellan, J.S.; Chen, M.; Joyce, M.G.; Sastry, M.; Stewart-Jones, G.B.; Yang, Y.; Zhang, B.; Chen, L.; Srivatsan, S.; Zheng, A.; et al. Structure-based design of a fusion glycoprotein vaccine for respiratory syncytial virus. Science 2013, 342, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Nuccitelli, A.; Cozzi, R.; Gourlay, L.J.; Donnarumma, D.; Necchi, F.; Norais, N.; Telford, J.L.; Rappuoli, R.; Bolognesi, M.; Maione, D.; et al. Structure-based approach to rationally design a chimeric protein for an effective vaccine against Group B Streptococcus infections. Proc. Natl. Acad. Sci. USA 2011, 108, 10278–10283. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Moyle, P.M. Bioconjugation Approaches to Producing Subunit Vaccines Composed of Protein or Peptide Antigens and Covalently Attached Toll-Like Receptor Ligands. Bioconjugate Chem. 2018, 29, 572–586. [Google Scholar] [CrossRef] [PubMed]
- Helgeby, A.; Robson, N.C.; Donachie, A.M.; Beackock-Sharp, H.; Lovgren, K.; Schon, K.; Mowat, A.; Lycke, N.Y. The combined CTA1-DD/ISCOM adjuvant vector promotes priming of mucosal and systemic immunity to incorporated antigens by specific targeting of B cells. J. Immunol. 2006, 176, 3697–3706. [Google Scholar] [CrossRef]
- Williams, N.A.; Hirst, T.R.; Nashar, T.O. Immune modulation by the cholera-like enterotoxins: From adjuvant to therapeutic. Immunol. Today 1999, 20, 95–101. [Google Scholar] [CrossRef]
- Lycke, N. From toxin to adjuvant: Basic mechanisms for the control of mucosal IgA immunity and tolerance. Immunol. Lett. 2005, 97, 193–198. [Google Scholar] [CrossRef]
- Coffman, R.L.; Sher, A.; Seder, R.A. Vaccine adjuvants: Putting innate immunity to work. Immunity 2010, 33, 492–503. [Google Scholar] [CrossRef]
- Kawamura, Y.I.; Kawashima, R.; Shirai, Y.; Kato, R.; Hamabata, T.; Yamamoto, M.; Furukawa, K.; Fujihashi, K.; McGhee, J.R.; Hayashi, H.; et al. Cholera toxin activates dendritic cells through dependence on GM1-ganglioside which is mediated by NF-kappaB translocation. Eur. J. Immunol. 2003, 33, 3205–3212. [Google Scholar] [CrossRef]
- Esposito, C.; Carullo, P.; Pedone, E.; Graziano, G.; Del Vecchio, P.; Berisio, R. Dimerisation and structural integrity of Heparin Binding Hemagglutinin A from Mycobacterium tuberculosis: Implications for bacterial agglutination. FEBS Lett. 2010, 584, 1091–1096. [Google Scholar] [CrossRef]
- Kim, H.M.; Park, B.S.; Kim, J.I.; Kim, S.E.; Lee, J.; Oh, S.C.; Enkhbayar, P.; Matsushima, N.; Lee, H.; Yoo, O.J.; et al. Crystal structure of the TLR4-MD-2 complex with bound endotoxin antagonist Eritoran. Cell 2007, 130, 906–917. [Google Scholar] [CrossRef]
- Osugi, Y.; Vuckovic, S.; Hart, D.N. Myeloid blood CD11c(+) dendritic cells and monocyte-derived dendritic cells differ in their ability to stimulate T lymphocytes. Blood 2002, 100, 2858–2866. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.; Lindgren, G.; Sandgren, K.J.; Thompson, E.A.; Francica, J.R.; Seubert, A.; De Gregorio, E.; Barnett, S.; O’Hagan, D.T.; Sullivan, N.J.; et al. Vaccine priming is restricted to draining lymph nodes and controlled by adjuvant-mediated antigen uptake. Sci. Transl. Med. 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- van Aalst, S.; Ludwig, I.S.; van Kooten, P.J.S.; van der Zee, R.; van Eden, W.; Broere, F. Dynamics of APC recruitment at the site of injection following injection of vaccine adjuvants. Vaccine 2017, 35, 1622–1629. [Google Scholar] [CrossRef] [PubMed]
- Abidin, B.M.; Hammami, A.; Stager, S.; Heinonen, K.M. Infection-adapted emergency hematopoiesis promotes visceral leishmaniasis. PLoS Pathog. 2017, 13, e1006422. [Google Scholar] [CrossRef] [PubMed]
- Burberry, A.; Zeng, M.Y.; Ding, L.; Wicks, I.; Inohara, N.; Morrison, S.J.; Nunez, G. Infection mobilizes hematopoietic stem cells through cooperative NOD-like receptor and Toll-like receptor signaling. Cell Host Microbe 2014, 15, 779–791. [Google Scholar] [CrossRef]
- Kaufmann, E.; Sanz, J.; Dunn, J.L.; Khan, N.; Mendonca, L.E.; Pacis, A.; Tzelepis, F.; Pernet, E.; Dumaine, A.; Grenier, J.C.; et al. BCG Educates Hematopoietic Stem Cells to Generate Protective Innate Immunity against Tuberculosis. Cell 2018, 172, 176–190.e119. [Google Scholar] [CrossRef]
- Novais, F.O.; Nguyen, B.T.; Beiting, D.P.; Carvalho, L.P.; Glennie, N.D.; Passos, S.; Carvalho, E.M.; Scott, P. Human classical monocytes control the intracellular stage of Leishmania braziliensis by reactive oxygen species. J. Infect. Dis. 2014, 209, 1288–1296. [Google Scholar] [CrossRef]
- Darrah, P.A.; Patel, D.T.; De Luca, P.M.; Lindsay, R.W.; Davey, D.F.; Flynn, B.J.; Hoff, S.T.; Andersen, P.; Reed, S.G.; Morris, S.L.; et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat. Med. 2007, 13, 843–850. [Google Scholar] [CrossRef]
- De Brito, R.C.F.; Cardoso, J.M.O.; Reis, L.E.S.; Mathias, F.A.S.; Aguiar-Soares, R.D.O.; Teixeira-Carvalho, A.; Roatt, B.M.; Correa-Oliveira, R.; Ruiz, J.C.; Resende, D.M.; et al. Synthetic Peptides Elicit Strong Cellular Immunity in Visceral Leishmaniasis Natural Reservoir and Contribute to Long-Lasting Polyfunctional T-Cells in BALB/c Mice. Vaccines 2019, 7, 162. [Google Scholar] [CrossRef]
- Sabur, A.; Bhowmick, S.; Chhajer, R.; Ejazi, S.A.; Didwania, N.; Asad, M.; Bhattacharyya, A.; Sinha, U.; Ali, N. Liposomal Elongation Factor-1alpha Triggers Effector CD4 and CD8 T Cells for Induction of Long-Lasting Protective Immunity against Visceral Leishmaniasis. Front. Immunol. 2018, 9, 18. [Google Scholar] [CrossRef]
- Sanchez-Sampedro, L.; Gomez, C.E.; Mejias-Perez, E.; Sorzano, C.O.; Esteban, M. High quality long-term CD4+ and CD8+ effector memory populations stimulated by DNA-LACK/MVA-LACK regimen in Leishmania major BALB/c model of infection. PLoS ONE 2012, 7, e38859. [Google Scholar] [CrossRef] [PubMed]
- Dey, R.; Dagur, P.K.; Selvapandiyan, A.; McCoy, J.P.; Salotra, P.; Duncan, R.; Nakhasi, H.L. Live attenuated Leishmania donovani p27 gene knockout parasites are nonpathogenic and elicit long-term protective immunity in BALB/c mice. J. Immunol. 2013, 190, 2138–2149. [Google Scholar] [CrossRef] [PubMed]
- Hofmeyer, K.A.; Duthie, M.S.; Laurance, J.D.; Favila, M.A.; Van Hoeven, N.; Coler, R.N.; Reed, S.G. Optimizing Immunization Strategies for the Induction of Antigen-Specific CD4 and CD8 T Cell Responses for Protection against Intracellular Parasites. Clin. Vaccine Immunol. Cvi 2016, 23, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Murray, H.W.; Nathan, C.F. Macrophage microbicidal mechanisms in vivo: Reactive nitrogen versus oxygen intermediates in the killing of intracellular visceral Leishmania donovani. J. Exp. Med. 1999, 189, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Peters, N.C.; Pagan, A.J.; Lawyer, P.G.; Hand, T.W.; Henrique Roma, E.; Stamper, L.W.; Romano, A.; Sacks, D.L. Chronic parasitic infection maintains high frequencies of short-lived Ly6C+CD4+ effector T cells that are required for protection against re-infection. PLoS Pathog. 2014, 10, e1004538. [Google Scholar] [CrossRef] [PubMed]
- Liew, F.Y.; Li, Y.; Millott, S. Tumor necrosis factor-alpha synergizes with IFN-gamma in mediating killing of Leishmania major through the induction of nitric oxide. J. Immunol. 1990, 145, 4306–4310. [Google Scholar]
- Wherry, E.J.; Teichgraber, V.; Becker, T.C.; Masopust, D.; Kaech, S.M.; Antia, R.; von Andrian, U.H.; Ahmed, R. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat. Immunol. 2003, 4, 225–234. [Google Scholar] [CrossRef]
- Millington, K.A.; Innes, J.A.; Hackforth, S.; Hinks, T.S.; Deeks, J.J.; Dosanjh, D.P.; Guyot-Revol, V.; Gunatheesan, R.; Klenerman, P.; Lalvani, A. Dynamic relationship between IFN-gamma and IL-2 profile of Mycobacterium tuberculosis-specific T cells and antigen load. J. Immunol. 2007, 178, 5217–5226. [Google Scholar] [CrossRef]
- Lindenstrom, T.; Agger, E.M.; Korsholm, K.S.; Darrah, P.A.; Aagaard, C.; Seder, R.A.; Rosenkrands, I.; Andersen, P. Tuberculosis subunit vaccination provides long-term protective immunity characterized by multifunctional CD4 memory T cells. J. Immunol. 2009, 182, 8047–8055. [Google Scholar] [CrossRef]
- Esch, K.J.; Juelsgaard, R.; Martinez, P.A.; Jones, D.E.; Petersen, C.A. Programmed death 1-mediated T cell exhaustion during visceral leishmaniasis impairs phagocyte function. J. Immunol. 2013, 191, 5542–5550. [Google Scholar] [CrossRef]
- Stager, S.; Alexander, J.; Kirby, A.C.; Botto, M.; Rooijen, N.V.; Smith, D.F.; Brombacher, F.; Kaye, P.M. Natural antibodies and complement are endogenous adjuvants for vaccine-induced CD8+ T-cell responses. Nat. Med. 2003, 9, 1287–1292. [Google Scholar] [CrossRef]
- Basu, R.; Roy, S.; Walden, P. HLA class I-restricted T cell epitopes of the kinetoplastid membrane protein-11 presented by Leishmania donovani-infected human macrophages. J. Infect. Dis. 2007, 195, 1373–1380. [Google Scholar] [CrossRef] [PubMed]
- Agallou, M.; Smirlis, D.; Soteriadou, K.P.; Karagouni, E. Vaccination with Leishmania histone H1-pulsed dendritic cells confers protection in murine visceral leishmaniasis. Vaccine 2012, 30, 5086–5093. [Google Scholar] [CrossRef] [PubMed]
- Margaroni, M.; Agallou, M.; Athanasiou, E.; Kammona, O.; Kiparissides, C.; Gaitanaki, C.; Karagouni, E. Vaccination with poly(D,L-lactide-co-glycolide) nanoparticles loaded with soluble Leishmania antigens and modified with a TNFalpha-mimicking peptide or monophosphoryl lipid A confers protection against experimental visceral leishmaniasis. Int. J. Nanomed. 2017, 12, 6169–6184. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, P.; Singh, V.; Naik, S. Immune response to leishmania: Paradox rather than paradigm. Fems Immunol. Med. Microbiol. 2007, 51, 229–242. [Google Scholar] [CrossRef]
- Gerth, A.J.; Lin, L.; Peng, S.L. T-bet regulates T-independent IgG2a class switching. Int. Immunol. 2003, 15, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Selvapandiyan, A.; Dey, R.; Nylen, S.; Duncan, R.; Sacks, D.; Nakhasi, H.L. Intracellular replication-deficient Leishmania donovani induces long lasting protective immunity against visceral leishmaniasis. J. Immunol. 2009, 183, 1813–1820. [Google Scholar] [CrossRef] [PubMed]
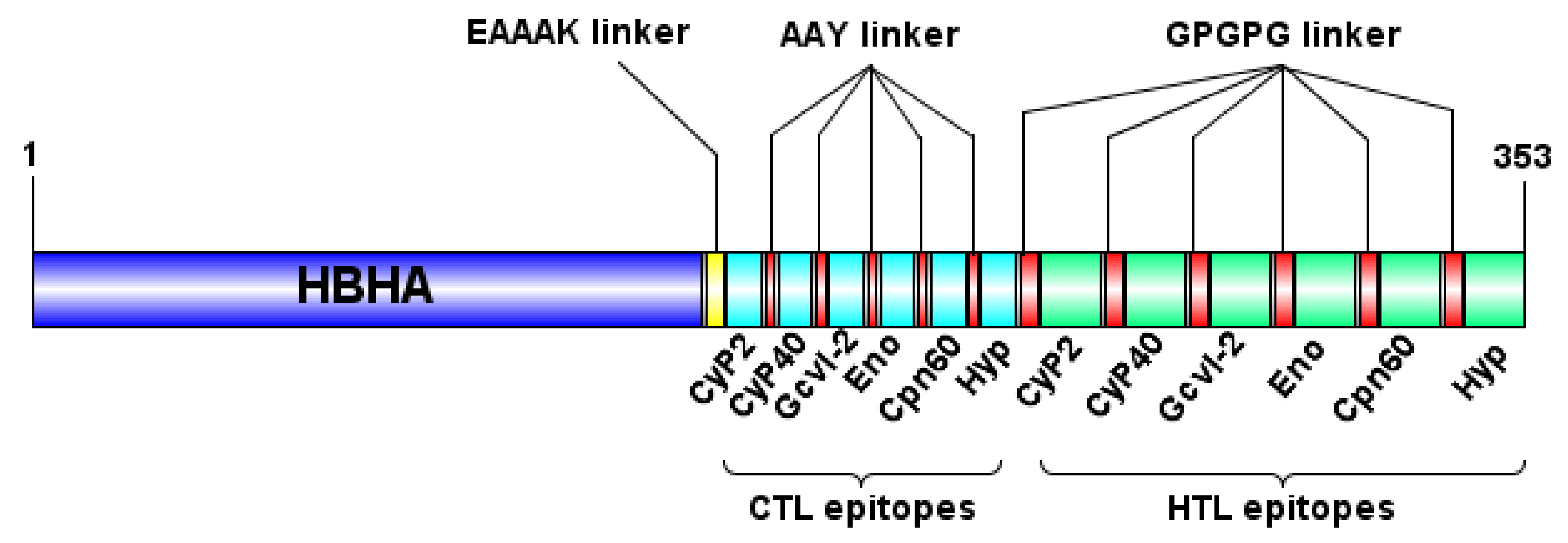
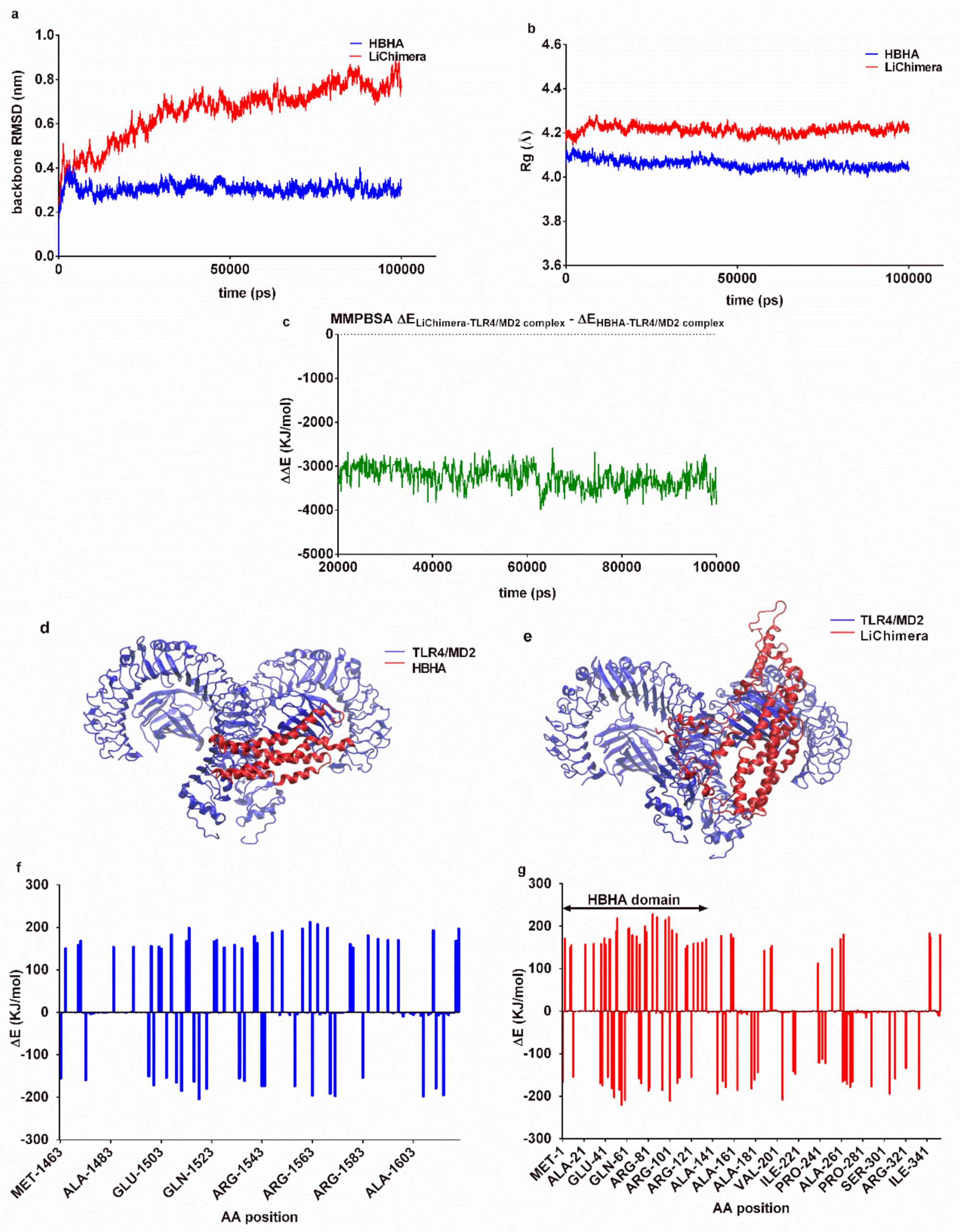

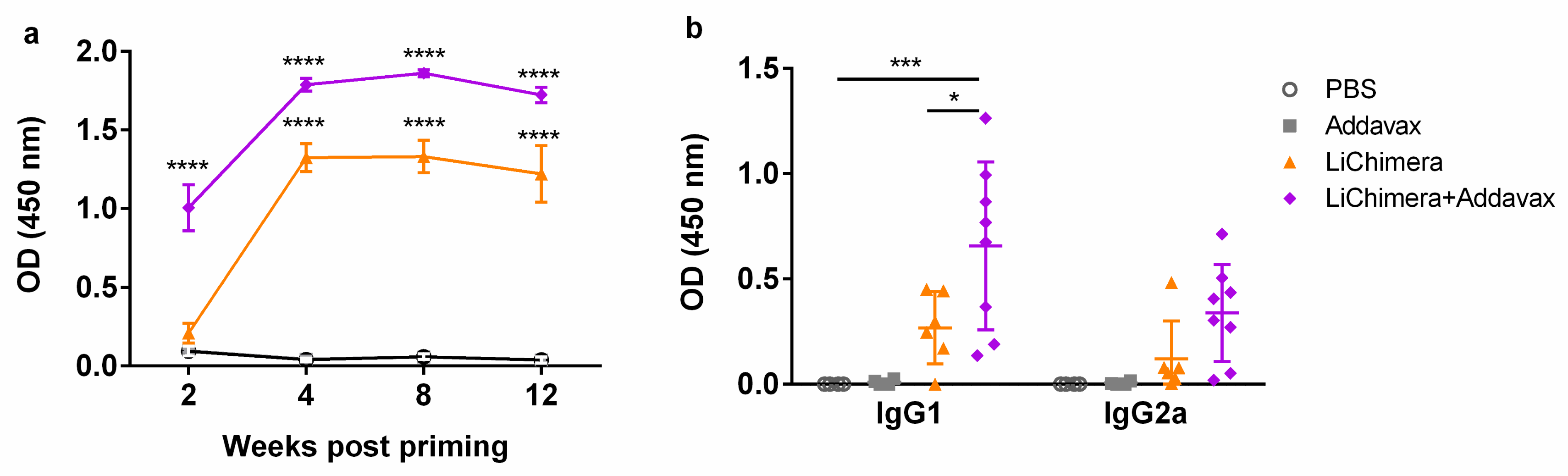
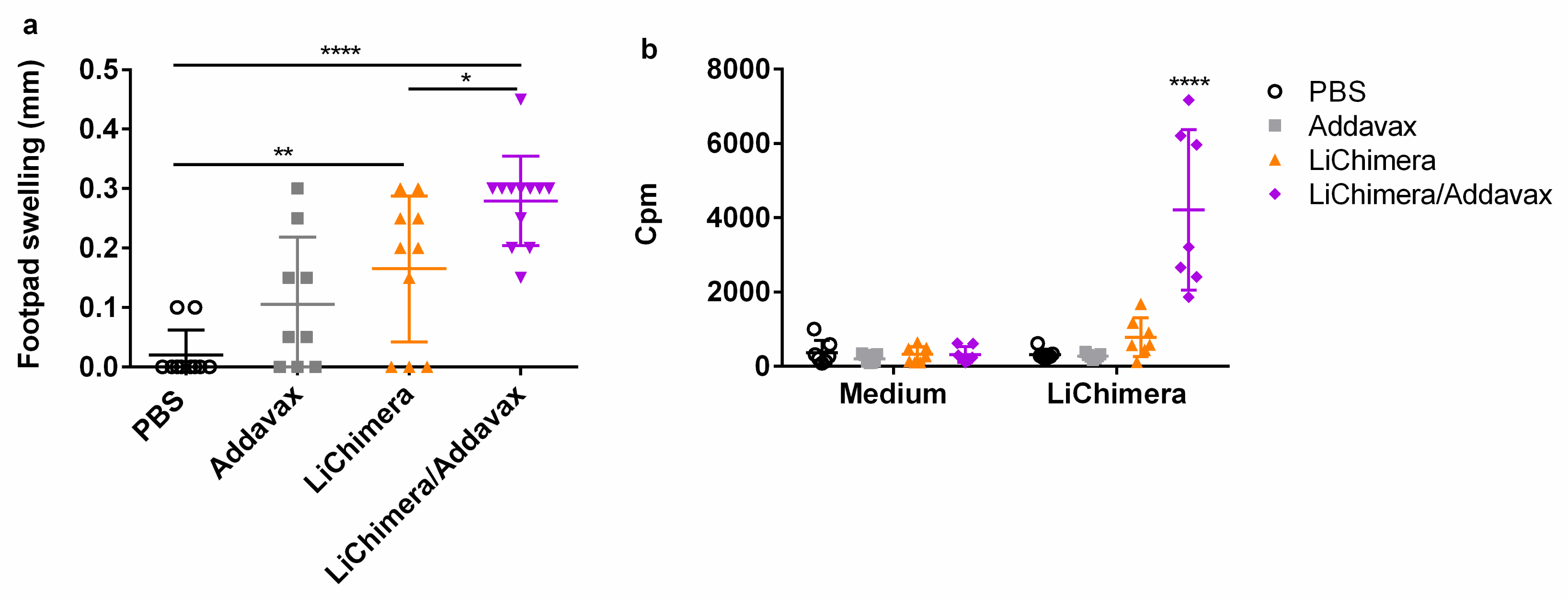

| Protein Name | Epitope Sequence | Population Coverage | Conservancy |
|---|---|---|---|
| CyP2 | 126-GPNTNGSQF-134 | 15.14% | 100% |
| CyP40 | 232-KYAKAVRYL-240 | 23.33% | 100% |
| Gcvl-2 | 439-EYGASSEDL-447 | 32.09% | 100% |
| Enol | 186-VYHALKVII-194 | 26.18% | 100% |
| Cpn60 | 38-LGPKGRNVI-46 | 76.14% | 100% |
| Hyp | 169-LFSCMLTSL-177 | 21.38% | 100% |
| Protein Name | Epitope Sequence | Population Coverage | Conservancy |
|---|---|---|---|
| CyP2 | 173-DRPVKPVKIVASGEL-187 | 59.52% | 100% |
| CyP40 | 332-SEAKEKVKAQKAKLA-340 | 71.01% | 100% |
| Gcvl-2 | 19-GGPGGYVAAIKAAQL-33 | 79.83% | 100% |
| Enol | 111-GCSMAISKAAAAKAG-125 | 80.49% | 100% |
| Cpn60 | 27-VTRAVAAVATTLGPK-41 | 66.37% | 100% |
| Hyp | 57-VAITEDVALAAVQAV-71 | 52.49% | 100% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agallou, M.; Margaroni, M.; Kotsakis, S.D.; Karagouni, E. A Canine-Directed Chimeric Multi-Epitope Vaccine Induced Protective Immune Responses in BALB/c Mice Infected with Leishmania infantum. Vaccines 2020, 8, 350. https://doi.org/10.3390/vaccines8030350
Agallou M, Margaroni M, Kotsakis SD, Karagouni E. A Canine-Directed Chimeric Multi-Epitope Vaccine Induced Protective Immune Responses in BALB/c Mice Infected with Leishmania infantum. Vaccines. 2020; 8(3):350. https://doi.org/10.3390/vaccines8030350
Chicago/Turabian StyleAgallou, Maria, Maritsa Margaroni, Stathis D. Kotsakis, and Evdokia Karagouni. 2020. "A Canine-Directed Chimeric Multi-Epitope Vaccine Induced Protective Immune Responses in BALB/c Mice Infected with Leishmania infantum" Vaccines 8, no. 3: 350. https://doi.org/10.3390/vaccines8030350
APA StyleAgallou, M., Margaroni, M., Kotsakis, S. D., & Karagouni, E. (2020). A Canine-Directed Chimeric Multi-Epitope Vaccine Induced Protective Immune Responses in BALB/c Mice Infected with Leishmania infantum. Vaccines, 8(3), 350. https://doi.org/10.3390/vaccines8030350





