Characteristics of Adverse Events Following Immunization Reporting in Children: The Japanese Adverse Drug Event Report Database
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Characteristics of the Reports in the JADER
3.2. Outcomes Associated with AEFI Reports
3.3. Ten Most Frequently Reported Vaccines
3.4. Ten Most Frequently Reported Reactions
3.5. Ten Most Frequently Reported Vaccine–Reaction Pairs
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhou, W.; Pool, V.; Iskander, J.K.; English-Bullard, R.; Ball, R.; Wise, R.P.; Haber, P.; Pless, R.P.; Mootrey, G.; Ellenberg, S.S.; et al. Surveillance for safety after immunization: Vaccine Adverse Event Reporting System (VAERS)–United States, 1991–2001. MMWR Surveill Summ. 2003, 52, 1–24. [Google Scholar] [PubMed]
- De Bie, S.; Ferrajolo, C.; Straus, S.M.J.M.; Verhamme, K.M.C.; Weeks, A.; Wong, I.C.; Sturkenboom, M.C.J.M.; Network, G. Pediatric drug safety surveillance in FDA-AERS: A description of adverse events from GRiP Project. PLoS ONE 2015, 10, e0130399. [Google Scholar] [CrossRef] [PubMed]
- Kimland, E.; Rane, A.; Ufer, M.; Panagiotidis, G. Paediatric adverse drug reactions reported in Sweden from 1987 to 2001. Pharmacol. Drug Saf. 2005, 14, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Hawcutt, D.B.H.; Russell, N.-J.R.; Maqsood, H.M.; Kouranloo, K.K.; Gomberg, S.G.; Waitt, C.W.; Sharp, A.S.; Riordan, A.R.; Turner, M.A.P. Spontaneous adverse drug reaction reports for neonates and infants in the UK 2001–2010: Content and utility analysis. Br. J. Clin. Pharmacol. 2016, 82, 1601–1612. [Google Scholar] [CrossRef] [PubMed]
- Rosli, R.; Ming, L.C.; Abd Aziz, N.A.; Manan, M.M. A retrospective analysis of spontaneous adverse drug reactions reports relating to paediatric patients. PLoS ONE 2016, 11, e0155385. [Google Scholar] [CrossRef] [PubMed]
- Aldea, A.; García Sánchez-Colomer, M.; Fernández Quintana, E.F.; Sáiz, M.G. Paediatric adverse drug reactions reported to the Spanish Pharmacovigilance System from 2004 to 2009. Eur. J. Clin. Pharmacol. 2012, 68, 1329–1338. [Google Scholar] [CrossRef] [PubMed]
- Larson, H.J.; de Figueiredo, A.; Xiahong, Z.; Schulz, W.S.; Verger, P.; Johnston, I.G.; Cook, W.C.; Jones, N.S. The state of vaccine confidence 2016: Global insights through a 67-country survey. EBioMedicine 2016, 12, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Hirota, Y.; Kaji, M. Scepticism about influenza vaccine efficacy in Japan. Lancet 1994, 344, 408–409. [Google Scholar] [CrossRef]
- Tanaka, D. Global Developments in Vaccine Pharmacovigilance-Activities in WHO. Jpn. J. Pharmacol. 2015, 20, 53. [Google Scholar]
- WHO. Global Vaccine Safety Blueprint 2.0. Available online: https://www.who.int/vaccine_safety/Draft_GVSB2.0_20190929.pdf (accessed on 23 May 2020).
- The ICH Steering Committee. Maintenance of the ICH Guideline on Clinical Safety data Management: Data Elements for Transmission of Individual Case Safety Reports E2B(M). Available online: https://www.pmda.go.jp/files/000156826.pdf (accessed on 23 May 2020).
- Aagaard, L.; Strandell, J.; Melskens, L.; Petersen, P.S.; Holme Hansen, E. Global Patterns of Adverse Drug Reactions Over a Decade: Analyses of Spontaneous Reports to VigiBase™. Drug Saf. 2012, 35, 1171–1182. [Google Scholar] [CrossRef] [PubMed]
- The Ministry of Health, Labour and Welfare. The Basic Plan for Vaccination. Available online: https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/kenkou_iryou/kenkou/kekkaku-kansenshou/kihonteki_keikaku/index.html (accessed on 21 May 2020).
- Cliff-Eribo, K.O.; Sammons, H.; Choonara, I. Systematic review of paediatric studies of adverse drug reactions from pharmacovigilance databases. Expert Opin. Drug Saf. 2016, 15, 1321–1328. [Google Scholar] [CrossRef] [PubMed]
- The Ministry of Health, Labour and Welfare. Pharmaceuticals and Medical Devices Safety Information No. 280. Available online: https://www.pmda.go.jp/files/000153635.pdf (accessed on 13 May 2020).
- The Japan Pediatric Society. Changes in the Immunization Schedule Recommended by the Japan Pediatric Society. Available online: http://www.jpeds.or.jp/uploads/files/20180801_JPS%20Schedule%20English.pdf (accessed on 13 May 2020).
- The Ministry of Health, Labour and Welfare. The Emergency Project for the Promotion of Vaccination Against Cervical Cancer, etc. Available online: https://www.mhlw.go.jp/bunya/kenkou/other/dl/101209i.pdf (accessed on 1 April 2020).
- Japan Pediatric Society. Concept of the Japan Pediatric Society for Simultaneous Administration of Multiple Vaccines. Available online: https://www.jpeds.or.jp/uploads/files/saisin_1101182.pdf (accessed on 16 February 2019).
- The Weber Effect and the United States Food and Drug Administration’s Adverse Event Reporting System (FAERS): Analysis of Sixty-Two Drugs Approved from 2006 to 2010. Drug Saf. 2014, 37, 283–294. [CrossRef] [PubMed]
- Suzuki, S.; Hosono, A. No association between HPV vaccine and reported post-vaccination symptoms in Japanese young women: Results of the Nagoya study. Papillomavirus Res. 2018, 5, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Larson, H.J. Japan’s HPV vaccine crisis: Act now to avert cervical cancer cases and deaths. Lancet Public Health 2020, 5, e184–e185. [Google Scholar] [CrossRef]
- Tsuchiya, M.; Obara, T.; Sakai, T.; Nomura, K.; Takamura, C.; Mano, M. Quality evaluation of adverse drug reaction reports using the Japanese Adverse Drug Event Report (JADER) database. Pharmacol. Drug Saf. 2020, 29, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, M.; Obara, T.; Miyazaki, M.; Noda, A.; Takamura, C.; Mano, M. The quality assessment of the Japanese Adverse Drug Event Report database using vigiGrade. Int. J. Clin. Pharm. 2020, 42, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Pharmaceutical and Medical Device Regulatory Science Society of Japan. Recommendation on the Reporting System of Adverse Reactions to Vaccines and the Development of Infrastructure related to Vaccine Risk Management in Japan <The Fifth Recommendation>. Available online: https://www.pmrj.jp/teigen/PMRJ_recommen5_sho_ENG.pdf (accessed on 17 June 2020).
- Sakai, T.; Ohtsu, F.; Mori, C.; Tanabe, K.; Goto, N. Signal of miscarriage with aripiprazole: A disproportionality analysis of the Japanese Adverse Drug Event Report Database. Drug Saf. 2017, 40, 1141–1146. [Google Scholar] [CrossRef] [PubMed]
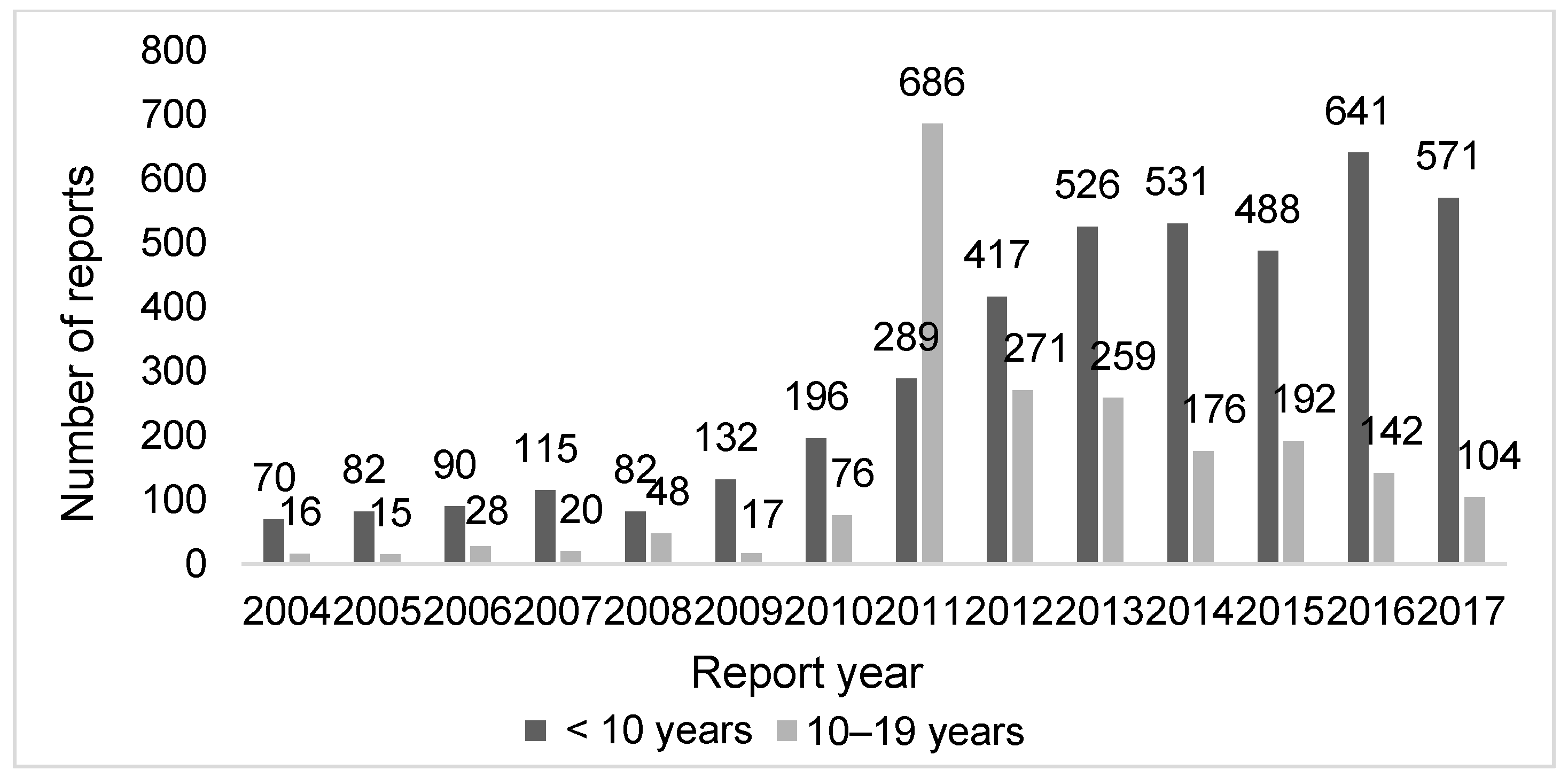
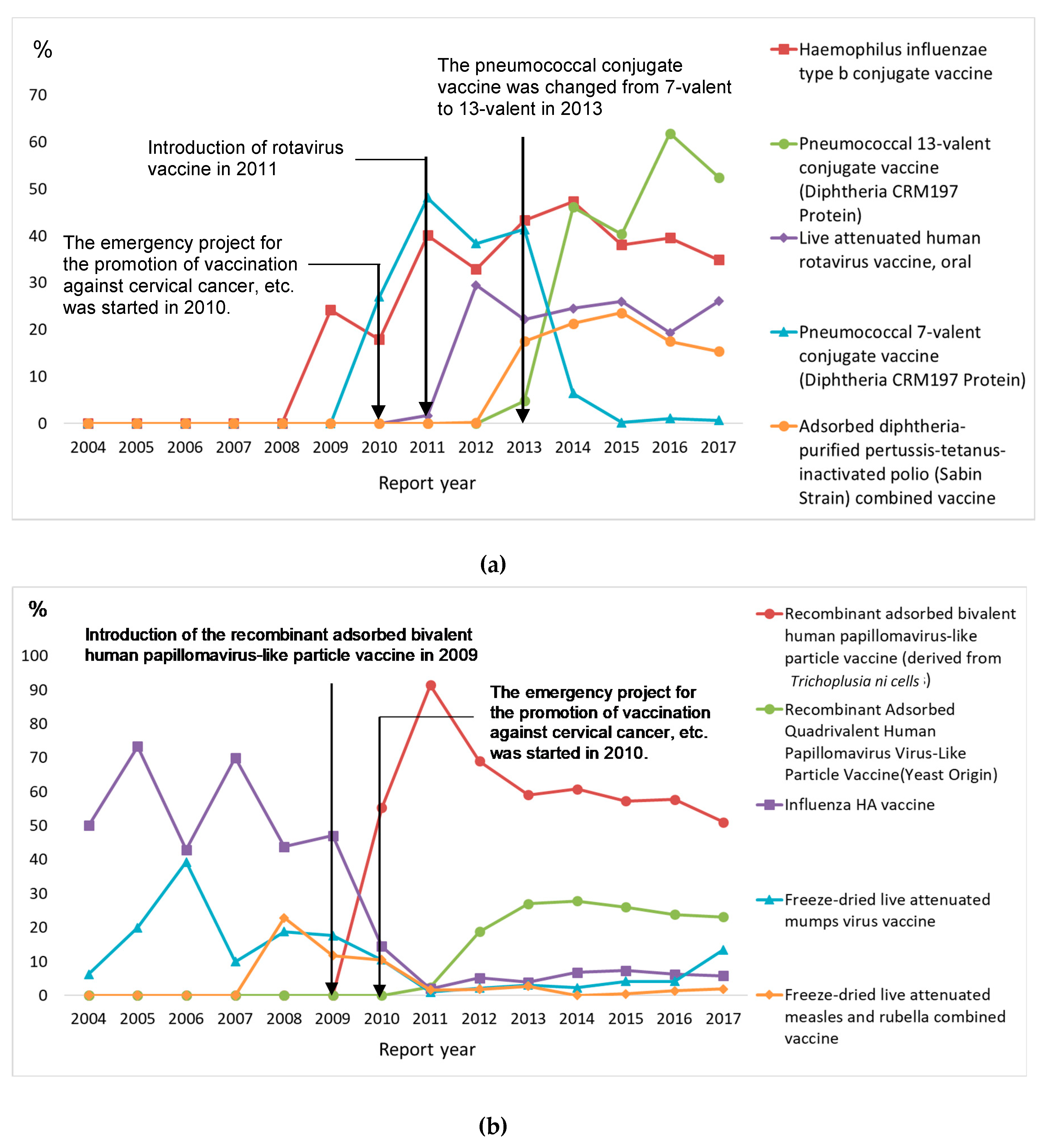
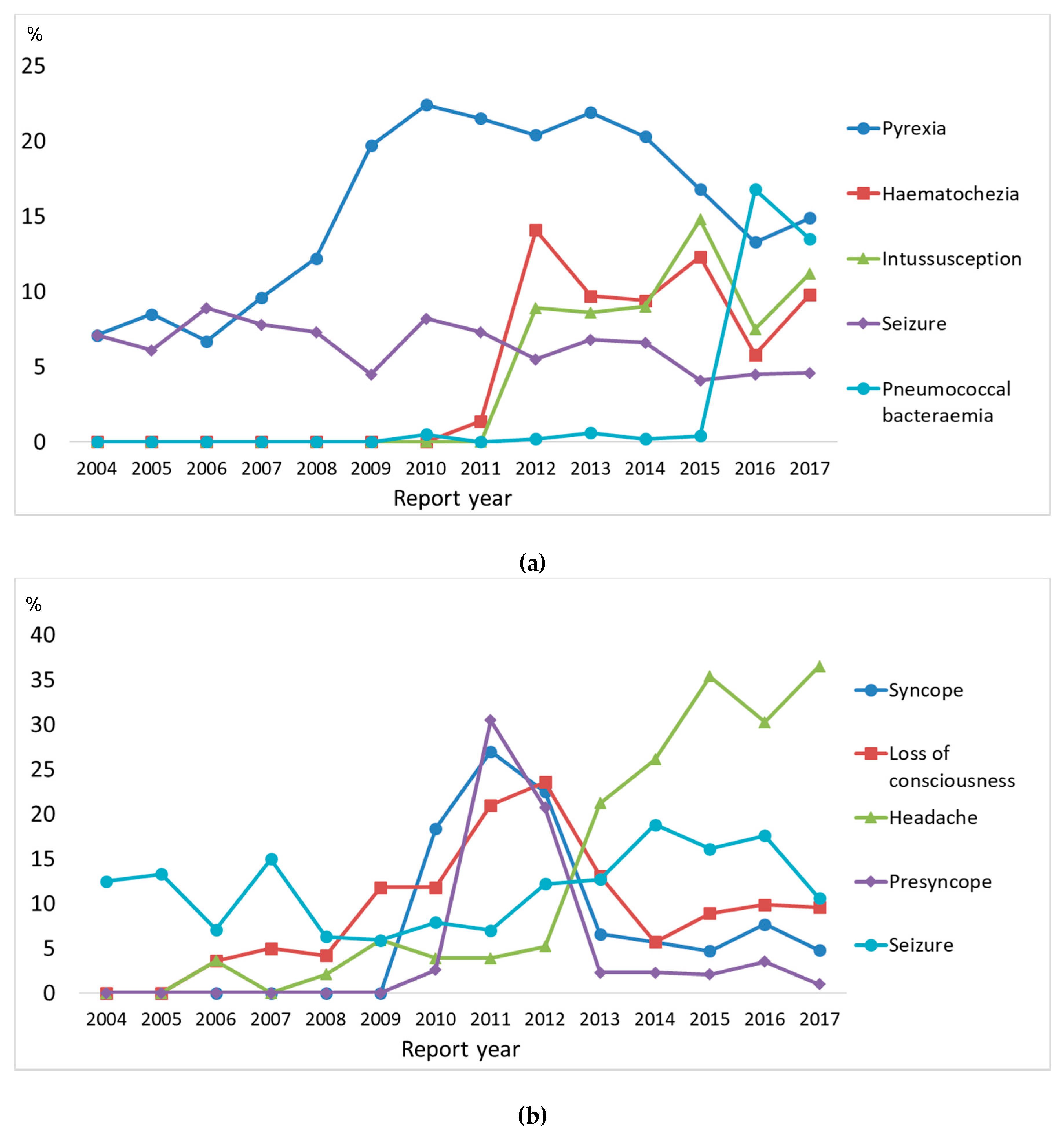
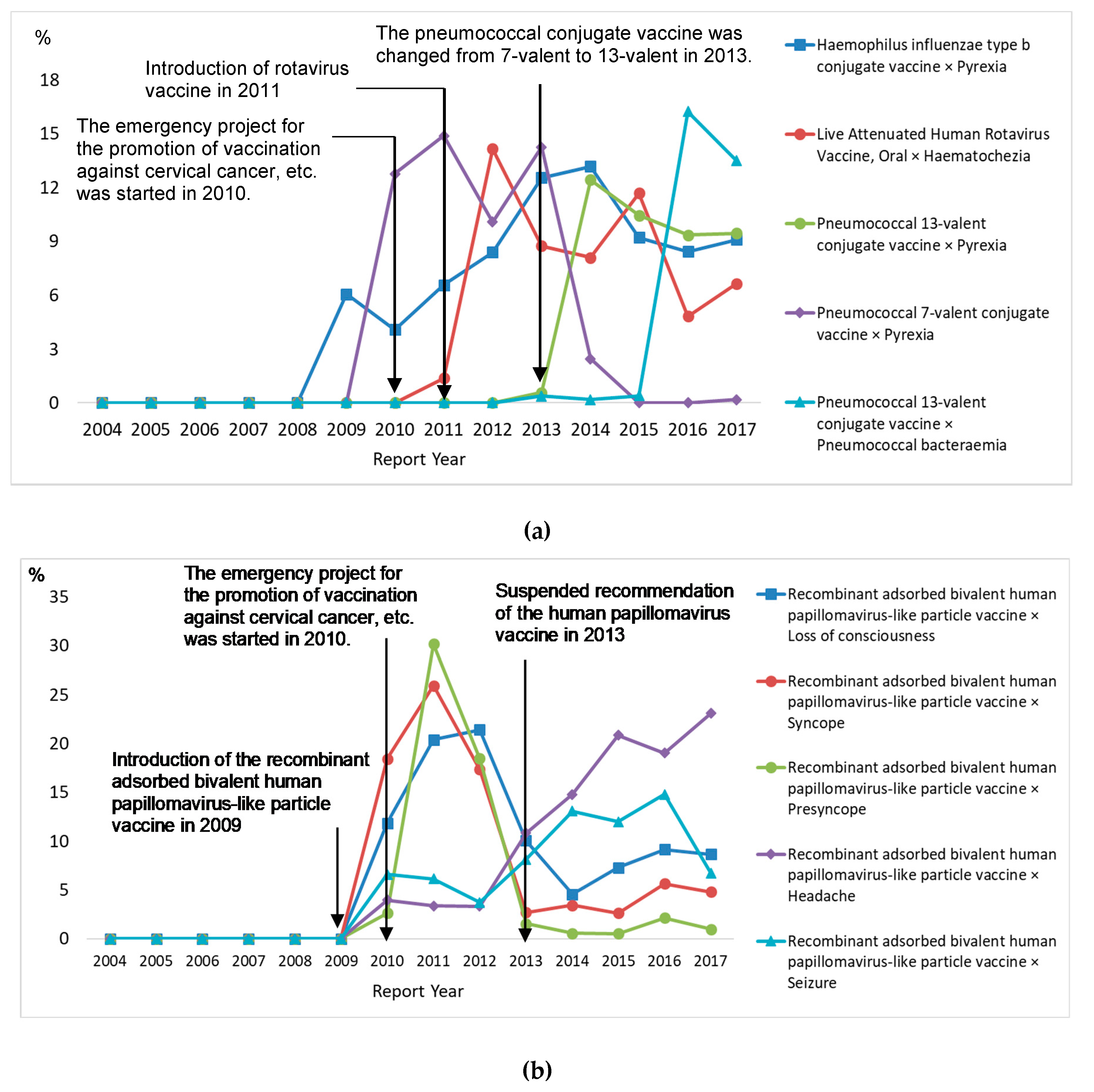
| Characteristics | Age Group | p | |
|---|---|---|---|
| <10 Years a n (%) | 10–19 Years b n (%) | ||
| Sex | |||
| Boy | 2272 (53.7) | 208 (10.1) | <0.0001 |
| Girl | 1801 (42.6) | 1838 (89.7) | |
| Unknown | 157 (3.7) | 4 (0.2) | |
| The type of report sender | |||
| Pharmaceutical company | 4155 (98.2) | 2047 (99.9) | <0.0001 |
| Healthcare facility | 75 (1.8) | 3 (0.1) | |
| The qualifications of the reporter | |||
| Doctor | 3548 (73.7) | 1807 (58.0) | <0.0001 |
| Pharmacist | 157 (3.3) | 99 (3.2) | |
| Healthcare professional | 358 (7.4) | 201 (6.5) | |
| Consumer | 335 (7.0) | 919 (29.5) | |
| Lawyer | 0 (0.0) | 21 (0.7) | |
| Unknown | 414 (8.6) | 67 (2.2) | |
| Total c | 4812 (100) | 3114 (100) | - |
| No. of suspected vaccines per AEFI report | |||
| 1 | 2673 (63.2) | 2028 (98.9) | <0.0001 |
| 2 | 554 (13.1) | 22 (1.1) | |
| 3 | 485 (11.5) | 0 (0.0) | |
| 4 | 347 (8.2) | 0 (0.0) | |
| ≥5 | 171 (4.0) | 0 (0.0) | |
| Total | 4230 (100) | 2050 (100) | - |
| Outcomes associated with AEFI report | |||
| Cured | 3934 (52.6) | 3734 (44.3) | <0.0001 |
| Recovering | 1585 (21.2) | 1024 (12.2) | |
| Did not recover | 276 (3.7) | 1776 (21.1) | |
| Recovering with sequelae | 91 (1.2) | 51 (0.6) | |
| Death | 321 (4.3) | 15 (0.2) | |
| Unknown | 1272 (17.0) | 1826 (21.7) | |
| Total d | 7479 (100) | 8426 (100) | - |
| <10 Years | n (%) |
| Vaccine Name a | |
| Haemophilus influenzae type b conjugate vaccine (monovalent) | 1438 (19.2) |
| Pneumococcal 13-valent conjugate vaccine (diphtheria CRM197 protein) | 1163 (15.5) |
| Live attenuated human rotavirus vaccine, oral (monovalent) | 775 (10.3) |
| Pneumococcal 7-valent conjugate vaccine (diphtheria CRM197 protein) | 616 (8.2) |
| Adsorbed diphtheria-purified pertussis-tetanus-inactivated polio (Sabin strain) combined vaccine | 521 (7.0) |
| Influenza hemagglutinin vaccine | 517 (6.9) |
| Recombinant adsorbed hepatitis B vaccine (yeast derived) | 412 (5.5) |
| Rotavirus vaccine, live, oral, pentavalent | 376 (5.0) |
| Freeze-dried live attenuated mumps virus vaccine | 313 (4.2) |
| Freeze-dried bacillus Calmette–Guérin vaccine | 267 (3.6) |
| Adverse reactions b | |
| Pyrexia | 731 (9.9) |
| Hematochezia | 317 (4.3) |
| Intussusception | 314 (4.3) |
| Seizure | 245 (3.3) |
| Pneumococcal bacteremia | 193 (2.6) |
| Immune thrombocytopenic purpura | 190 (2.6) |
| Anaphylactic reaction | 188 (2.6) |
| Meningitis aseptic | 179 (2.4) |
| Vomiting | 178 (2.4) |
| Febrile convulsion | 173 (2.3) |
| 10–19 years | n (%) |
| Vaccine name c | |
| Recombinant adsorbed bivalent human papillomavirus-like particle vaccine (derived from Trichoplusia ni cells) | 1361 (65.7) |
| Recombinant adsorbed quadrivalent human papillomavirus virus-like particle vaccine (yeast origin) | 295 (14.2) |
| Influenza hemagglutinin vaccine | 164 (7.9) |
| Freeze-dried live attenuated mumps virus vaccine | 90 (4.3) |
| Freeze-dried live attenuated measles and rubella combined vaccine | 50 (2.4) |
| Freeze-dried, cell culture-derived Japanese encephalitis vaccine | 31 (1.5) |
| Adsorbed diphtheria–tetanus combined toxoid | 30 (1.4) |
| Japanese encephalitis vaccine (not otherwise specified) | 11 (0.5) |
| Recombinant adsorbed hepatitis B vaccine (yeast derived) | 10 (0.5) |
| Dried live attenuated measles vaccine | 9 (0.4) |
| Adverse reactions d | |
| Syncope | 312 (3.8) |
| Loss of consciousness | 308 (3.8) |
| Headache | 297 (3.6) |
| Presyncope | 287 (3.5) |
| Seizure | 233 (2.8) |
| Malaise | 196 (2.4) |
| Pyrexia | 193 (2.4) |
| Pain | 178 (2.2) |
| Hypoesthesia | 148 (1.8) |
| Dizziness | 146 (1.8) |
| Generic Name | Adverse Reactions | n (%) |
|---|---|---|
| <10 Years | ||
| Haemophilus influenzae type b conjugate vaccine | Pyrexia | 357 (2.6) |
| Live attenuated human rotavirus vaccine, oral | Hematochezia | 278 (2.1) |
| Pneumococcal 13-valent conjugate vaccine (diphtheria CRM197 protein) | Pyrexia | 234 (1.7) |
| Pneumococcal 7-valent conjugate vaccine (diphtheria CRM197 protein) | Pyrexia | 199 (1.5) |
| Pneumococcal 13-valent conjugate vaccine (diphtheria CRM197 protein) | Pneumococcal bacteremia | 186 (1.4) |
| Live attenuated human rotavirus vaccine, oral | Intussusception | 182 (1.3) |
| Freeze-dried live attenuated mumps virus vaccine | Meningitis aseptic | 162 (1.2) |
| Live attenuated human rotavirus vaccine, oral | Pyrexia | 161 (1.2) |
| Rotavirus vaccine, live, oral, pentavalent | Intussusception | 130 (1.0) |
| Adsorbed diphtheria-purified pertussis-tetanus-inactivated polio (Sabin strain) combined vaccine | Pyrexia | 129 (1.0) |
| 10–19 years | ||
| Recombinant adsorbed bivalent human papillomavirus-like particle vaccine (derived from Trichoplusia ni cells) | Loss of consciousness | 277 (3.4) |
| Syncope | 270 (3.3) | |
| Presyncope | 269 (3.3) | |
| Headache | 180 (2.2) | |
| Seizure | 152 (1.8) | |
| Depressed level of consciousness | 135 (1.6) | |
| Malaise | 132 (1.6) | |
| Pyrexia | 122 (1.5) | |
| Pain | 120 (1.5) | |
| Arthralgia | 108 (1.3) | |
| Generic Name | Adverse Reactions | n (%) |
|---|---|---|
| <10 years | ||
| Haemophilus influenzae type b conjugate vaccine | Cardio-respiratory arrest | 51 (4.3) |
| Haemophilus influenzae type b conjugate vaccine | Sudden infant death syndrome | 39 (3.3) |
| Pneumococcal 13-valent conjugate vaccine (diphtheria CRM197 protein) | Cardio-respiratory arrest | 30 (2.5) |
| Haemophilus influenzae type b conjugate vaccine | Death | 29 (2.4) |
| Adsorbed diphtheria-purified pertussis-tetanus-inactivated polio (Sabin strain) combined vaccine | Cardio-respiratory arrest | 23 (1.9) |
| Pneumococcal 13-valent conjugate vaccine (diphtheria CRM197 protein) | Sudden infant death syndrome | 21 (1.8) |
| Haemophilus influenzae type b conjugate vaccine | Respiratory arrest | 21(1.8) |
| Pneumococcal 13-valent conjugate vaccine (diphtheria CRM197 protein) | Death | 20 (1.7) |
| Pneumococcal 7-valent conjugate vaccine (diphtheria CRM197 protein) | Cardio-respiratory arrest | 19 (1.6) |
| Adsorbed diphtheria-purified pertussis-tetanus-inactivated polio (Sabin strain) combined vaccine | Sudden infant death syndrome | 18 (1.5) |
| Pneumococcal 13-valent conjugate vaccine (diphtheria CRM197 protein) | Sudden infant death syndrome | 18 (1.5) |
| 10–19 years | ||
| Recombinant adsorbed bivalent human papillomavirus-like particle vaccine (derived from Trichoplusia ni cells) | Ventricular fibrillation | 2 (6.7) |
| Respiratory failure | 2 (6.7) | |
| Altered state of consciousness, Bulbar palsy, Ventricular tachycardia, Amyotrophic lateral sclerosis, Cardio-respiratory arrest, Bundle branch block left, Musculoskeletal stiffness, Tension headache, Sinus arrhythmia, Electroencephalogram abnormal, Osteosarcoma, Loss of consciousness, Ventricular arrhythmia, Dyspnoea, Completed suicide, Arrhythmia supraventricular, Respiratory arrest, Muscular weakness, Seizure, Ventricular extrasystoles | 1 (3.3) for each adverse reaction | |
| Influenza HA vaccine | Myocarditis | 1 (3.3) |
| Freeze-dried, Cell Culture-derived Japanese. Encephalitis Vaccine | Syncope | 1 (3.3) |
| Freeze-dried live attenuated measles and rubella combined vaccine | Cardio-respiratory arrest | 1 (3.3) |
| Pneumococcal vaccine | Pneumococcal infection, Post procedural infection, Sudden death | 1 (3.3) for each adverse reaction |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noda, A.; Sakai, T.; Tsuchiya, M.; Oyanagi, G.; Obara, T.; Mano, N. Characteristics of Adverse Events Following Immunization Reporting in Children: The Japanese Adverse Drug Event Report Database. Vaccines 2020, 8, 357. https://doi.org/10.3390/vaccines8030357
Noda A, Sakai T, Tsuchiya M, Oyanagi G, Obara T, Mano N. Characteristics of Adverse Events Following Immunization Reporting in Children: The Japanese Adverse Drug Event Report Database. Vaccines. 2020; 8(3):357. https://doi.org/10.3390/vaccines8030357
Chicago/Turabian StyleNoda, Aoi, Takamasa Sakai, Masami Tsuchiya, Gen Oyanagi, Taku Obara, and Nariyasu Mano. 2020. "Characteristics of Adverse Events Following Immunization Reporting in Children: The Japanese Adverse Drug Event Report Database" Vaccines 8, no. 3: 357. https://doi.org/10.3390/vaccines8030357
APA StyleNoda, A., Sakai, T., Tsuchiya, M., Oyanagi, G., Obara, T., & Mano, N. (2020). Characteristics of Adverse Events Following Immunization Reporting in Children: The Japanese Adverse Drug Event Report Database. Vaccines, 8(3), 357. https://doi.org/10.3390/vaccines8030357





