Preventing the Risk of Hospitalization for Respiratory Complications of Influenza among the Elderly: Is There a Better Influenza Vaccination Strategy? A Retrospective Population Study
Abstract
1. Introduction
2. Material and Methods
2.1. Design, Setting and Data Sources
2.2. Study Population and Influenza Vaccination Grouping Criteria
2.3. Study and Follow-up Periods
2.4. Outcomes
2.5. Statistical Analysis
2.6. Ethics Statement
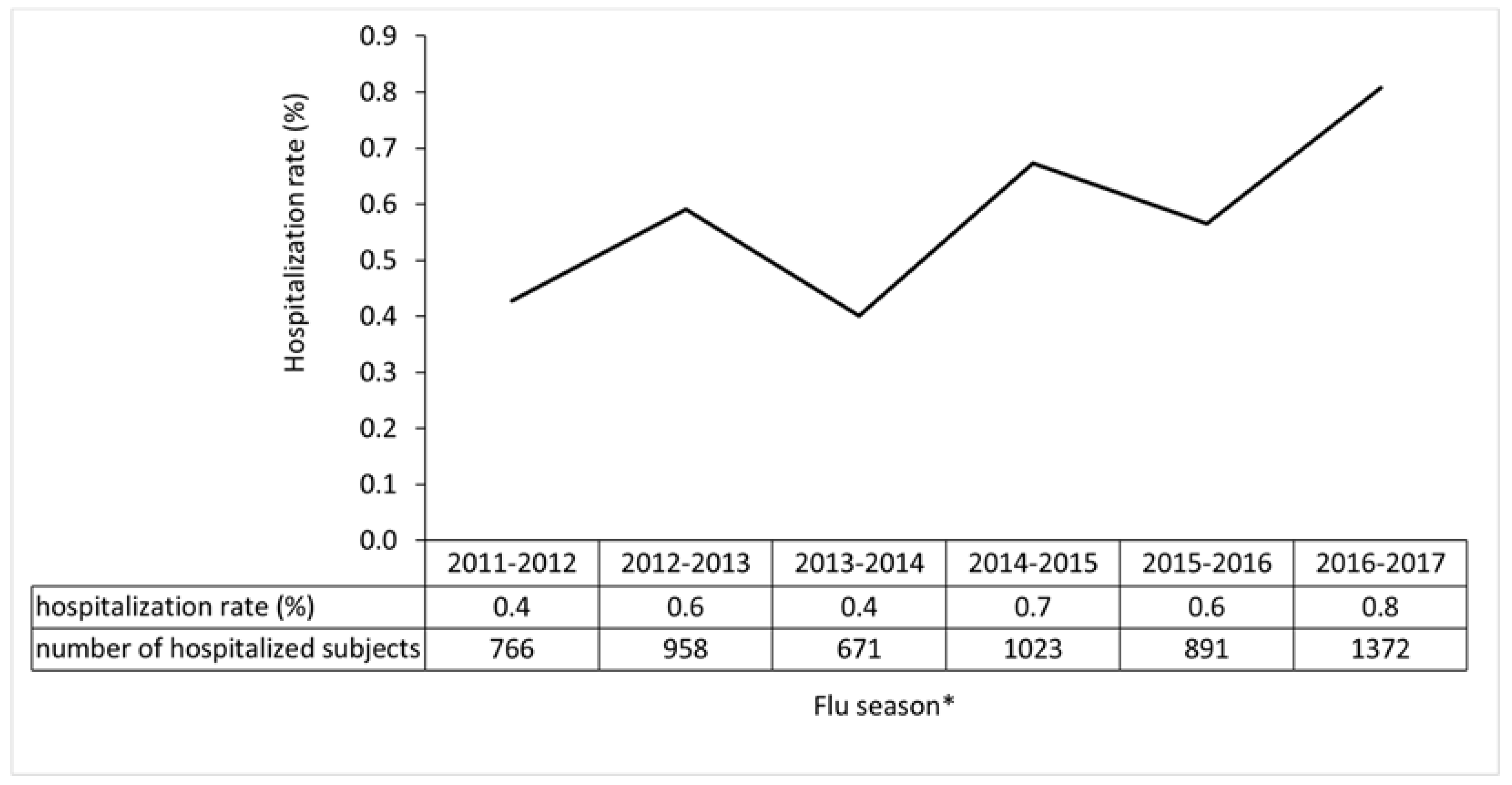
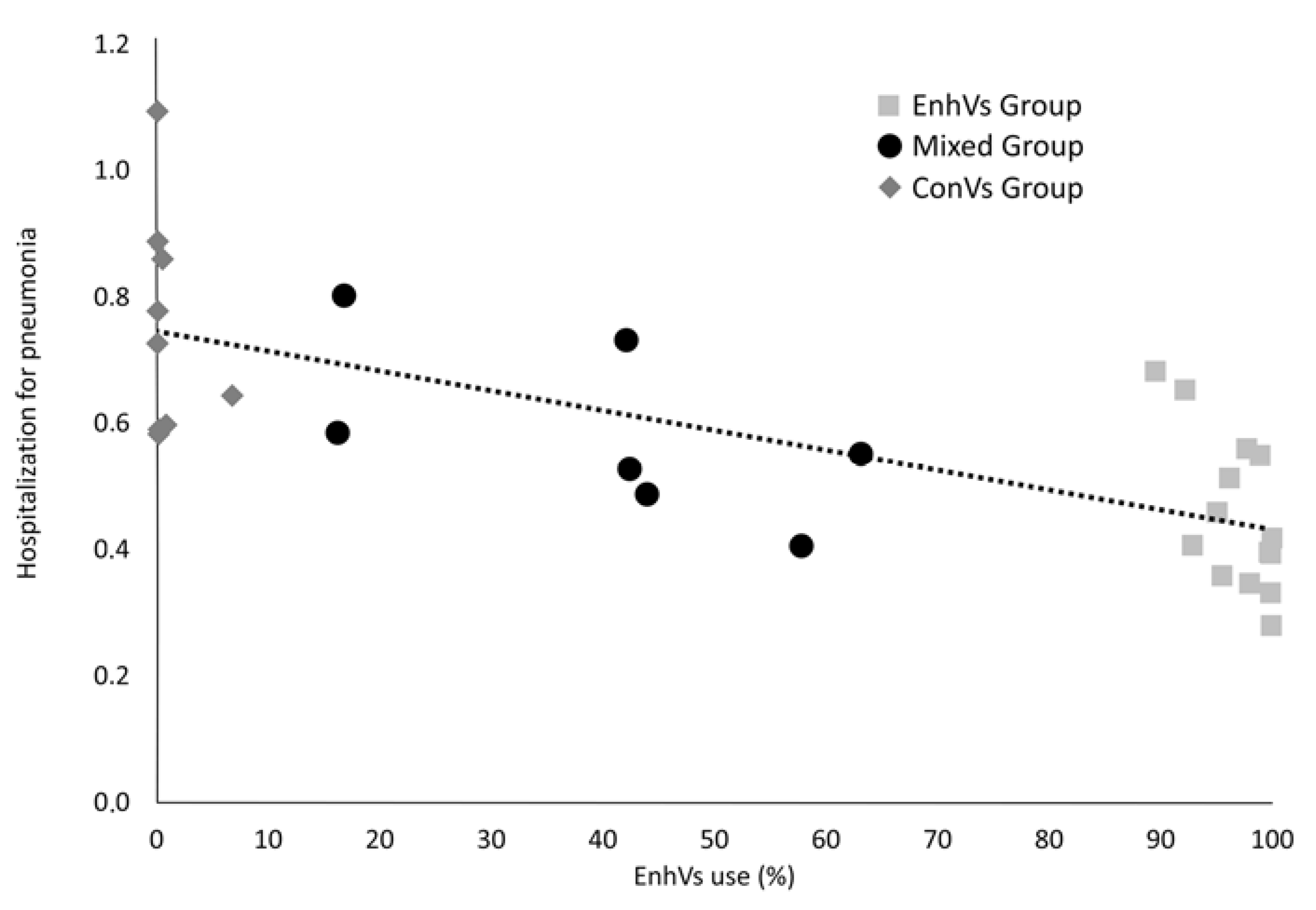
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. Influenza (Seasonal) 2009. Available online: https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal) (accessed on 12 May 2020).
- Influenza. Nature Reviews Disease Primers. December 2018. Available online: http://www.nature.com/articles/s41572-018-0002-y (accessed on 26 June 2020).
- Burden of Influenza and Impact of Seasonal Vaccination in Europe-A Ten Year Forecast. Available online: http://erj.ersjournals.com/lookup/doi/10.1183/13993003.congress-2018.PA4503. (accessed on 26 June 2020).
- Jackson, M.L.; Phillips, C.H.; Benoit, J.; Jackson, L.A.; Gaglani, M.; Murthy, K.; McLean, H.Q.; Belongia, E.A.; Malosh, R.; Zimmerman, R.; et al. Burden of medicaly attended influenza infection and cases averted by vaccination-United States, 2013/14 through 2015/16 influenza seasons. Vaccine 2018, 36, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Gasparini, R.; Bonanni, P.; Amicizia, D.; Bella, A.; Donatelli, I.; Cristina, M.L.; Panatto, D.; Lai, P.L. Influenza epidemiology in Italy two years after the 2009-2010 pandemic: Need to improve vaccination coverage. Hum. Vaccines Immunother. 2013, 9, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Campigotto, A.; Mubareka, S. Influenza-associated bacterial pneumonia; managing and controlling infection on two fronts. Expert Rev. Antiinfect. Ther. 2015, 13, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Metersky, M.L.; Masterton, R.G.; Lode, H.; File, T.M.; Babinchak, T. Epidemiology, microbiology, and treatment considerations for bacterial pneumonia complicating influenza. Int. J. Infect. Dis. 2012, 16, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Baldo, V.; Cocchio, S.; Gallo, T.; Furlan, P.; Clagnan, E.; Del Zotto, S.; Saia, M.; Bertoncello, C.; Buja, A.; Baldovin, T. Impact of pneumococcal conjugate vaccination: A retrospective study of hospitalization for pneumonia in North-East Italy. J. Prev. Med. Hyg. 2016, 57, E61–E68. [Google Scholar]
- Baldo, V.; Cocchio, S.; Baldovin, T.; Buja, A.; Furlan, P.; Bertoncello, C.; Russo, F.; Saia, M. A population-based study on the impact of hospitalization for pneumonia in different age groups. BMC Infect. Dis. 2014, 14, 485. [Google Scholar] [CrossRef][Green Version]
- Iuliano, A.D.; Roguski, K.M.; Chang, H.H.; Muscatello, D.J.; Palekar, R.; Tempia, S.; Cohen, C.; Gran, J.M.; Schanzer, D.; Cowling, B.J.; et al. Estimates of global seasonal influenza-associated respiratory mortality: A modelling study. Lancet 2018, 391, 1285–1300. [Google Scholar] [CrossRef]
- Vaccines against influenza WHO position paper-November 2012. Wkly. Epidemiol. Rec. 2012, 87, 461–476.
- European Commission (EC). State of Play on Implementation of the Council Recommendation of 22 December 2009 on Seasonal Influenza Vaccination (2009/1019/EU). Available online: http://ec.europa.eu/health/vaccination/docs/seasonflu_staffwd2014_en.pdf (accessed on 28 June 2020).
- Italian Ministry of Health (MOH. Prevenzione e Controllo dell’Influenza: Raccomandazioni per la Stagione 2018–2019 [Influenza Prevention and Control: Recommendations for the 2018–2019 Season]. Available online: http://www.salute.gov.it/portale/influenza/dettaglioContenutiInfluenza.jsp?lingua=italiano&id=686&area=influenza&menu=vuoto (accessed on 28 June 2020).
- Ribaudo, A.S.; Hayney, M.S. Influenza vaccine formulation and effectiveness. J. Am. Pharm. Assoc. 2017, 57, 637–639. [Google Scholar] [CrossRef]
- Host Factors Impact Vaccine Efficacy: Implications for Seasonal and Universal Influenza Vaccine Programs. Available online: https://jvi.asm.org/content/93/21/e00797-19 (accessed on 26 June 2020).
- Demicheli, V.; Jefferson, T.; Di Pietrantonj, C.; Ferroni, E.; Thorning, S.; Thomas, R.E.; Rivetti, A. Vaccines for preventing influenza in the elderly. Cochrane Database Syst. Rev. 2018, 2, CD004876. [Google Scholar] [CrossRef]
- Fabiani, M.; Volpe, E.; Faraone, M.; Bella, A.; Pezzotti, P.; Chini, F. Effectiveness of influenza vaccine in reducing influenza-associated hospitalizations and deaths among the elderly population; Lazio region, Italy, season 2016–2017. Expert Rev. Vaccines 2020, 19, 479–489. [Google Scholar] [CrossRef]
- Bordano, C.D. Aggiornamento Dell’offerta Vaccinale Per Adulti, Anziani e Soggetti a Rischio Nella Regione Friuli Venezia Giulia. In Regione Friuli Venezia Giulia, 2nd ed.; del Gemonese, C.M., del Ferro e Val Canale, C., Eds.; MAGGIO: Udine, Italy, 2018. [Google Scholar]
- Garante per la protezione dei dati personali Autorizzazione generale al trattamento dei dati personali effettuato per scopi di ricerca scientifica. Gazzetta Ufficiale della Repubb. Ital. 2012, 72, 47–52.
- Troeger, C.; Forouzanfar, M.; Rao, P.C.; Khalil, I.; Brown, A.; Swartz, S.; Fullman, N.; Mosser, J.; Thompson, R.L.; Reiner, R.C.; et al. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory tract infections in 195 countries: A systematic analysis for the Global Burden of Disease Study 2015. Lancet Infect. Dis. 2017, 17, 1133–1161. [Google Scholar] [CrossRef]
- Beyer, W.E.P.; Nauta, J.J.P.; Palache, A.M.; Giezeman, K.M.; Osterhaus, A.D.M.E. Immunogenicity and safety of inactivated influenza vaccines in primed populations: A systematic literature review and meta-analysis. Vaccine 2011, 29, 5785–5792. [Google Scholar] [CrossRef]
- Estimated Influenza Illnesses, Medical Visits, Hospitalizations, and Deaths Averted by Vaccination in the United States. Available online: https://www.cdc.gov/flu/about/disease/2016-17.htm (accessed on 24 June 2020).
- Global and Regional Burden of Hospital Admissions for Pneumonia in Older Adults: A Systematic Review and Meta-Analysis. J. Infect. Dis. Available online: https://academic.oup.com/jid/advance-article/doi/10.1093/infdis/jiz053/5372488 (accessed on 12 May 2020).
- Pneumonia prevention in the elderly patients: The other sides. Aging Clinical and Experimental Research. Available online: http://link.springer.com/10.1007/s40520-019-01437-7 (accessed on 12 May 2020).
- Ahrenfeldt, L.J.; Möller, S.; Thinggaard, M.; Christensen, K.; Lindahl-Jacobsen, R. Sex Differences in Comorbidity and Frailty in Europe. Int. J. Public Health 2019, 64, 1025–1036. [Google Scholar] [CrossRef] [PubMed]
- Ekström, M.P.; Jogréus, C.; Ström, K.E. Comorbidity and Sex-Related Differences in Mortality in Oxygen-Dependent Chronic Obstructive Pulmonary Disease. PLoS ONE 2012, 7, e35806. [Google Scholar] [CrossRef]
- The Lancet Respiratory Medicine Null. Elderly at risk as severe influenza season looms large. Lancet Respir. Med. 2018, 6, 1. [Google Scholar] [CrossRef]
- End-of-Season Influenza Vaccine Effectiveness in Adults and Children, United Kingdom, 2016/17. Available online: http://www.eurosurveillance.org/content/10.2807/1560-7917.ES.2017.22.44.17-00306 (accessed on 12 May 2020).
- Valent, F.; Gallo, T. Influenza vaccine effectiveness in an Italian elderly population during the 2016–2017 season. Annali dell’Istituto Superiore di Sanita 2018, 54, 67–71. [Google Scholar] [PubMed]
- Low 2016/17 Season Vaccine Effectiveness Against Hospitalised Influenza A(H3N2) Among Elderly: Awareness Warranted for 2017/18 Season. Available online: http://www.eurosurveillance.org/content/10.2807/1560-7917.ES.2017.22.41.17-00645 (accessed on 12 May 2020).
- DiazGranados, C.A.; Dunning, A.J.; Kimmel, M.; Kirby, D.; Treanor, J.; Collins, A.; Pollak, R.; Christoff, J.; Earl, J.; Landolfi, V.; et al. Efficacy of High-Dose versus Standard-Dose Influenza Vaccine in Older Adults. N. Engl. J. Med. 2014, 371, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Domnich, A.; Arata, L.; Amicizia, D.; Puig-Barberà, J.; Gasparini, R.; Panatto, D. Effectiveness of MF59-adjuvanted seasonal influenza vaccine in the elderly: A systematic review and meta-analysis. Vaccine 2017, 35, 513–520. [Google Scholar] [CrossRef]
- Van Buynder, P.G.; Konrad, S.; Van Buynder, J.L.; Brodkin, E.; Krajden, M.; Ramler, G.; Bigham, M. The comparative effectiveness of adjuvanted and unadjuvanted trivalent inactivated influenza vaccine (TIV) in the elderly. Vaccine 2013, 31, 6122–6128. [Google Scholar] [CrossRef]
- Gasparini, R.; Amicizia, D.; Lai, P.L.; Rossi, S.; Panatto, D. Effectiveness of adjuvanted seasonal influenza vaccines (Inflexal V® and Fluad® ) in preventing hospitalization for influenza and pneumonia in the elderly: A matched case-control study. Hum. Vaccines Immunother. 2013, 9, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Puig-Barberà, J.; Natividad-Sancho, A.; Calabuig-Pérez, J.; Lluch-Rodrigo, J.A.; Pastor-Villalba, E.; Martínez-Úbeda, S.; Díez-Domingo, J. Intradermal and virosomal influenza vaccines for preventing influenza hospitalization in the elderly during the 2011-2012 influenza season: A comparative effectiveness study using the Valencia health care information system. Vaccine 2014, 32, 5447–5454. [Google Scholar] [CrossRef] [PubMed]
- Lapi, F.; Marconi, E.; Simonetti, M.; Baldo, V.; Rossi, A.; Sessa, A.; Cricelli, C. Adjuvanted versus nonadjuvanted influenza vaccines and risk of hospitalizations for pneumonia and cerebro/cardiovascular events in the elderly. Expert Rev. Vaccines 2019, 18, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Baldo, V.; Baldovin, T.; Pellegrini, M.; Angiolelli, G.; Majori, S.; Floreani, A.; Busana, M.C.; Bertoncello, C.; Trivello, R. Immunogenicity of three different influenza vaccines against homologous and heterologous strains in nursing home elderly residents. Clin. Dev. Immunol. 2010, 2010, e517198. [Google Scholar] [CrossRef]
- Durando, P.; Icardi, G.; Ansaldi, F. MF59-adjuvanted vaccine: A safe and useful tool to enhance and broaden protection against seasonal influenza viruses in subjects at risk. Expert Opin. Biol. Ther. 2010, 10, 639–651. [Google Scholar] [CrossRef]
| Characteristics | Total | Vaccination Strategy | ||||||
|---|---|---|---|---|---|---|---|---|
| ConVs | MixVs | EnhVs | ||||||
| (n = 987,266) | (n = 383,286) | (n = 160,854) | (n = 443,126) | |||||
| Sex (n (%)) | ||||||||
| Male | 423,151 | (42.9) | 128,740 | (42.6) | 104,256 | (43.1) | 190,155 | (42.9) |
| Female | 564,115 | (57.1) | 173,325 | (57.4) | 137,819 | (56.9) | 252,971 | (57.1) |
| Age group (n (%)) | ||||||||
| 65–74 years old | 389,943 | (39.5) | 113,661 | (37.6) | 96,083 | (39.7) | 180,199 | (40.7) |
| 75–84 years old | 396,511 | (40.2) | 125,337 | (41.5) | 97,067 | (40.1) | 174,107 | (39.3) |
| 85+ years old | 200,812 | (20.3) | 63,067 | (20.9) | 48,925 | (20.2) | 88,820 | (20.0) |
| Days of follow-up (mean (SD)) | 118.2 | (15.3) | 116.7 | (16,1) | 118.8 | (14.6) | 119.0 | (15.0) |
| Prior pneumonia vaccination | 529,004 | (53.6) | 194,447 | (50.7) | 85,361 | (53.1) | 249,196 | (56.2) |
| Hospitalization for pneumonia | 5681 | (0.58) | 2251 | (0.75) | 1429 | (0.59) | 2001 | (0.45) |
| Variables | Hospitalization | OR (95% CI) | Adj OR (95% CI) | |
|---|---|---|---|---|
| Yes | No | |||
| Age (means ± SD) | 83.5 ± 7.8 | 77.9 ± 7.7 | 1.091 (1.087–1.094) | 1.099 (1.096–1.103) |
| Sex [n (%)] | ||||
| Female | 2785 (0.5) | 561,330 (99.5) | Ref. | Ref. |
| Male | 2896 (0.7) | 420,255 (99.3) | 1.389 (1.312–1.463) | 1.822 (1.727–1.922) |
| Vaccination group [n (%)] | ||||
| ConVs group | 2819 (0.7) | 380,467 (99.3) | Ref. | Ref. |
| MixVs group | 861 (0.5) | 159,993 (99.5) | 0.726 (0.672–0.784) | 0.813 (0.760–0.869) |
| EnhVs group | 2001 (0.5) | 441,125 (99.5 | 0.612 (0.578–0.648) | 0.622 (0.58–0.661) |
| H3N2 mismatch [n (%)] | ||||
| no | 3286 (0.5) | 662,288 (99.5) | Ref. | Ref. |
| yes | 2395 (0.7) | 319,297 (99.3) | 1.512 (1.434–1.594) | 1.471 (1.379–1.570) |
| B mismatch [n (%)] | ||||
| No | 1023 (0.7) | 150,964 (99.3) | Ref. | Ref. |
| Yes | 4658 (0.6) | 830,621(99.4) | 1.207 (1.128–1.291) | 1.041 (0.958–1.131) |
| Previous vaccination against influenza [n (%)] | ||||
| No | 733 (0.5) | 136,373 (99.5) | Ref | Ref. |
| Yes | 4948 (0.6) | 845,012 (99.4) | 0.917 (0.842–0.992) | 0.752 (0.695–0.814) |
| Pneumococcal vaccination (n (%)) | ||||
| No | 2517 (0.5) | 455,743 (99.5) | Ref. | Ref. |
| Yes | 3164 (0.6) | 525,807 (99.4) | 1.090 (1.034–1.148) | 1.032 (0.979–1.089) |
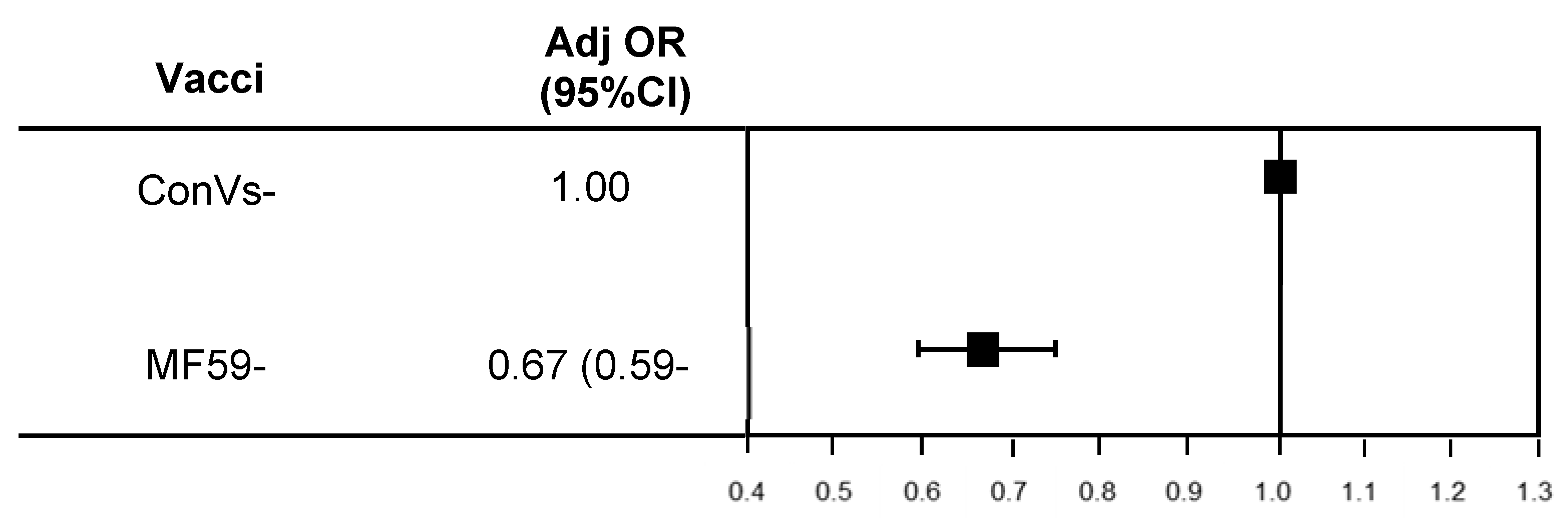
| Characteristics | Total | Type of Vaccination | ||||
|---|---|---|---|---|---|---|
| ConVs Group | MF59-TIV | |||||
| (n = 479,397) | (n = 410,737) | (n = 68,660) | ||||
| Sex (n (%)) | ||||||
| Male | 204,738 | (42.7) | 176,154 | (42.9) | 28,584 | (41.6) |
| Female | 274,659 | (57.3) | 234,583 | (57.1) | 40,076 | (58.4) |
| Age group (n (%)) | ||||||
| 65–74 years old | 162,486 | (33.9) | 139,914 | (34.1) | 22,572 | (32.9) |
| 75–84 years old | 202,083 | (42.2) | 174,475 | (42.5) | 27,608 | (40.2) |
| 85+ years old | 114,828 | (24.0) | 96,348 | (23.5) | 18,480 | (26.9) |
| Days of follow-up (mean (SD)) | 117.1 | (15.9) | 116.8 | (16.1) | 118.7 | (14.8) |
| Prior vaccination for pneumonia | 247,939 | (51.7) | 204,188 | (49.7) | 43,751 | (63.7) |
| Hospitalization for pneumonia | 3176 | (0.66) | 2849 | (0.69) | 327 | (0.48) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cocchio, S.; Gallo, T.; Del Zotto, S.; Clagnan, E.; Iob, A.; Furlan, P.; Fonzo, M.; Bertoncello, C.; Baldo, V. Preventing the Risk of Hospitalization for Respiratory Complications of Influenza among the Elderly: Is There a Better Influenza Vaccination Strategy? A Retrospective Population Study. Vaccines 2020, 8, 344. https://doi.org/10.3390/vaccines8030344
Cocchio S, Gallo T, Del Zotto S, Clagnan E, Iob A, Furlan P, Fonzo M, Bertoncello C, Baldo V. Preventing the Risk of Hospitalization for Respiratory Complications of Influenza among the Elderly: Is There a Better Influenza Vaccination Strategy? A Retrospective Population Study. Vaccines. 2020; 8(3):344. https://doi.org/10.3390/vaccines8030344
Chicago/Turabian StyleCocchio, Silvia, Tolinda Gallo, Stefania Del Zotto, Elena Clagnan, Andrea Iob, Patrizia Furlan, Marco Fonzo, Chiara Bertoncello, and Vincenzo Baldo. 2020. "Preventing the Risk of Hospitalization for Respiratory Complications of Influenza among the Elderly: Is There a Better Influenza Vaccination Strategy? A Retrospective Population Study" Vaccines 8, no. 3: 344. https://doi.org/10.3390/vaccines8030344
APA StyleCocchio, S., Gallo, T., Del Zotto, S., Clagnan, E., Iob, A., Furlan, P., Fonzo, M., Bertoncello, C., & Baldo, V. (2020). Preventing the Risk of Hospitalization for Respiratory Complications of Influenza among the Elderly: Is There a Better Influenza Vaccination Strategy? A Retrospective Population Study. Vaccines, 8(3), 344. https://doi.org/10.3390/vaccines8030344








