Sexual Dimorphism Has Low Impact on the Response against Rotavirus Infection in Suckling Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Design
2.3. Clinical Indexes and Fecal Specimen Collection
2.4. Fecal SA-11 Shedding
2.5. Sample Collection and Processing
2.6. Anti-RV Antibodies in Plasma
2.7. Immunoglobulins in Plasma
2.8. Intestinal Permeability
2.9. Gene Expression Analysis
2.10. Statistical Analysis
3. Results
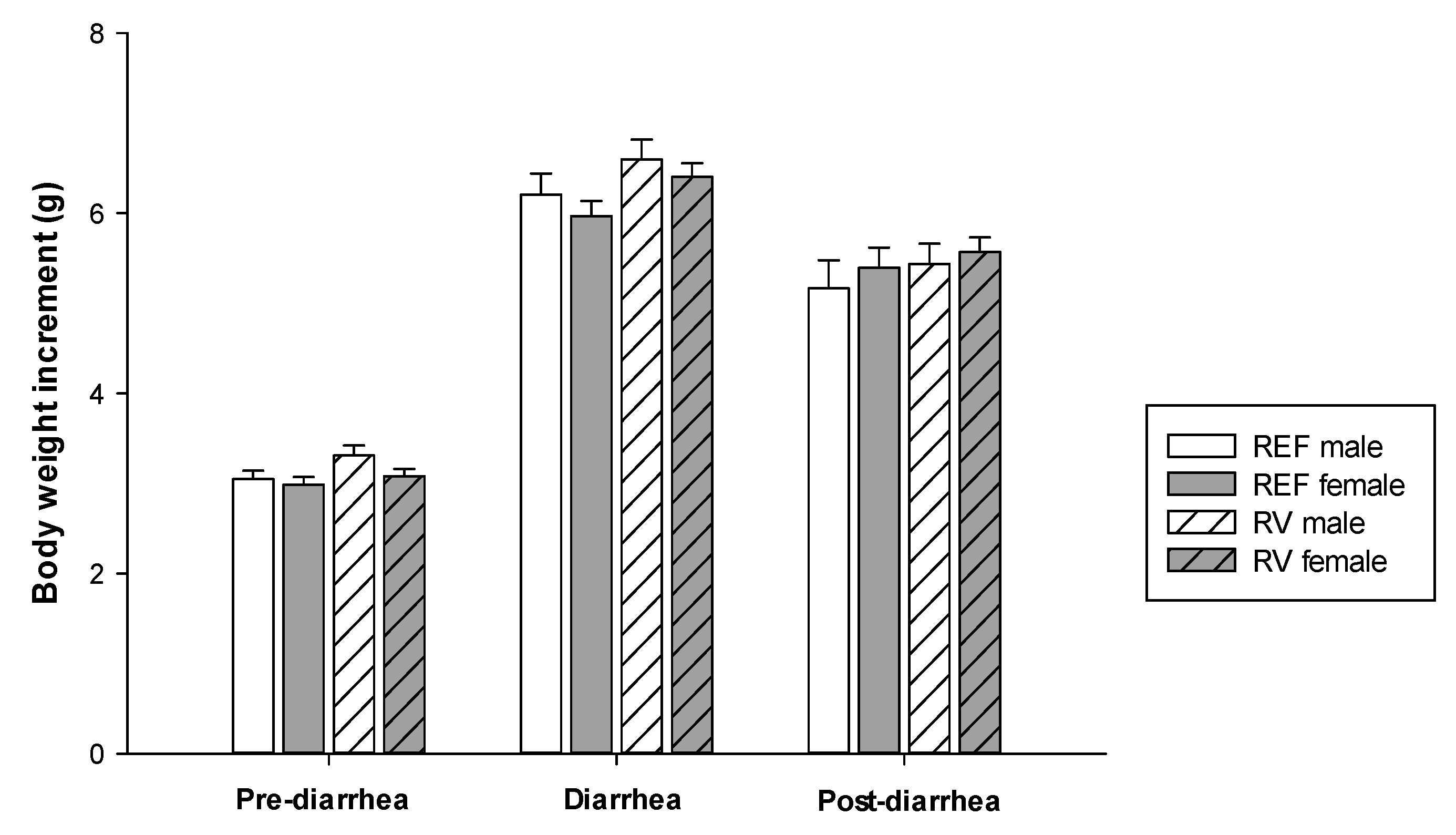
3.1. Body Weight
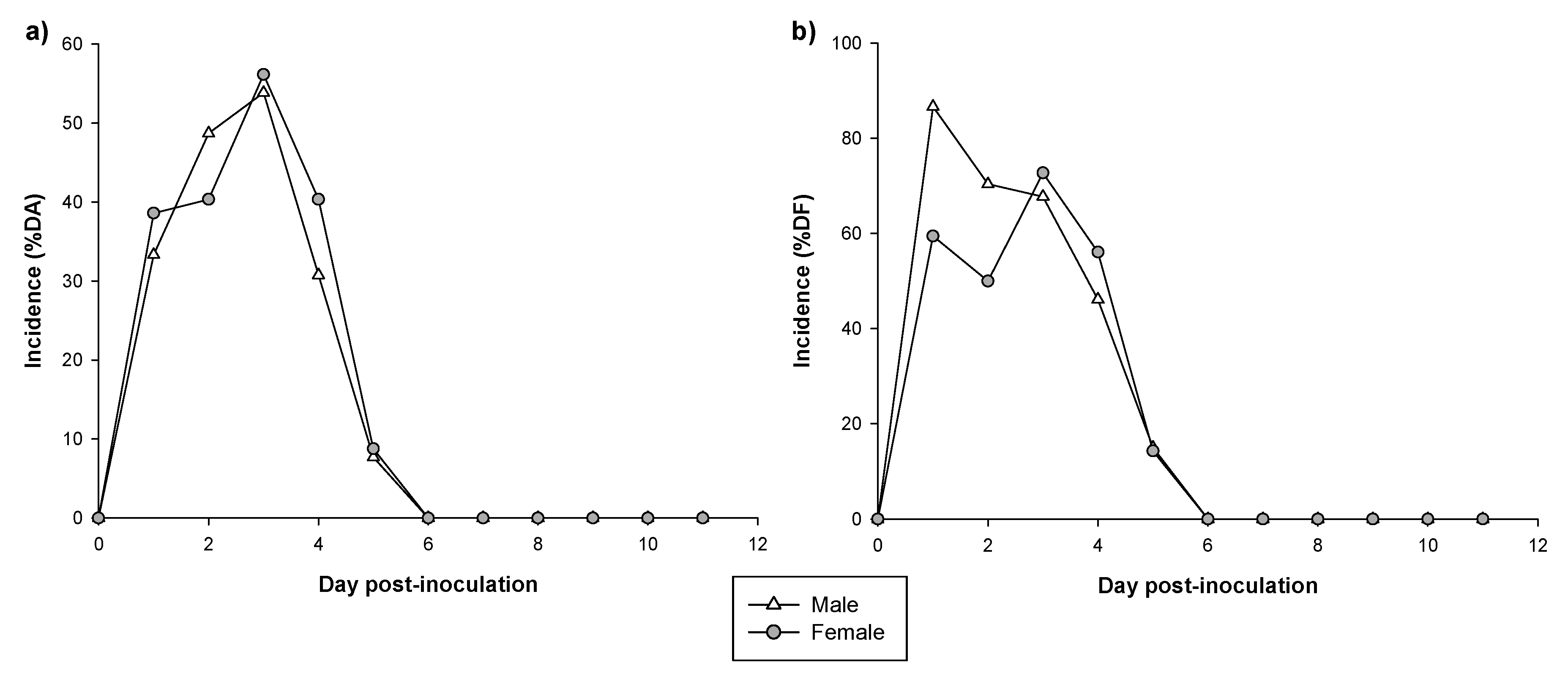
3.2. Incidence of Diarrhea
3.3. Severity of Diarrhea
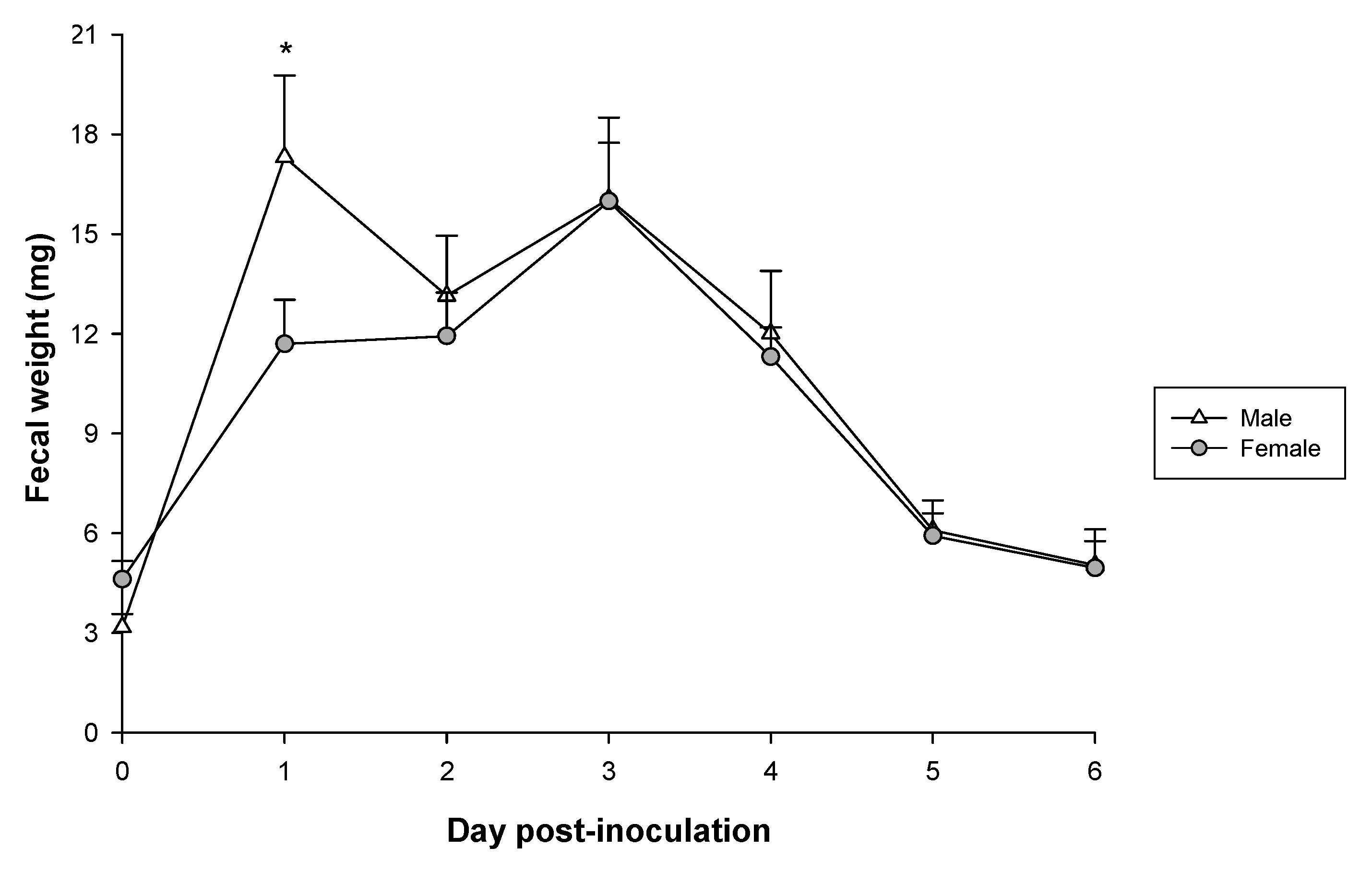
3.4. Fecal Weight
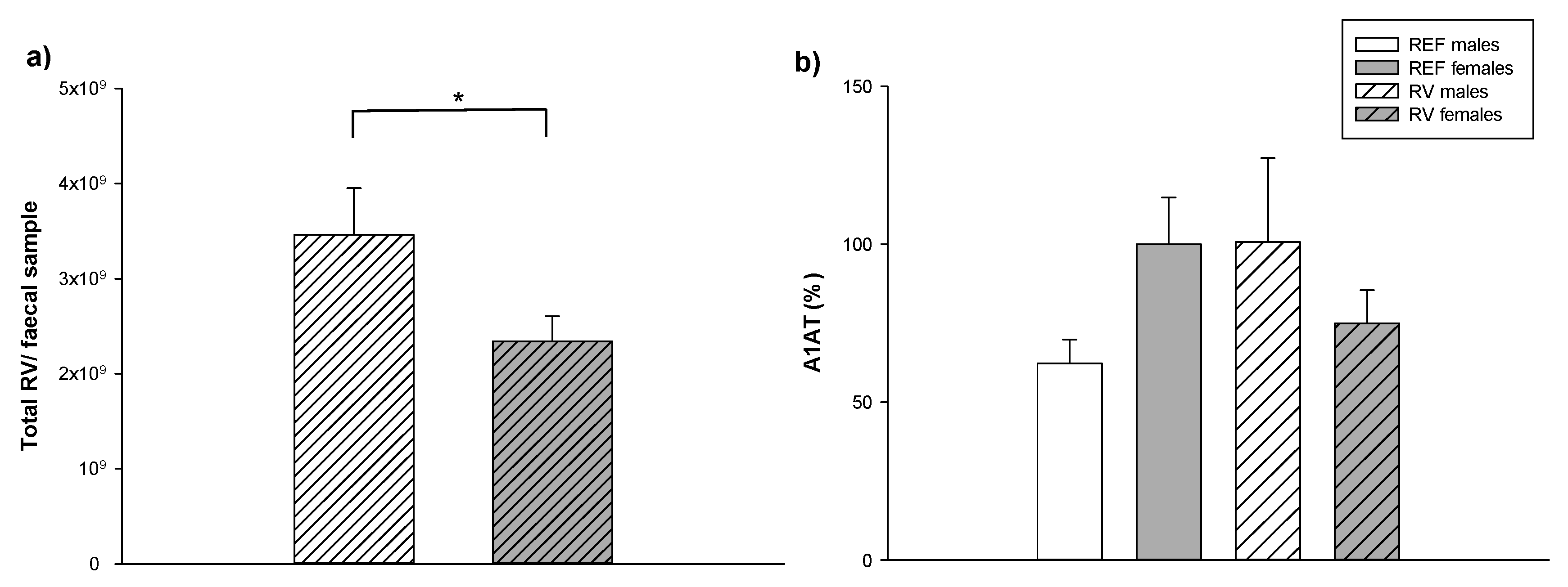
3.5. Fecal SA-11 Shedding and Intestinal Barrier Function
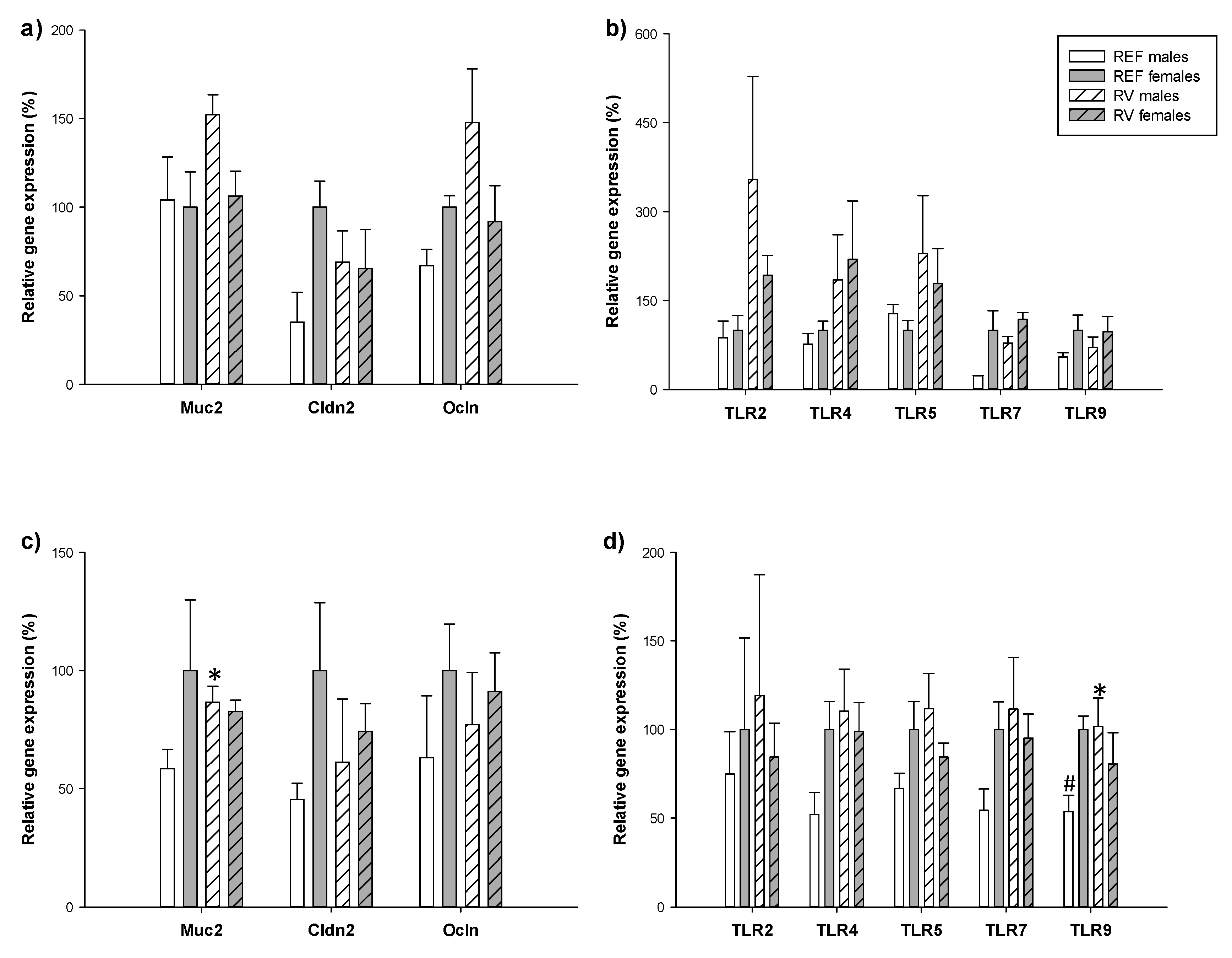
3.6. Intestinal Gene Expression
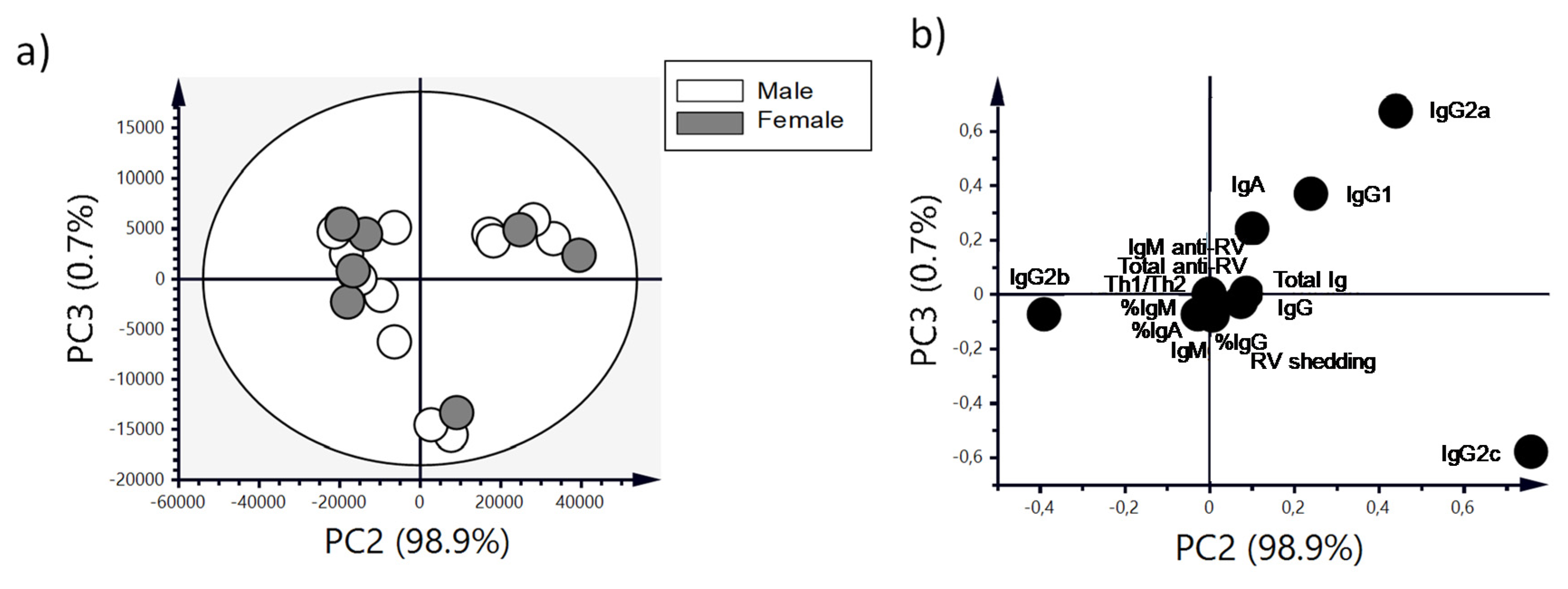
3.7. Antibody Production
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jaillon, S.; Berthenet, K.; Garlanda, C. Sexual dimorphism in innate immunity. Clin. Rev. Allergy Immunol. 2019, 56, 308–321. [Google Scholar] [CrossRef]
- Giefing-Kröll, C.; Berger, P.; Lepperdinger, G.; Grubeck-Loebenstein, B. How sex and age affect immune responses, susceptibility to infections, and response to vaccination. Aging Cell 2015, 14, 309–321. [Google Scholar] [CrossRef] [PubMed]
- McCombe, P.; Greer, J.; Mackay, I. Sexual dimorphism in autoimmune disease. Curr. Mol. Med. 2009, 9, 1058–1079. [Google Scholar] [CrossRef] [PubMed]
- Markle, J.G.; Fish, E.N. SeXX matters in immunity. Trends Immunol. 2014, 35, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.; Jung, N.; Robinson, N.; Lehmann, C. Sex differences in immune responses to infectious diseases. Infection 2015, 43, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Ruggierii, A.; Anticoli, S.; D’ambrosio, A.; Giordani, L.; Mora, M. The influence of sex and gender on immunity, infection and vaccination. Ann. Ist. Super. Sanita 2016, 52, 198–204. [Google Scholar]
- Grebely, J.; Page, K.; Sacks-davis, R.; van der Loeff, M.S.; Rice, T.M.; Bruneau, J.; Morris, M.D.; Hajarizadeh, B.; Amin, J.; Cox, A.L.; et al. The effects of female sex, viral genotype and IL28B genotype on spontaneous clearance of acute hepatitis C virus infection. Hepatology 2014, 59, 109–120. [Google Scholar] [CrossRef]
- Klein, S.L.; Flanagan, K.L. Sex Differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef]
- Posma, E.; Moes, H.; Heineman, M.J.; Faas, M.M. The effect of testosterone on cytokine production in the specific and non-specific immune response. Am. J. Reprod. Immunol. 2004, 52, 237–243. [Google Scholar] [CrossRef]
- Elderman, M.; Hugenholtz, F.; Belzer, C.; Boekschoten, M.; van Beek, A.; de Haan, B.; Savelkoul, H.; de Vos, P.; Faas, M. Sex and strain dependent differences in mucosal immunology and microbiota composition in mice. Biol. Sex Differ. 2018, 9, 26. [Google Scholar] [CrossRef]
- Yurkovetskiy, L.; Burrows, M.; Khan, A.A.; Graham, L.; Volchkov, P.; Becker, L.; Antonopoulos, D.; Umesaki, Y.; Chervonsky, A.V. Gender bias in autoimmunity is influenced by microbiota. Immunity 2013, 39, 400–412. [Google Scholar] [CrossRef]
- Haro, C.; Rangel-Zúñiga, O.A.; Alcalá-Díaz, J.F.; Gómez-Delgado, F.; Pérez-Martínez, P.; Delgado-Lista, J.; Quintana-Navarro, G.M.; Landa, B.B.; Navas-Cortés, J.A.; Tena-Sempere, M.; et al. Intestinal microbiota is influenced by gender and body mass index. PLoS ONE 2016, 11, e0154090. [Google Scholar] [CrossRef]
- Greenberg, H.B.; Estes, M.K. Rotaviruses: From pathogenesis to vaccination. Gastroenterology 2009, 136, 1939–1951. [Google Scholar] [CrossRef]
- Lundgren, O.; Svensson, L. Pathogenesis of rotavirus diarrhea. Microbes Infect. 2001, 3, 1145–1156. [Google Scholar] [CrossRef]
- Crawford, S.E.; Ramani, S.; Tate, J.E.; Parashar, U.D.; Svensson, L.; Hagbom, M.; Franco, M.A.; Greenberg, H.B.; O’Ryan, M.; Kang, G.; et al. Rotavirus infection. Nat. Rev. Dis. Prim. 2017, 3. [Google Scholar] [CrossRef]
- Sadiq, A.; Bostan, N.; Yinda, K.C.; Naseem, S.; Sattar, S. Rotavirus: Genetics, pathogenesis and vaccine advances. Rev. Med. Virol. 2018, 28, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Odimayo, M.S.; Olanrewaju, W.I.; Omilabu, S.A.; Adegboro, B. Prevalence of rotavirus-induced diarrhoea among children under 5 years in Ilorin, Nigeria. J. Trop. Pediatr. 2008, 54, 343–346. [Google Scholar] [CrossRef]
- Carneiro, N.B.; Diniz-Santos, D.R.; Fagundes, S.Q.; Neves, L.L.; Reges, R.M.B.; Lima, E.K.P.; De Oliva Quadros, V.H.; De Jesus Soares, L.E.; Silva, F.S.R.; De Gouvêa Schneiter, H.; et al. Clinical and epidemiological aspects of children hospitalized with severe rotavirus-associated gastroenteritis in Salvador, BA, Brazil. Braz. J. Infect. Dis. 2005, 9, 525–528. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kirkwood, C.D.; Ma, L.F.; Carey, M.E.; Steele, A.D. The rotavirus vaccine development pipeline. Vaccine 2019, 37, 7328–7335. [Google Scholar] [CrossRef] [PubMed]
- Rigo-Adrover, M.D.M.; Knipping, K.; Garssen, J.; Saldaña-Ruíz, S.; Franch, À.; Castell, M.; Pérez-Cano, F.J. Rotavirus double infection model to study preventive dietary interventions. Nutrients 2019, 11, 131. [Google Scholar] [CrossRef]
- Azagra-Boronat, I.; Massot-Cladera, M.; Knipping, K.; Van’t Land, B.; Stahl, B.; Garssen, J.; Rodríguez-Lagunas, M.J.; Franch, À.; Castell, M.; Pérez-Cano, F.J. Supplementation with 2’-FL and ScGOS/LcFOS ameliorates rotavirus-induced diarrhea in suckling rats. Front. Cell. Infect. Microbiol. 2018, 8, 372. [Google Scholar] [CrossRef]
- Ciarlet, M.; Conner, M.E.; Finegold, M.J.; Estes, M.K. Group a rotavirus infection and age-dependent diarrheal disease in rats: A new animal model to study the pathophysiology of rotavirus infection. J. Virol. 2002, 76, 41–57. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Cano, F.J.; Castell, M.; Castellote, C.; Franch, À. Characterization of clinical and immune response in a rotavirus diarrhea model in suckling Lewis rats. Pediatr. Res. 2007, 62, 658–663. [Google Scholar] [CrossRef] [PubMed]
- Ventola, H.; Lehtoranta, L.; Madetoja, M.; Simonen-Tikka, M.L.; Maunula, L.; Roivainen, M.; Korpela, R.; Holma, R. Effects of the viability of lactobacillus Rhamnosus GG on rotavirus infection in neonatal rats. World J. Gastroenterol. 2012, 18, 5925–5931. [Google Scholar] [CrossRef]
- Azagra-Boronat, I.; Massot-Cladera, M.; Knipping, K.; Garssen, J.; Ben Amor, K.; Knol, J.; Franch, À.; Castell, M.; Rodríguez-Lagunas, M.J.; Pérez-Cano, F.J. Strain-specific probiotic properties of bifidobacteria and lactobacilli for the prevention of diarrhea caused by rotavirus in a preclinical model. Nutrients 2020, 12, 498. [Google Scholar] [CrossRef] [PubMed]
- Rigo-Adrover, M.; Saldaña-Ruíz, S.; van Limpt, K.; Knipping, K.; Garssen, J.; Knol, J.; Franch, A.; Castell, M.; Pérez-Cano, F.J. A combination of ScGOS/LcFOS with bifidobacterium breve M-16V protects suckling rats from rotavirus gastroenteritis. Eur. J. Nutr. 2017, 56, 1657–1670. [Google Scholar] [CrossRef]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C. AIN-93 Purified diets for laboratory rodents: Final report of the american institute of nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef]
- Azagra-Boronat, I.; Rodríguez-Lagunas, M.J.; Castell, M.; Pérez-Cano, F.J. Prebiotics for Gastrointestinal Infections and Acute Diarrhea. In Dietary Interventions in Gastrointestinal Diseases; Watson, R., Preedy, V., Eds.; Academic Press: London, UK, 2019; pp. 179–191. [Google Scholar]
- Jacobi, S.K.; Moeser, A.J.; Blikslager, A.T.; Rhoads, J.M.; Corl, B.A.; Harrell, R.J.; Odle, J. Acute effects of rotavirus and malnutrition on intestinal barrier function in neonatal piglets. World J. Gastroenterol. 2013, 19, 5094–5102. [Google Scholar] [CrossRef]
- Iturriza-Gómara, M.; Dallman, T.; Bányai, K.; Böttiger, B.; Buesa, J.; Diedrich, S.; Fiore, L.; Johansen, K.; Korsun, N.; Kroneman, A.; et al. Rotavirus surveillance in Europe, 2005–2008: Web-enabled reporting and real-time analysis of genotyping and epidemiological data. J. Infect. Dis. 2009, 200, S215–S221. [Google Scholar] [CrossRef]
- Staat, M.A.; Azimi, P.H.; Berke, T.; Roberts, N.; Bernstein, D.I.; Ward, R.L.; Pickering, L.K.; Matson, D.O. Clinical presentations of rotavirus infection among hospitalized children. Pediatr. Infect. Dis. J. 2002, 21, 221–227. [Google Scholar] [CrossRef]
- Sabbaj, D.L.; DePetre, E.E.; Gómez, J.A.; Sordo, M.E. Artículo original rotavirus En La diarrea aguda. Arch. Argent. Pediatr. 2001, 99, 486–490. [Google Scholar]
- Hussein, A.M.; Hassan, M.K. Rotavirus infection among hospitalized children with acute watery diarrhea in Basrah-Iraq, Bahrain Med. Bull 2000, 22, 170–173. [Google Scholar]
- Rytlewska, M.; Bako, W.; Ratajczak, B.; Marek, A.; Gwizdek, A.; Czarnecka-Rudnik, D.; Swiatkowska, H.; Tyl, J.; Korzon, M. Epidemiological and clinical characteristics of rotaviral diarrhoea in children from Gdansk, Gdynia and Sopot. Med. Sci. Monit. 2000, 6, 117–122. [Google Scholar]
- Junaid, S.A.; Umeh, C.; Olabode, A.O.; Banda, J.M. Incidence of rotavirus infection in children with gastroenteritis attending Jos University Teaching Hospital, Nigeria. Virol. J. 2011, 8. [Google Scholar] [CrossRef]
- Hasso, S.A.; Pandey, R. Possible sex differences in the susceptibility of calves to rotavirus infection. Can. J. Vet. Res. 1986, 50, 287–288. [Google Scholar] [PubMed]
- Muendo, C.; Laving, A.; Kumar, R.; Osano, B.; Egondi, T.; Njuguna, P. Prevalence of rotavirus infection among children with acute diarrhoea after rotavirus vaccine introduction in Kenya, a Hospital Cross-Sectional Study. BMC Pediatr. 2018, 18. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Kabir, A.R.M.L.; Rahman, A.; Hussain, M.; Khatoon, S.; Hannan, A. Severity of rotavirus diarrhea in children: One year experience in a Children Hospital of Bangladesh. Iran. J. Pediatr. 2009, 19, 108–116. [Google Scholar]
- Klein, S.L. The effects of hormones on sex differences in infection: From genes to behavior. Neurosci. Biobehav. Rev. 2000, 24, 627–638. [Google Scholar] [CrossRef]
- Azagra-Boronat, I.; Massot-Cladera, M.; Mayneris-Perxachs, J.; Knipping, K.; Van’t Land, B.; Tims, S.; Stahl, B.; Garssen, J.; Franch, À.; Castell, M.; et al. Immunomodulatory and prebiotic effects of 2′-fucosyllactose in suckling rats. Front. Immunol. 2019, 10, 1773. [Google Scholar] [CrossRef]
- Azagra-Boronat, I.; Tres, A.; Massot-Cladera, M.; Franch, À.; Castell, M.; Guardiola, F.; Pérez-Cano, F.J.; Rodríguez-Lagunas, M.J. Associations of breast milk microbiota, immune factors, and fatty acids in the rat mother–offspring pair. Nutrients 2020, 12, 319. [Google Scholar] [CrossRef]
- Christoforidou, Z.; Mora Ortiz, M.; Poveda, C.; Abbas, M.; Walton, G.; Bailey, M.; Lewis, M.C. Sexual dimorphism in immune development and in response to nutritional intervention in neonatal piglets. Front. Immunol. 2019, 10, 1–17. [Google Scholar] [CrossRef]
- Simon, A.K.; Hollander, G.A.; McMichael, A. Evolution of the immune system in humans from infancy to old age. Proc. R. Soc. B Biol. Sci. 2015, 282. [Google Scholar] [CrossRef] [PubMed]
- Hewagama, A.; Patel, D.; Yarlagadda, S.; Strickland, F.; Richardson, B. Stronger Inflammatory/Cytotoxic T cell response in women identified by microarray analysis. Genes Immun. 2009, 10, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Oertelt-Prigione, S. The influence of sex and gender on the immune response. Autoimmun. Rev. 2012, 11. [Google Scholar] [CrossRef]
- Azagra-Boronat, I.; Massot-Cladera, M.; Knipping, K.; van‘t Land, B.; Tims, S.; Stahl, B.; Knol, J.; Garssen, J.; Franch, À.; Castell, M.; et al. Oligosaccharides modulate rotavirus-associated dysbiosis and TLR gene expression in neonatal rats. Cells 2019, 8, 876. [Google Scholar] [CrossRef] [PubMed]
- Bouman, A.; Jan Heineman, M.; Faas, M.M. Sex hormones and the immune response in humans. Hum. Reprod. Update 2005, 11, 411–423. [Google Scholar] [CrossRef]
- Cross, T.W.L.; Kasahara, K.; Rey, F.E. Sexual dimorphism of cardiometabolic dysfunction: Gut microbiome in the play? Mol. Metab. 2018, 15, 70–81. [Google Scholar] [CrossRef]
- Cui, M.; Xiao, H.; Li, Y.; Zhang, S.; Dong, J.; Wang, B.; Zhu, C.; Jiang, M.; Zhu, T.; He, J.; et al. Sexual dimorphism of gut microbiota dictates therapeutics efficacy of radiation injuries. Adv. Sci. 2019, 6. [Google Scholar] [CrossRef]
- Elderman, M.; de Vos, P.; Faas, M. Role of microbiota in sexually dimorphic immunity. Front. Immunol. 2018, 9, 1–6. [Google Scholar] [CrossRef]
- Dominianni, C.; Sinha, R.; Goedert, J.J.; Pei, Z.; Yang, L.; Hayes, R.B.; Ahn, J. Sex, Body mass index, and dietary fiber intake influence the human gut microbiome. PLoS ONE 2015, 10, e0124599. [Google Scholar] [CrossRef]
- Martin, R.; Makino, H.; Yavuz, A.C.; Ben-Amor, K.; Roelofs, M.; Ishikawa, E.; Kubota, H.; Swinkels, S.; Sakai, T.; Oishi, K.; et al. Early-life events, including mode of delivery and type of feeding, siblings and gender, shape the developing gut microbiota. PLoS ONE 2016, 11, e0158498. [Google Scholar] [CrossRef] [PubMed]
- Jašarević, E.; Morrison, K.E.; Bale, T.L. Sex differences in the gut microbiome—Brain axis across the lifespan. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Clarke, E.; Desselberger, U. Correlates of protection against human rotavirus disease and the factors influencing protection in low-income settings. Mucosal Immunol. 2015, 8, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Bines, J.E.; Kirkwood, C.D. Conquering rotavirus: From discovery to global vaccine implementation. J. Paediatr. Child Health 2015, 51, 34–39. [Google Scholar] [CrossRef]
- Mrozek-Budzyn, D.; Kieltyka, A.; Majewska, R.; Augustyniak, M. The effectiveness of rotavirus vaccine in preventing acute gastroenteritis during rotavirus seasons among Polish children. Arch. Med. Sci. 2016, 12, 614–620. [Google Scholar] [CrossRef]



| Clinical Outcome | Variable | Males (n = 39) | Females (n = 57) |
|---|---|---|---|
| Incidence | MDA | 53.85% | 56.14% |
| MDAd | 3 DPI | 3 DPI | |
| daAUC | 174.36 | 184.21 | |
| MDF | 86.67% | 72.73% | |
| MDFd | 1 DPI | 3 DPI * | |
| dfAUC | 285.93 | 252.57 | |
| Duration | DDB | 1.78 ± 0.16 DPI | 2.05 ± 0.17 DPI |
| DDE | 3.37 ± 0.21 DPI | 3.45 ± 0.16 DPI | |
| DP | 1.48 ± 0.24 DPI | 1.28 ± 0.19 DPI | |
| DwD | 2.03 ± 0.21 DPI | 2.00 ± 0.17 DPI | |
| Severity | MDI | 2.49 ± 0.09 * | 2.28 ± 0.06 |
| MDId | 2.26 ± 0.20 * DPI | 2.76 ± 0.14 DPI | |
| sAUC | 4.53 ± 0.35 * | 3.76 ± 0.21 |
| Immunoglobulins | REF Males | REF Females | RV Males | RV Females |
|---|---|---|---|---|
| IgA | 18.5 ± 1.82 | 38.0 ± 9.5 | 47.7 ± 1.74 | 46.0 ± 11.3 |
| IgM | 23.6 ± 2.73 | 22.3 ± 1.43 | 21.9 ± 1.72 | 20.4 ± 0.75 |
| IgG | 3070 ± 520 | 3400 ± 191 | 3820 ± 895 | 3610 ± 498 |
| IgG1 | 279 ± 30.1 | 239 ± 28.4 | 159 ± 21.0 * | 150 ± 11.4 * |
| IgG2a | 549 ± 67.6 | 648 ± 60.6 | 512 ± 65.9 | 492 ± 45.4 * |
| IgG2b | 1180 ± 207 | 1280 ± 99.2 | 2090 ± 813 | 1930 ± 45.2 |
| IgG2c | 1070 ± 252 | 1230 ± 1114 | 1060 ± 126 | 1040 ± 67.7 |
| Th1/Th2 # | 2.61 ± 0.29 | 2.88 ± 0.20 | 4.55 ± 0.93 * | 4.56 ± 0.52 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morales-Ferré, C.; Azagra-Boronat, I.; Massot-Cladera, M.; Franch, À.; Castell, M.; Rodríguez-Lagunas, M.J.; Pérez-Cano, F.J. Sexual Dimorphism Has Low Impact on the Response against Rotavirus Infection in Suckling Rats. Vaccines 2020, 8, 345. https://doi.org/10.3390/vaccines8030345
Morales-Ferré C, Azagra-Boronat I, Massot-Cladera M, Franch À, Castell M, Rodríguez-Lagunas MJ, Pérez-Cano FJ. Sexual Dimorphism Has Low Impact on the Response against Rotavirus Infection in Suckling Rats. Vaccines. 2020; 8(3):345. https://doi.org/10.3390/vaccines8030345
Chicago/Turabian StyleMorales-Ferré, Carla, Ignasi Azagra-Boronat, Malén Massot-Cladera, Àngels Franch, Margarida Castell, María José Rodríguez-Lagunas, and Francisco J. Pérez-Cano. 2020. "Sexual Dimorphism Has Low Impact on the Response against Rotavirus Infection in Suckling Rats" Vaccines 8, no. 3: 345. https://doi.org/10.3390/vaccines8030345
APA StyleMorales-Ferré, C., Azagra-Boronat, I., Massot-Cladera, M., Franch, À., Castell, M., Rodríguez-Lagunas, M. J., & Pérez-Cano, F. J. (2020). Sexual Dimorphism Has Low Impact on the Response against Rotavirus Infection in Suckling Rats. Vaccines, 8(3), 345. https://doi.org/10.3390/vaccines8030345









