Liver Stiffness Hinders Normalization of Systemic Inflammation and Endothelial Activation after Hepatitis C Virus (HCV) Eradication in HIV/HCV Coinfected Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants and Sample Collection
2.2. Liver Stiffness Assessment
2.3. Measurement of Plasma Markers of Inflammation, Pro-Coagulation and Endothelial Activation
2.4. Statistical Analysis
3. Results
3.1. Characteristics of Patients Included in the Study
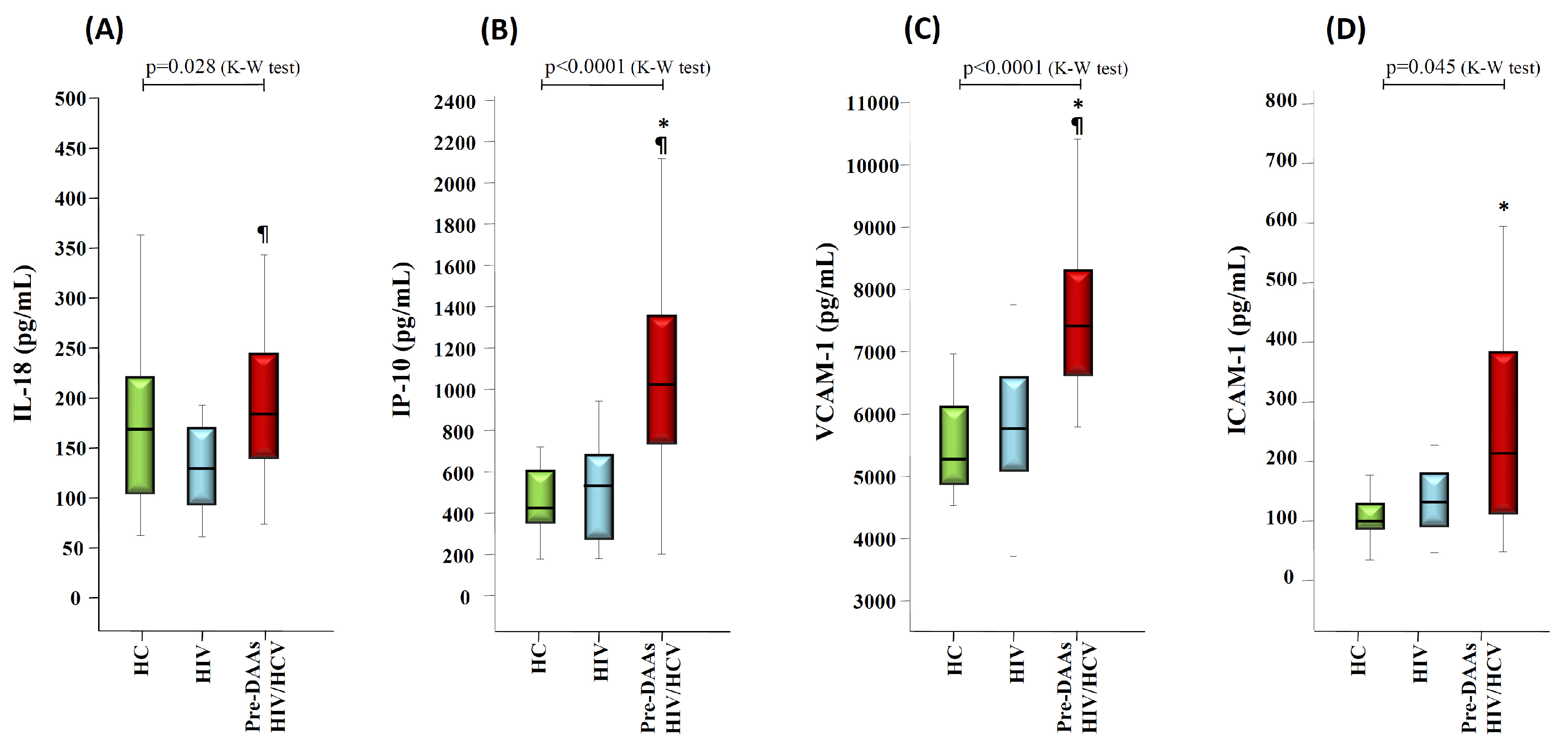
3.2. HCV Coinfection and Liver Stiffness Significantly Impacts on Markers of Inflammation and Endothelial Activation
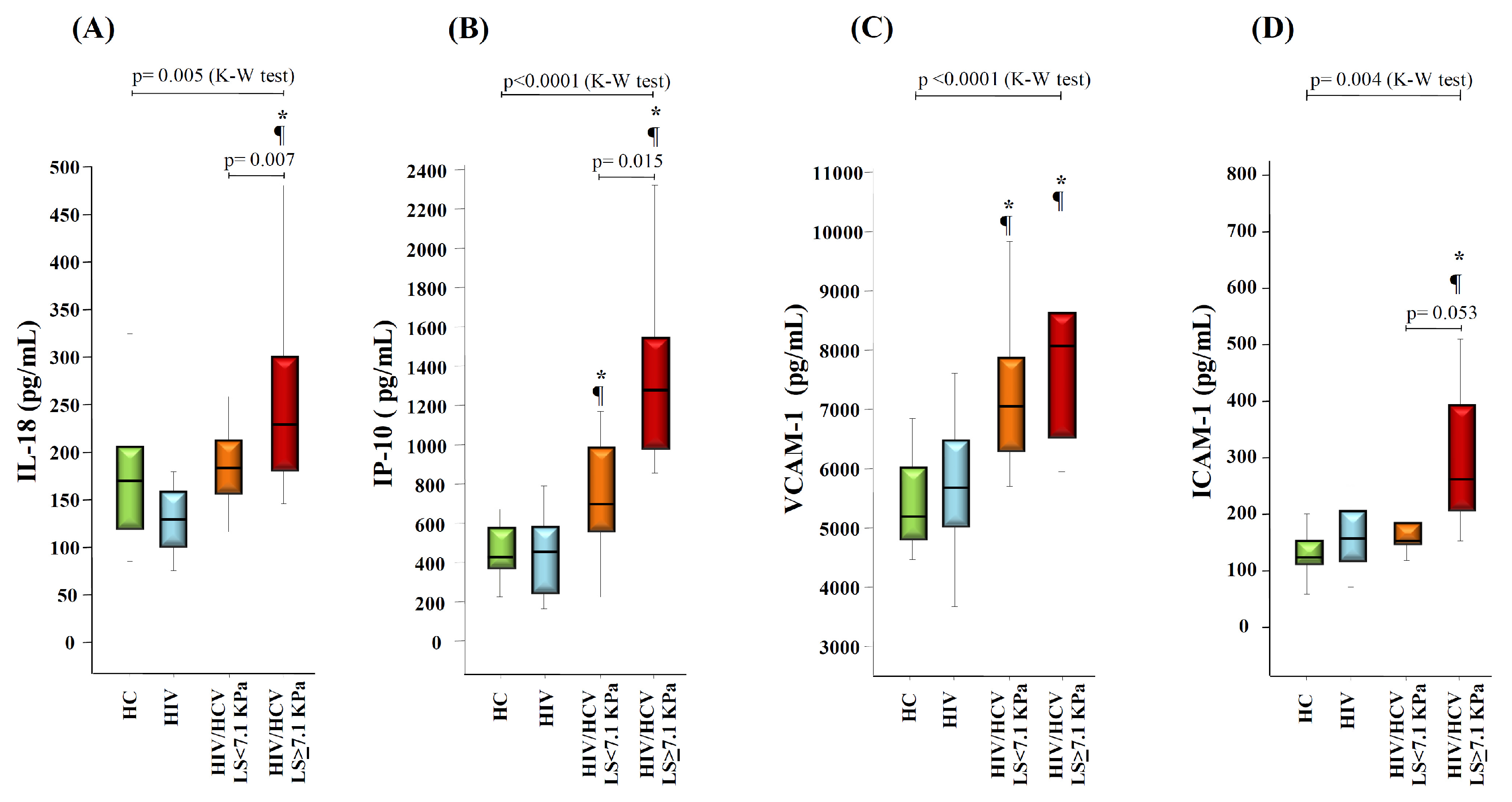
3.3. Liver Stiffness Correlates with Markers of Liver Disease Severity
3.4. Correlations between Baseline Levels of IL-18, IP-10, VCAM-1 and ICAM-1 with Markers of Liver Damage and with Virological Parameters
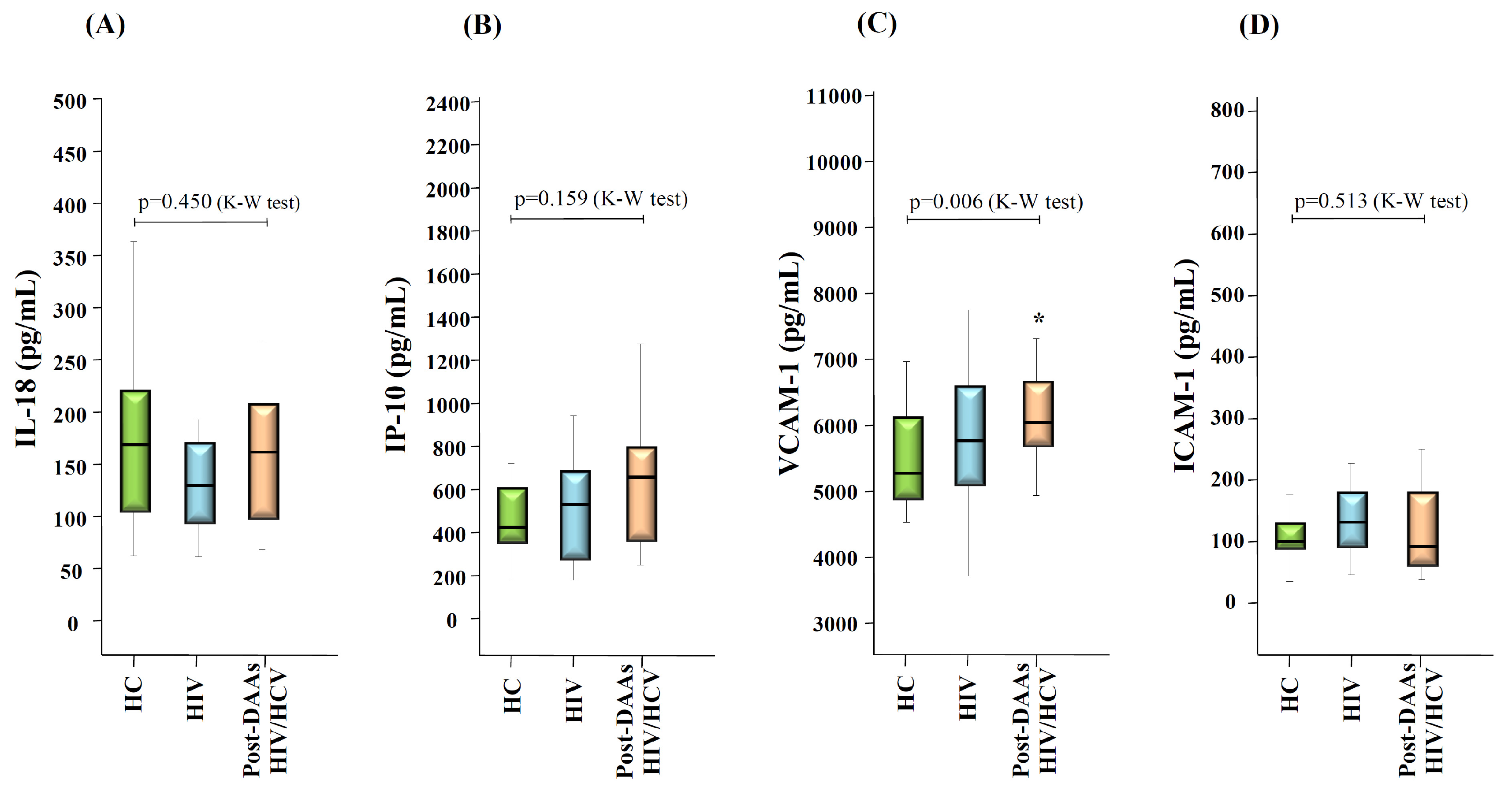
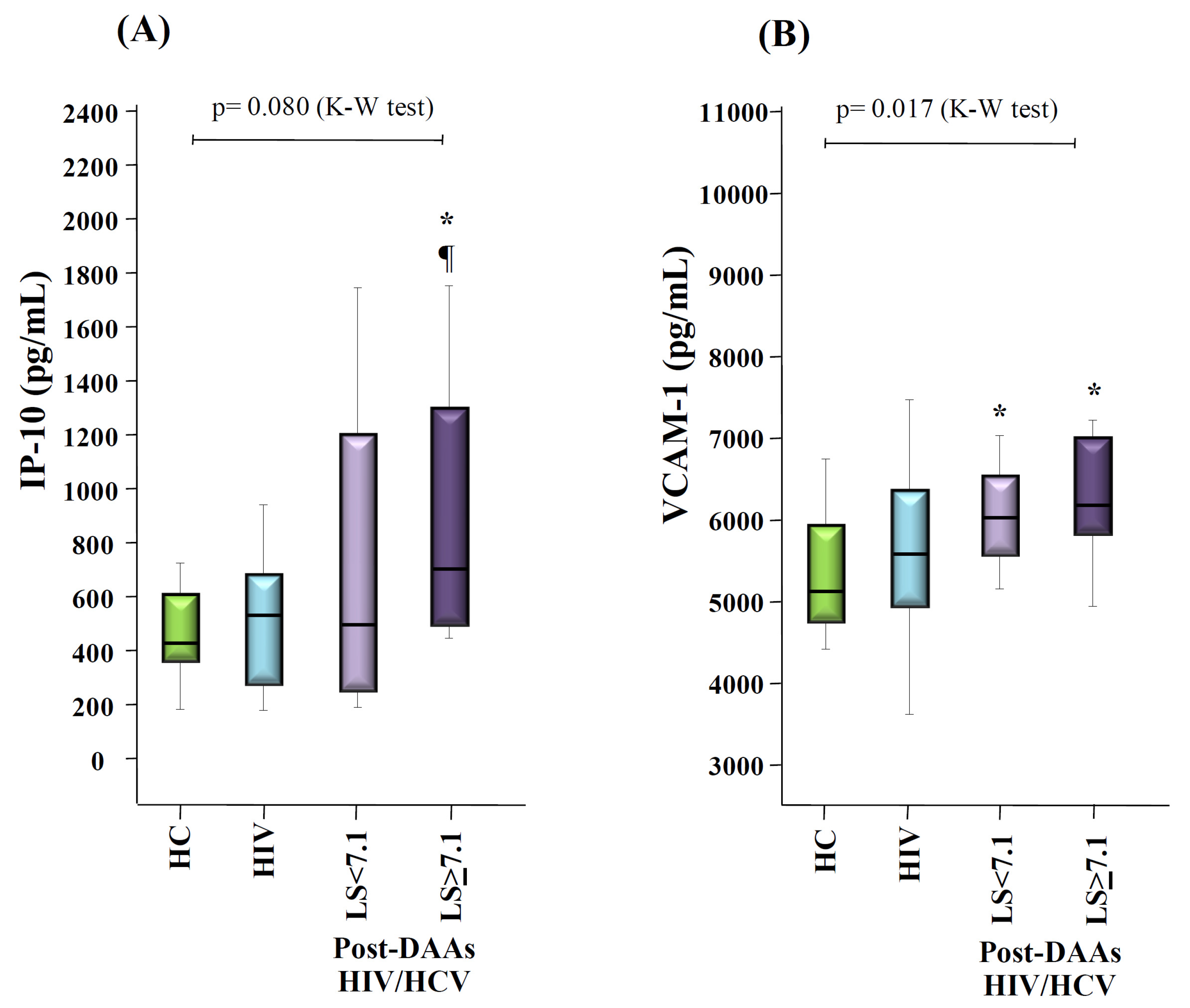
3.5. Changes in Levels of Inflammation and Endothelial Activation Makers after HCV Eradication with DAAs Therapy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sabin, C.A. Do people with HIV infection have a normal life expectancy in the era of combination antiretroviral therapy? BMC Med. 2013, 11, 251. [Google Scholar] [CrossRef] [PubMed]
- Hileman, C.O.; Funderburg, N.T. Inflammation, Immune Activation, and Antiretroviral Therapy in HIV. Curr HIV/AIDS Rep. 2017, 14, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Zicari, S.; Sessa, L.; Cotugno, N.; Ruggiero, A.; Morrocchi, E.; Concato, C.; Rocca, S.; Zangari, P.; Manno, E.C.; Palma, P. Immune Activation, Inflammation, and Non-AIDS Co-Morbidities in HIV-Infected Patients under Long-Term ART. Viruses 2019, 112, 200. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, K. Current status of HIV/AIDS in the ART era. J. Infect. Chemother. 2017, 23, 12–16. [Google Scholar] [CrossRef]
- Peterson, T.E.; Baker, J.V. Assessing inflammation and its role in comorbidities among persons living with HIV. Curr. Opin. Infect. Dis. 2019, 32, 8–15. [Google Scholar] [CrossRef]
- Platt, L.; Easterbrook, P.; Gower, E.; McDonald, B.; Sabin, K.; McGowan, C.; Yanny, I.; Razavi, H.; Vickerman, P. Prevalence and burden of HCV co-infection in people living with HIV: A global systematic review and meta-analysis. Lancet Infect. Dis. 2016, 16, 797–808. [Google Scholar] [CrossRef]
- Thein, H.H.; Yi, Q.; Dore, G.J.; Krahn, M.D. Natural history of hepatitis C virus infection in HIV-infected individuals and the impact of HIV in the era of highly active antiretroviral therapy: A meta-analysis. AIDS 2008, 22, 1979–1991. [Google Scholar] [CrossRef]
- van der Helm, J.; Geskus, R.; Sabin, C.; Meyer, L.; Del Amo, J.; Chêne, G.; Dorrucci, M.; Muga, R.; Porter, K.; Prins, M. CASCADE Collaboration in EuroCoord. Effect of HCV infection on cause-specific mortality after HIV seroconversion, before and after 1997. Gastroenterology 2013, 144, 751–760. [Google Scholar] [CrossRef]
- Feuth, T.; Arends, J.E.; Fransen, J.H.; Nanlohy, N.M.; van Erpecum, K.J.; Siersema, P.D.; Hoepelman, A.I.; van Baarle, D. Complementary role of HCV and HIV in T-cell activation and exhaustion in HIV/HCV coinfection. PLoS ONE 2013, 8, e59302. [Google Scholar] [CrossRef]
- Rallón, N.; García, M.; García-Samaniego, J.; Rodríguez, N.; Cabello, A.; Restrepo, C.; Álvarez, B.; García, R.; Górgolas, M.; Benito, J.M. HCV coinfection contributes to HIV pathogenesis by increasing immune exhaustion in CD8 T-cells. PLoS ONE 2017, 12, e0173943. [Google Scholar] [CrossRef]
- Zampino, R.; Marrone, A.; Restivo, L.; Guerrera, B.; Sellitto, A.; Rinaldi, L.; Romano, C.; Adinolfi, L.E. Chronic HCV infection and inflammation: Clinical impact on hepatic and extra-hepatic manifestations. World J. Hepatol. 2013, 5, 528–540. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Fulgencio, M.; Berenguer, J.; de Castro, I.F.; Micheloud, D.; López, J.C.; Cosín, J.; Miralles, P.; Lorente, R.; Aldamiz-Echevarría, T.; Muñoz-Fernández, M.Á.; et al. Sustained virological response to interferon-α plus ribavirin decreases inflammation and endothelial dysfunction markers in HIV/HCV co-infected patients. J. Antimicrob. Chemother. 2011, 66, 645–649. [Google Scholar] [CrossRef] [PubMed]
- Shmagel, K.V.; Saidakova, E.V.; Shmagel, N.G.; Korolevskaya, L.B.; Chereshnev, V.A.; Robinson, J.; Grivel, J.C.; Douek, D.C.; Margolis, L.; Anthony, D.D.; et al. Systemic inflammation and liver damage in HIV/hepatitis C virus coinfection. HIV Med. 2016, 17, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Veenhuis, R.T.; Astemborski, J.; Chattergoon, M.A.; Greenwood, P.; Jarosinski, M.; Moore, R.D.; Mehta, S.H.; Cox, A.L. Systemic Elevation of Proinflammatory Interleukin 18 in HIV/HCV Coinfection versus HIV or HCV Monoinfection. Clin. Infect. Dis. 2017, 64, 589–596. [Google Scholar] [CrossRef]
- Mascia, C.; Lichtner, M.; Zuccalà, P.; Vita, S.; Tieghi, T.; Marocco, R.; Savinelli, S.; Rossi, R.; Iannetta, M.; Campagna, M.; et al. Active HCV infection is associated with increased circulating levels of interferon-gamma (IFN-γ)-inducible protein-10 (IP-10), soluble CD163 and inflammatory monocytes regardless of liver fibrosis and HIV coinfection. Clin. Res. Hepatol. Gastroenterol. 2017, 41, 644–655. [Google Scholar] [CrossRef]
- Medrano, L.M.; Garcia-Broncano, P.; Berenguer, J.; González-García, J.; Jiménez-Sousa, M.Á.; Guardiola, J.M.; Crespo, M.; Quereda, C.; Sanz, J.; Canorea, I.; et al. GESIDA 3603b Study Group Elevated liver stiffness is linked to increased biomarkers of inflammation and immune activation in HIV/hepatitis C virus-coinfected patients. AIDS 2018, 32, 1095–1105. [Google Scholar] [CrossRef]
- Parisi, S.G.; Andreis, S.; Mengoli, C.; Menegotto, N.; Cavinato, S.; Scaggiante, R.; Andreoni, M.; Palù, G.; Basso, M.; Cattelan, A.M. Soluble CD163 and soluble CD14 plasma levels but not cellular HIV-DNA decrease during successful interferon-free anti-HCV therapy in HIV-1-HCV co-infected patients on effective combined anti-HIV treatment. Med. Microbiol. Immunol. 2018, 207, 183–194. [Google Scholar] [CrossRef]
- López-Cortés, L.F.; Trujillo-Rodríguez, M.; Báez-Palomo, A.; Benmarzouk-Hidalgo, O.J.; Dominguez-Molina, B.; Milanés-Guisado, Y.; Espinosa, N.; Viciana, P.; Gutiérrez-Valencia, A. Eradication of Hepatitis C Virus (HCV) Reduces Immune Activation, Microbial Translocation, and the HIV DNA Level in HIV/HCV-Coinfected Patients. J. Infect. Dis. 2018, 218, 624–632. [Google Scholar] [CrossRef]
- Thomas, H.; Foster, G.; Platis, D. Mechanisms of action of interferon and nucleoside analogues [published correction appears in J Hepatol. 2004 Feb;40(2):364). J. Hepatol. 2003, 39, S93–S98. [Google Scholar] [CrossRef]
- Sikavi, C.; Chen, P.H.; Lee, A.D.; Saab, E.G.; Choi, G.; Saab, S. Hepatitis C and human immunodeficiency virus coinfection in the era of direct-acting antiviral agents: No longer a difficult-to-treat population. Hepatology 2018, 67, 847–857. [Google Scholar] [CrossRef]
- Castéra, L.; Vergniol, J.; Foucher, J.; Le Bail, B.; Chanteloup, E.; Haaser, M.; Darriet, M.; Couzigou, P.; De Lédinghen, V. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology 2005, 128, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Roe, B.; Coughlan, S.; Hassan, J.; Grogan, A.; Farrell, G.; Norris, S.; Bergin, C.; Hall, W.W. Elevated serum levels of interferon-gamma-inducible protein-10 in patients coinfected with hepatitis C virus and HIV. J. Infect. Dis. 2007, 196, 1053–1057. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Griesbeck, M.; Valantin, M.A.; Lacombe, K.; Samri-Hassimi, A.; Bottero, J.; Blanc, C.; Sbihi, Z.; Zoorob, R.; Katlama, C.; Guiguet, M.; et al. HepACT-VIH study group. Hepatitis C virus drives increased type I interferon-associated impairments associated with fibrosis severity in antiretroviral treatment-treated HIV-1-hepatitis C virus-coinfected individuals. AIDS 2017, 31, 1223–1234. [Google Scholar] [CrossRef] [PubMed]
- de Larrañaga, G.F.; Wingeyer, S.D.; Puga, L.M.; Alonso, B.S.; Benetucci, J.A. Relationship between hepatitis C virus (HCV) and insulin resistance, endothelial perturbation, and platelet activation in HIV-HCV-coinfected patients under highly active antiretroviral treatment. Eur. J. Clin. Microbiol. Infect. Dis. 2006, 25, 98–103. [Google Scholar] [CrossRef] [PubMed]
- de Castro, I.F.; Micheloud, D.; Berenguer, J.; Guzmán-Fulgencio, M.; Catalán, P.; Miralles, P.; Alvarez, E.; López, J.C.; Cosín, J.; Lorente, R.; et al. Hepatitis C virus infection is associated with endothelial dysfunction in HIV/hepatitis C virus coinfected patients. AIDS 2010, 24, 2059–2067. [Google Scholar] [CrossRef]
- Sharma, A.; Chakraborti, A.; Das, A.; Dhiman, R.K.; Chawla, Y. Elevation of interleukin-18 in chronic hepatitis C: Implications for hepatitis C virus pathogenesis. Immunology 2009, 128, e514–e522. [Google Scholar] [CrossRef] [PubMed]
- Said, E.M.; Soliman, M.S.; Shousha, H.I.; Rashed, M.S.; Elazm, A.A.; Aamer, R.Z.; Kamel, M.H.; Abdelsalam, F.M. Interleukin-18 and its gene single nucleotide polymorphisms (SNPs) influence chronic hepatitis C progression. J. Infect. Dev. Ctries. 2018, 12, 257–264. [Google Scholar] [CrossRef]
- Gracie, J.A.; Robertson, S.E.; McInnes, I.B. Interleukin-18. J. Leukoc. Biol. 2003, 73, 213–224. [Google Scholar] [CrossRef]
- Shrivastava, S.; Mukherjee, A.; Ray, R.; Ray, R.B. Hepatitis C virus induces interleukin-1β (IL-1β)/IL-18 in circulatory and resident liver macrophages. J. Virol. 2013, 87, 12284–12290. [Google Scholar] [CrossRef]
- Harvey, C.E.; Post, J.J.; Palladinetti, P.; Freeman, A.J.; Ffrench, R.A.; Kumar, R.K.; Marinos, G.; Lloyd, A.R. Expression of the chemokine IP-10 (CXCL10) by hepatocytes in chronic hepatitis C virus infection correlates with histological severity and lobular inflammation. J. Leukoc. Biol. 2003, 74, 360–369. [Google Scholar] [CrossRef]
- Pirozyan, M.R.; Nguyen, N.; Cameron, B.; Luciani, F.; Bull, R.A.; Zekry, A.; Lloyd, A.R. Chemokine-Regulated Recruitment of Antigen-Specific T-Cell Subpopulations to the Liver in Acute and Chronic Hepatitis C Infection. J. Infect. Dis. 2019, 219, 1430–1438. [Google Scholar] [CrossRef]
- Zeremski, M.; Petrovic, L.M.; Chiriboga, L.; Brown, Q.B.; Yee, H.T.; Kinkhabwala, M.; Jacobson, I.M.; Dimova, R.; Markatou, M.; Talal, A.H. Intrahepatic levels of CXCR3-associated chemokines correlate with liver inflammation and fibrosis in chronic hepatitis C. Hepatology 2008, 48, 1440–1450. [Google Scholar] [CrossRef]
- Romero, A.I.; Lagging, M.; Westin, J.; Dhillon, A.P.; Dustin, L.B.; Pawlotsky, J.M.; Neumann, A.U.; Ferrari, C.; Missale, G.; DITTO-HCV Study Group; et al. Interferon (IFN)-gamma-inducible protein-10: Association with histological results, viral kinetics, and outcome during treatment with pegylated IFN-alpha 2a and ribavirin for chronic hepatitis C virus infection. J. Infect. Dis. 2006, 194, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Koenig, G.; Seneff, S. Gamma-Glutamyltransferase: A Predictive Biomarker of Cellular Antioxidant Inadequacy and Disease Risk. Dis. Markers. 2015, 2015, 818570. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.K. Linking endothelial dysfunction with endothelial cell activation. J. Clin. Investig. 2013, 123, 540–541. [Google Scholar] [CrossRef] [PubMed]
- Pircher, J.; Czermak, T.; Merkle, M.; Mannell, H.; Krötz, F.; Ribeiro, A.; Vielhauer, V.; Nadjiri, J.; Gaitzsch, E.; Niemeyer, M.; et al. Hepatitis C virus induced endothelial inflammatory response depends on the functional expression of TNFα receptor subtype 2. PLoS ONE 2014, 9, e113351. [Google Scholar] [CrossRef]
- Constans, J.; Conri, C. Circulating markers of endothelial function in cardiovascular disease. Clin. Chim. Acta 2006, 368, 33–47. [Google Scholar] [CrossRef]
- Vos, A.G.; Idris, N.S.; Barth, R.E.; Klipstein-Grobusch, K.; Grobbee, D.E. Pro-Inflammatory Markers in Relation to Cardiovascular Disease in HIV Infection. A Systematic Review. PLoS ONE 2016, 11, e0147484. [Google Scholar] [CrossRef]
- Bedimo, R.; Westfall, A.O.; Mugavero, M.; Drechsler, H.; Khanna, N.; Saag, M. Hepatitis C virus coinfection and the risk of cardiovascular disease among HIV-infected patients. HIV Med. 2010, 11, 462–468. [Google Scholar] [CrossRef]
- Tapper, E.B.; Cohen, A.B.; Patel, K.; Bacon, B.; Gordon, S.; Lawitz, E.; Nelson, D.; Nasser, I.A.; Challies, T.; Afdhal, N. Levels of Alanine Aminotransferase Confound Use of Transient Elastography to Diagnose Fibrosis in Patients with Chronic HCV Infection. Clin. Gastroenterol. Hepatol. 2012, 10, 932–937. [Google Scholar] [CrossRef]
- Fabbri, G.; Mastrorosa, I.; Vergori, A.; Timelli, L.; Lorenzini, P.; Zaccarelli, M.; Cicalini, S.; Bellagamba, R.; Plazzi, M.M.; Mazzotta, V.; et al. Liver stiffness reduction and serum fibrosis score improvement in HIV/hepatitis C virus-coinfected patients treated with direct-acting antivirals. HIV Med. 2018, 19, 578–584. [Google Scholar] [CrossRef] [PubMed]
- El-Garem, H.; AbdAllah, M.; Omar, H.; Cordie, A.; Abdel Alem, S.; Mohey Eldin Elzahry, M.A.; Ghaith, D.; Abou El-Soud, N.H.; Kamal, W.; Elsharkawy, A.; et al. DAAs therapy associated with improved hepatic fibrosis in HCV-GT4 patients co-infected with HIV. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Potter, M.; Odueyungbo, A.; Yang, H.; Saeed, S.; Klein, M.B. Canadian Co-infection Cohort Study Investigators. Impact of hepatitis C viral replication on CD4+ T-lymphocyte progression in HIV-HCV coinfection before and after antiretroviral therapy. AIDS 2010, 24, 1857–1865. [Google Scholar] [CrossRef] [PubMed]
- Mascia, C.; Vita, S.; Zuccalà, P.; Marocco, R.; Tieghi, T.; Savinelli, S.; Rossi, R.; Iannetta, M.; Pozzetto, I.; Furlan, C.; et al. Changes in inflammatory biomarkers in HCV-infected patients undergoing direct acting antiviral-containing regimens with or without interferon. PLoS ONE 2017, 12, e0179400. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, F.P.; Zimmermann, T.; Wenz, T.; Schnorbus, B.; Ostad, M.A.; Feist, C.; Grambihler, A.; Schattenberg, J.M.; Sprinzl, M.F.; Münzel, T.; et al. Interferon- and ribavirin-free therapy with new direct acting antivirals (DAA) for chronic hepatitis C improves vascular endothelial function. Int. J. Cardiol. 2018, 271, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Ning, G.; Li, Y.T.; Chen, Y.M.; Zhang, Y.; Zeng, Y.F.; Lin, C.S. Dynamic Changes of the Frequency of Classic and Inflammatory Monocytes Subsets and Natural Killer Cells in Chronic Hepatitis C Patients Treated by Direct-Acting Antiviral Agents. Can. J. Gastroenterol. Hepatol. 2017, 2017, 3612403. [Google Scholar] [CrossRef] [PubMed]
- Spaan, M.; van Oord, G.; Kreefft, K.; Hou, J.; Hansen, B.E.; Janssen, H.L.; de Knegt, R.J.; Boonstra, A. Immunological Analysis During Interferon-Free Therapy for Chronic Hepatitis C Virus Infection Reveals Modulation of the Natural Killer Cell Compartment. J. Infect. Dis. 2016, 213, 216–223. [Google Scholar] [CrossRef]
- Aldámiz-Echevarría, T.; Berenguer, J.; Miralles, P.; Jiménez-Sousa, M.A.; Carrero, A.; Pineda-Tenor, D.; Díez, C.; Tejerina, F.; Pérez-Latorre, L.; Bellón, J.M.; et al. Soluble Adhesion Molecules in Patients Coinfected with HIV and HCV: A Predictor of Outcome. PLoS ONE 2016, 11, e0148537. [Google Scholar] [CrossRef]
| Characteristic | HIV Group (n = 25) | HIV/HCV Group (n = 25) | p-Value |
|---|---|---|---|
| Age (years) | 48 (42–55) | 44 (39–48) | 0.11 |
| Gender (% of males) | 88% | 100% | 0.24 |
| Time since HIV diagnosis (years) | 9 (6–14) | 7 (2–10) | 0.07 |
| Time on cART (years) | 5 (3.5–7.5) | 4 (2–9) | 0.63 |
| Time since HCV diagnosis (years) | NA | 2 (1.5–5) | NA |
| CD4 count (cells/μL) | 816 (605–992) | 735 (577–902) | 0.31 |
| CD4/CD8 ratio | 0.84 (0.56–1.34) | 0.75 (0.60–1.09) | 0.49 |
| ALT level (IU/L) | 32 (24–37) | 74 (49–162) | <0.0001 |
| AST level (IU/L) | 27 (22–31) | 58 (37–122) | <0.0001 |
| GGT level (IU/L) | 36 (22–54) | 55 (25–119) | 0.06 |
| Total cholesterol level (mg/dL) | 194 (162–227) | 157 (125–177) | <0.0001 |
| HDL level (mg/dL) | 42 (37–50) | 46 (33–50) | 0.815 |
| LDL level (mg/dL) | 119 (93–146) | 85 (71–104) | 0.001 |
| Triglycerides level (mg/dL) | 138 (88–208) | 95 (70–140) | 0.050 |
| Body Mass Index (BMI) | 25.1 (22.7–26.8) | 24.1 (23–24.2) | 0.835 |
| HIV transmission route (%) | 1 | ||
| Sexual | 100% | 96% | |
| Parenteral | 0% | 4% | |
| HCV-RNA (log copies/mL) | NA | 6.1 (5.8–6.4) | NA |
| HCV genotype (%) | NA | ||
| 1a | NA | 60% | |
| 1b | NA | 12% | |
| 4 | NA | 28% | |
| Liver stiffness (KPa) | NA | 5.8 (4.5–7.9) | NA |
| <7.1 KPa (F0-F1) (%) | NA | 60% | |
| ≥7.1 KPa (F2-F4) (%) | NA | 40% | |
| APRI score | 0.33 (0.27–0.50) | 0.68 (0.43–1.58) | <0.0001 |
| FIB-4 index | 1.12 (0.78–1.36) | 1.31 (1.01–1.97) | 0.123 |
| Dependent Variable | Independent Variables | Percentage of Variation Explained by the Model (R2) | Regression Coefficient (β ± SD) | p-Value | |
|---|---|---|---|---|---|
| Individual | Accumulated | ||||
| IL-18 | AST level (IU/mL) | 0.517 | 0.517 | 0.71 ± 0.14 | <0.0001 |
| HCV-RNA (Log cop/mL) | 0.115 | 0.632 | 47.9 ± 18.3 | 0.016 | |
| IP-10 | GGT level (IU/mL) | 0.307 | 0.307 | 1.60 ± 0.45 | 0.002 |
| LS ≥ 7.1 KPa | 0.186 | 0.493 | 509 ± 179 | 0.010 | |
| VCAM-1 | AST level (IU/mL) | 0.199 | 0.199 | 5.78 ± 2.42 | 0.025 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Álvarez, B.; Restrepo, C.; García, M.; Navarrete-Muñoz, M.A.; Jiménez-Sousa, M.A.; Prieto, L.; Cabello, A.; Nistal, S.; Resino, S.; Górgolas, M.; et al. Liver Stiffness Hinders Normalization of Systemic Inflammation and Endothelial Activation after Hepatitis C Virus (HCV) Eradication in HIV/HCV Coinfected Patients. Vaccines 2020, 8, 323. https://doi.org/10.3390/vaccines8020323
Álvarez B, Restrepo C, García M, Navarrete-Muñoz MA, Jiménez-Sousa MA, Prieto L, Cabello A, Nistal S, Resino S, Górgolas M, et al. Liver Stiffness Hinders Normalization of Systemic Inflammation and Endothelial Activation after Hepatitis C Virus (HCV) Eradication in HIV/HCV Coinfected Patients. Vaccines. 2020; 8(2):323. https://doi.org/10.3390/vaccines8020323
Chicago/Turabian StyleÁlvarez, Beatriz, Clara Restrepo, Marcial García, María A. Navarrete-Muñoz, María A. Jiménez-Sousa, Laura Prieto, Alfonso Cabello, Sara Nistal, Salvador Resino, Miguel Górgolas, and et al. 2020. "Liver Stiffness Hinders Normalization of Systemic Inflammation and Endothelial Activation after Hepatitis C Virus (HCV) Eradication in HIV/HCV Coinfected Patients" Vaccines 8, no. 2: 323. https://doi.org/10.3390/vaccines8020323
APA StyleÁlvarez, B., Restrepo, C., García, M., Navarrete-Muñoz, M. A., Jiménez-Sousa, M. A., Prieto, L., Cabello, A., Nistal, S., Resino, S., Górgolas, M., Rallón, N., & Benito, J. M. (2020). Liver Stiffness Hinders Normalization of Systemic Inflammation and Endothelial Activation after Hepatitis C Virus (HCV) Eradication in HIV/HCV Coinfected Patients. Vaccines, 8(2), 323. https://doi.org/10.3390/vaccines8020323






