Chimeric Vaccines Designed by Immunoinformatics-Activated Polyfunctional and Memory T Cells That Trigger Protection against Experimental Visceral Leishmaniasis
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Epitope Mapping and Chimeric Proteins Design
2.3. Prediction of Epitope Conservancy among Leishmania Species
2.4. Prediction of Antigenicity, Allergenicity, and Physicochemical Properties of the Two Chimeras
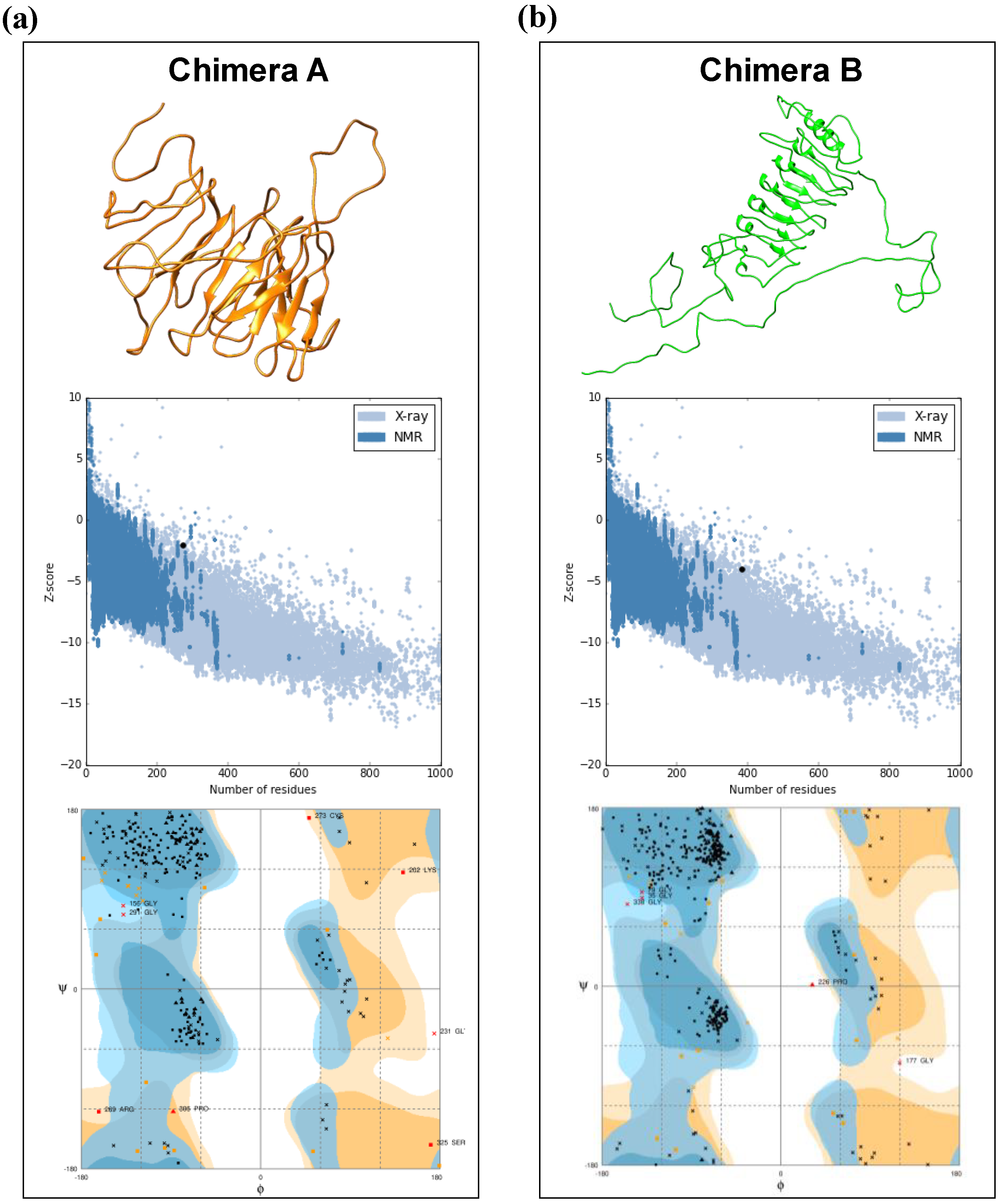
2.5. Tertiary Structure Prediction and Refinement of the Models
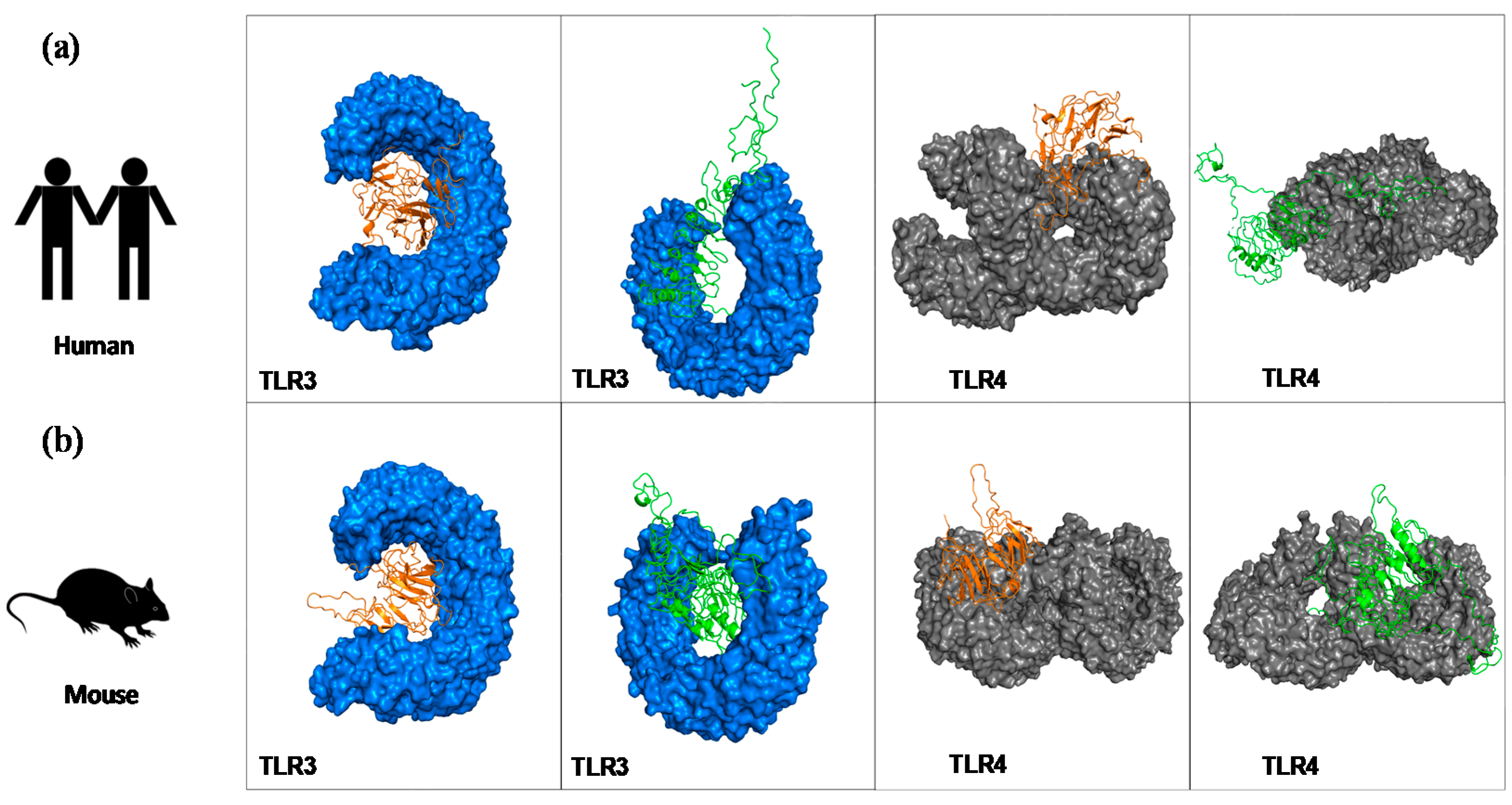
2.6. Molecular Docking to Highlight the Binding Affinity between the Chimeras and Mouse and Human TLR Receptors
2.7. Chimera Proteins Synthesis
2.8. Parasites
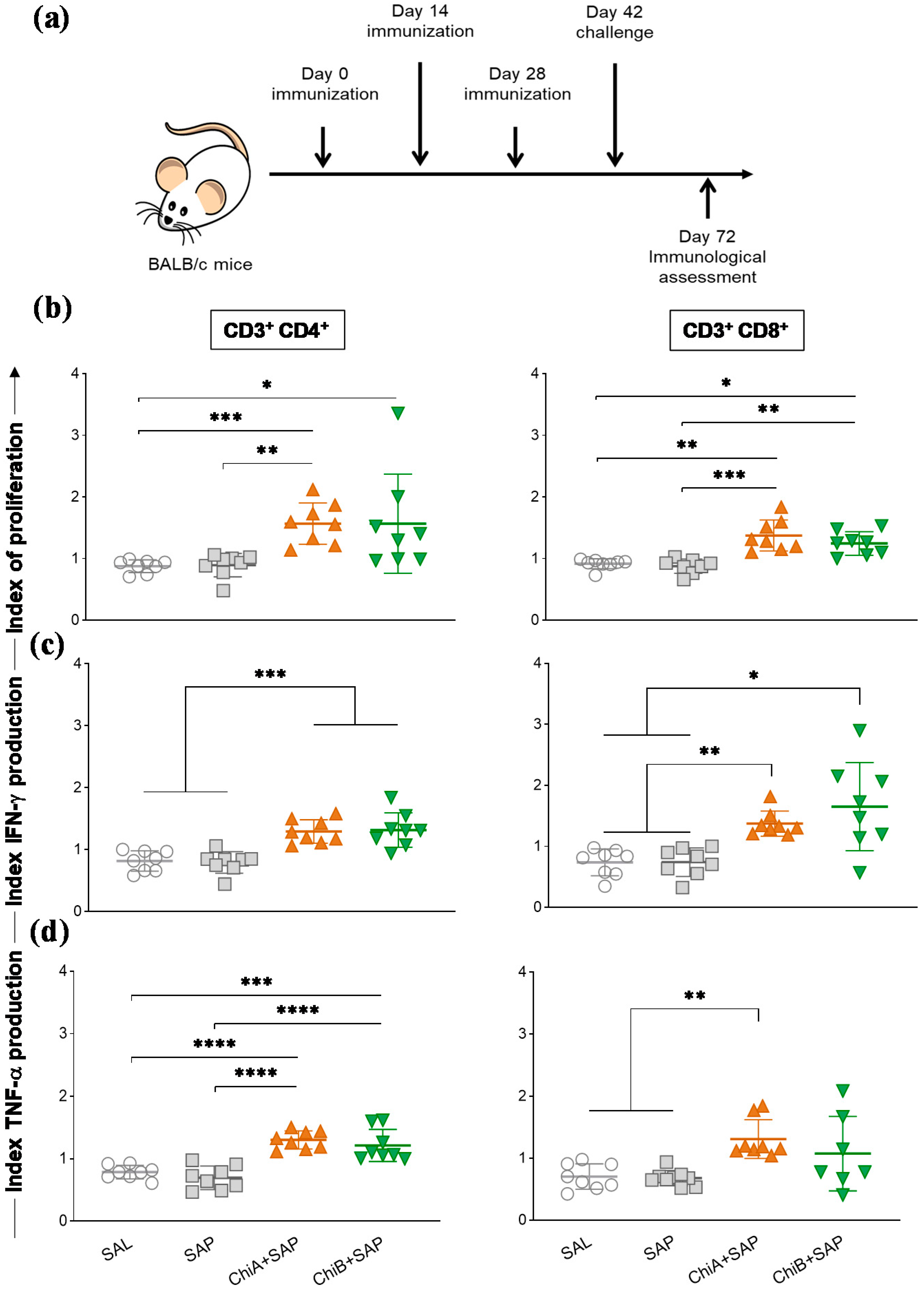
2.9. Immunization Regimens and Challenge of the Mice
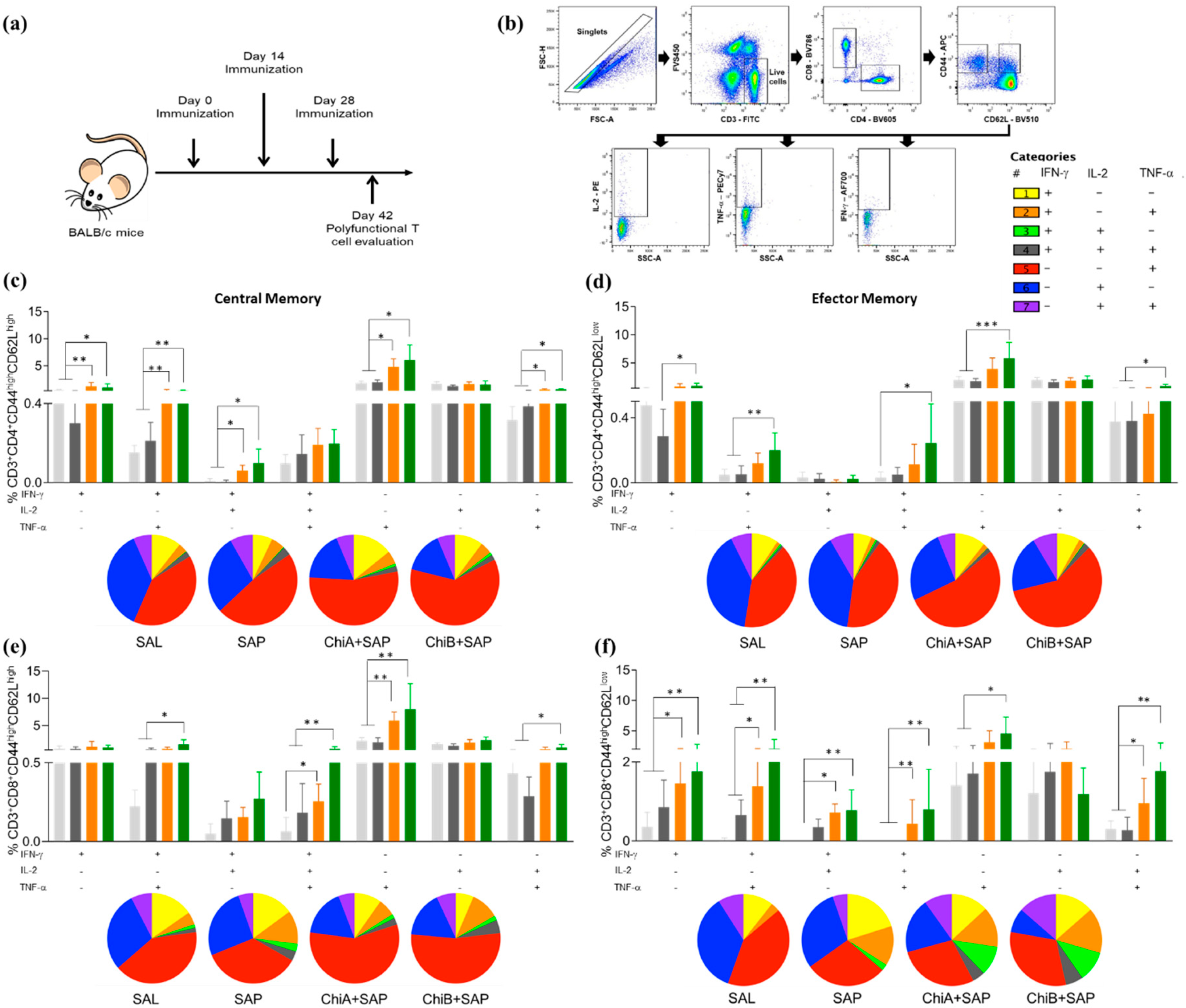
2.10. Analyses of Polyfunctional T cell Phenotypes
2.11. Proliferation Assay and Intracellular Cytokine Stain
2.12. Analyses of Memory T cell Phenotypes
2.13. Evaluation of the Parasite Burden in the Spleen by Quantitative PCR
2.14. Statistical Analysis
3. Results
3.1. In Silico Construction of the Chimeras A and B
3.2. Tertiary Structure Prediction and Binding Affinity between the Chimeras and Mouse and Human TLR Receptors
3.3. Chimeras A and B Designed by Immunoinformatics Tools Elicited a Different Pattern of Polyfunctional T Cells in the Spleen of Immunized Mice
3.4. Chimeras a and B Not Only Induced Proliferation of T Cells but Also Enhanced the Production of Intracellular Cytokines by These Cells in the Spleen of Immunized Mice and after Being Challenged with L. Infantum
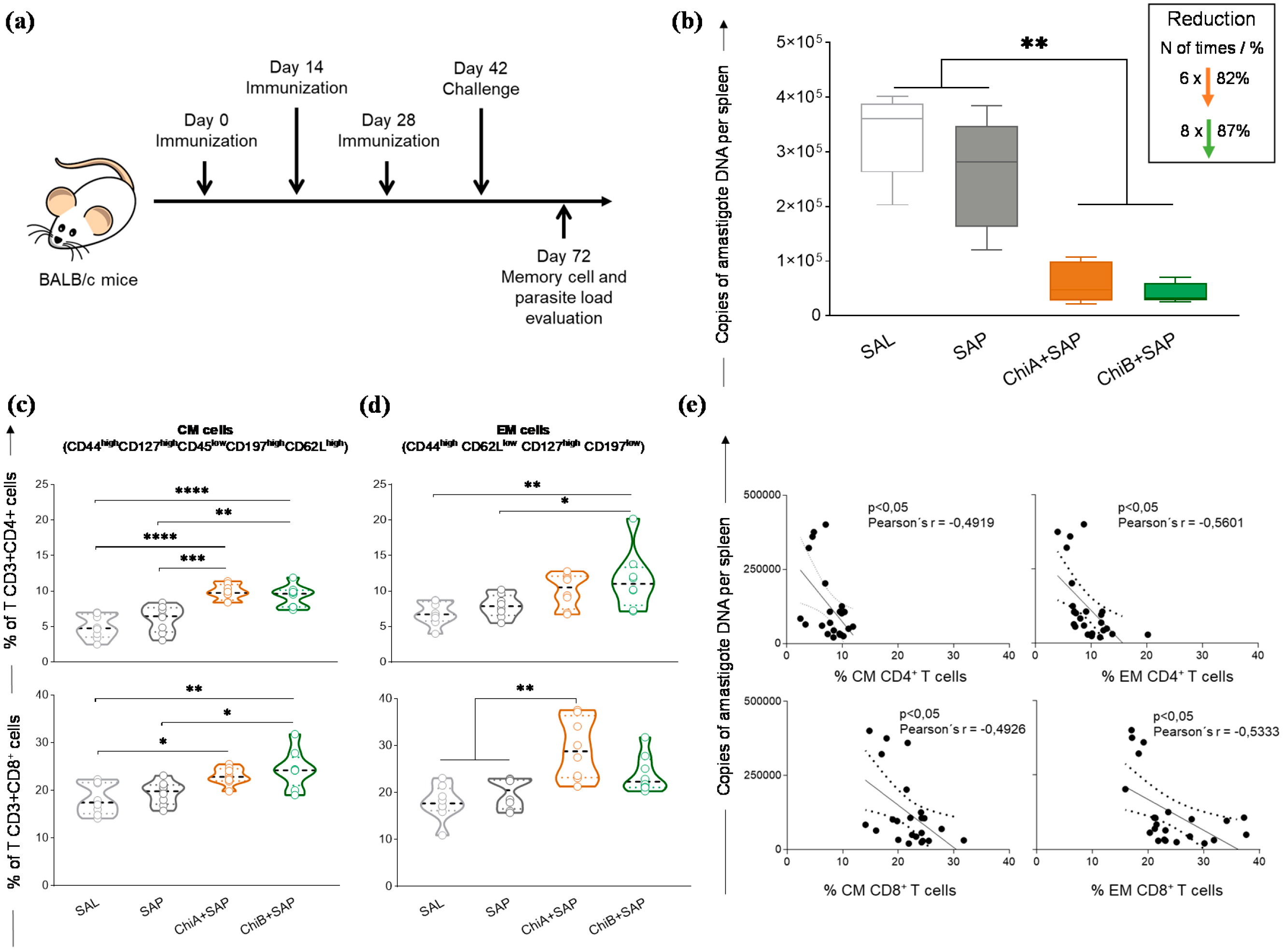
3.5. Chimeras A and B Promoted a Strong Reduction in Parasite Burden and Developed Central and Effector Memory of T Cells in the Spleen of Immunized and Challenged Mice
4. Discussion
5. Conclusions
6. Patent
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zutshi, S.; Kumar, S.; Chauhan, P.; Bansode, Y.; Nair, A.; Roy, S.; Sarkar, A.; Saha, B. Anti-Leishmanial Vaccines: Assumptions, Approaches, and Annulments. Vaccines (Basel) 2019, 7, 156. [Google Scholar] [CrossRef]
- Sachdeva, R.; Banerjea, A.C.; Malla, N.; Dubey, M.L. Immunogenicity and efficacy of single antigen Gp63, polytope and polytopeHSP70 DNA vaccines against visceral leishmaniasis in experimental mouse model. PLoS ONE 2009, 4, e7880. [Google Scholar] [CrossRef] [PubMed]
- Seyed, N.; Taheri, T.; Vauchy, C.; Dosset, M.; Godet, Y.; Eslamifar, A.; Sharifi, I.; Adotevi, O.; Borg, C.; Rohrlich, P.S.; et al. Immunogenicity evaluation of a rationally designed polytope construct encoding HLA-A*0201 restricted epitopes derived from Leishmania major related proteins in HLA-A2/DR1 transgenic mice: Steps toward polytope vaccine. PLoS ONE 2014, 9, e108848. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Freier, A.; Boussoffara, T.; Das, S.; Oswald, D.; Losch, F.O.; Selka, M.; Sacerdoti-Sierra, N.; Schonian, G.; Wiesmuller, K.H.; et al. Modular multiantigen T cell epitope-enriched DNA vaccine against human leishmaniasis. Sci. Transl. Med. 2014, 6, 234ra56. [Google Scholar] [CrossRef] [PubMed]
- Seder, R.A.; Darrah, P.A.; Roederer, M. T-cell quality in memory and protection: Implications for vaccine design. Nat. Rev. Immunol. 2008, 8, 247–258. [Google Scholar] [CrossRef]
- Darrah, P.A.; Patel, D.T.; De Luca, P.M.; Lindsay, R.W.; Davey, D.F.; Flynn, B.J.; Hoff, S.T.; Andersen, P.; Reed, S.G.; Morris, S.L.; et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat. Med. 2007, 13, 843–850. [Google Scholar] [CrossRef]
- De Groot, A.S. Immunomics: Discovering new targets for vaccines and therapeutics. Drug Discov. Today 2006, 11, 203–209. [Google Scholar] [CrossRef]
- De Groot, A.S.; Berzofsky, J.A. From genome to vaccine—New immunoinformatics tools for vaccine design. Methods 2004, 34, 425–428. [Google Scholar] [CrossRef]
- Brito, R.C.; Guimaraes, F.G.; Velloso, J.P.; Correa-Oliveira, R.; Ruiz, J.C.; Reis, A.B.; Resende, D.M. Immunoinformatics Features Linked to Leishmania Vaccine Development: Data Integration of Experimental and In Silico Studies. Int. J. Mol. Sci. 2017, 18, 371. [Google Scholar] [CrossRef]
- Alves-Silva, M.V.; Nico, D.; Morrot, A.; Palatnik, M.; Palatnik-de-Sousa, C.B. A chimera containing CD4+ and CD8+ T-cell epitopes of the Leishmania donovani nucleoside hydrolase (NH36) optimizes cross-protection against Leishmania amazonesis infection. Front. Immunol. 2017, 8, 100. [Google Scholar] [CrossRef]
- De Brito, R.C.F.; Cardoso, J.M.O.; Reis, L.E.S.; Mathias, F.A.S.; Aguiar-Soares, R.D.O.; Teixeira-Carvalho, A.; Roatt, B.M.; Correa-Oliveira, R.; Ruiz, J.C.; Resende, D.M.; et al. Synthetic Peptides Elicit Strong Cellular Immunity in Visceral Leishmaniasis Natural Reservoir and Contribute to Long-Lasting Polyfunctional T-Cells in BALB/c Mice. Vaccines (Basel) 2019, 7, 162. [Google Scholar] [CrossRef] [PubMed]
- Larsen, M.V.; Lundegaard, C.; Lamberth, K.; Buus, S.; Lund, O.; Nielsen, M. Large-scale validation of methods for cytotoxic T-lymphocyte epitope prediction. BMC Bioinform. 2007, 8, 424. [Google Scholar] [CrossRef] [PubMed]
- Buus, S.; Lauemoller, S.L.; Worning, P.; Kesmir, C.; Frimurer, T.; Corbet, S.; Fomsgaard, A.; Hilden, J.; Holm, A.; Brunak, S. Sensitive quantitative predictions of peptide-MHC binding by a ‘Query by Committee’ artificial neural network approach. Tissue Antigens 2003, 62, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.; Lundegaard, C.; Lund, O. Prediction of MHC class II binding affinity using SMM-align, a novel stabilization matrix alignment method. BMC Bioinform. 2007, 8, 238. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Ding, F.; Zhang, Q.; Pan, X.; Qu, L.; Pan, W. Stability and potency of the Plasmodium falciparum MSP1-19/AMA-1(III) chimeric vaccine candidate with Montanide ISA720 adjuvant. Vaccine 2010, 28, 3152–3168. [Google Scholar] [CrossRef]
- Real, F.; Vidal, R.O.; Carazzolle, M.F.; Mondego, J.M.; Costa, G.G.; Herai, R.H.; Wurtele, M.; de Carvalho, L.M.; Carmona e Ferreira, R.; Mortara, R.A.; et al. The genome sequence of Leishmania (Leishmania) amazonensis: Functional annotation and extended analysis of gene models. DNA Res. 2013, 20, 567–581. [Google Scholar] [CrossRef]
- Khan, M.; Khan, S.; Ali, A.; Akbar, H.; Sayaf, A.M.; Khan, A.; Wei, D.Q. Immunoinformatics approaches to explore Helicobacter Pylori proteome (Virulence Factors) to design B and T cell multi-epitope subunit vaccine. Sci. Rep. 2019, 9, 13321. [Google Scholar] [CrossRef]
- Kallberg, M.; Wang, H.; Wang, S.; Peng, J.; Wang, Z.; Lu, H.; Xu, J. Template-based protein structure modeling using the RaptorX web server. Nat. Protoc. 2012, 7, 1511–1522. [Google Scholar] [CrossRef]
- Heo, L.; Park, H.; Seok, C. GalaxyRefine: Protein structure refinement driven by side-chain repacking. Nucleic Acids Res. 2013, 41, W384–W388. [Google Scholar] [CrossRef]
- Wiederstein, M.; Sippl, M.J. ProSA-web: Interactive web service for the recognition of errors in three-dimensional structures of proteins. Nucleic Acids Res. 2007, 35, W407–W410. [Google Scholar] [CrossRef]
- Lovell, S.C.; Davis, I.W.; Arendall, W.B., 3rd; de Bakker, P.I.; Word, J.M.; Prisant, M.G.; Richardson, J.S.; Richardson, D.C. Structure validation by Calpha geometry: Phi, psi and Cbeta deviation. Proteins 2003, 50, 437–450. [Google Scholar] [CrossRef] [PubMed]
- Vajda, S.; Yueh, C.; Beglov, D.; Bohnuud, T.; Mottarella, S.E.; Xia, B.; Hall, D.R.; Kozakov, D. New additions to the ClusPro server motivated by CAPRI. Proteins 2017, 85, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [PubMed]
- PDB. Available online: http://www.rcsb.org/ (accessed on 11 November 2019).
- Moreira, N.; Vitoriano-Souza, J.; Roatt, B.M.; Vieira, P.M.; Ker, H.G.; de Oliveira Cardoso, J.M.; Giunchetti, R.C.; Carneiro, C.M.; de Lana, M.; Reis, A.B. Parasite burden in hamsters infected with two different strains of leishmania (Leishmania) infantum: “Leishman Donovan units” versus real-time PCR. PLoS ONE 2012, 7, e47907. [Google Scholar] [CrossRef][Green Version]
- Reis, A.B.; Teixeira-Carvalho, A.; Vale, A.M.; Marques, M.J.; Giunchetti, R.C.; Mayrink, W.; Guerra, L.L.; Andrade, R.A.; Correa-Oliveira, R.; Martins-Filho, O.A. Isotype patterns of immunoglobulins: Hallmarks for clinical status and tissue parasite density in Brazilian dogs naturally infected by Leishmania (Leishmania) chagasi. Vet. Immunol. Immunopathol. 2006, 112, 102–116. [Google Scholar] [CrossRef]
- Coler, R.N.; Goto, Y.; Bogatzki, L.; Raman, V.; Reed, S.G. Leish-111f, a recombinant polyprotein vaccine that protects against visceral Leishmaniasis by elicitation of CD4+ T cells. Infect. Immun. 2007, 75, 4648–4654. [Google Scholar] [CrossRef]
- Dias, D.S.; Ribeiro, P.A.F.; Martins, V.T.; Lage, D.P.; Costa, L.E.; Chavez-Fumagalli, M.A.; Ramos, F.F.; Santos, T.T.O.; Ludolf, F.; Oliveira, J.S.; et al. Vaccination with a CD4(+) and CD8(+) T-cell epitopes-based recombinant chimeric protein derived from Leishmania infantum proteins confers protective immunity against visceral leishmaniasis. Transl. Res. 2018, 200, 18–34. [Google Scholar] [CrossRef]
- Sanchez-Sampedro, L.; Gomez, C.E.; Mejias-Perez, E.; Sorzano, C.O.; Esteban, M. High quality long-term CD4+ and CD8+ effector memory populations stimulated by DNA-LACK/MVA-LACK regimen in Leishmania major BALB/c model of infection. PLoS ONE 2012, 7, e38859. [Google Scholar] [CrossRef]
- Reis, L.E.S.; Brito, R.C.F.; Cardoso, J.M.O.; Mathias, F.A.S.; Aguiar Soares, R.D.O.; Carneiro, C.M.; de Abreu Vieira, P.M.; Ramos, G.S.; Frezard, F.J.G.; Roatt, B.M.; et al. Mixed Formulation of Conventional and Pegylated Meglumine Antimoniate-Containing Liposomes Reduces Inflammatory Process and Parasite Burden in Leishmania infantum-Infected BALB/c Mice. Antimicrob. Agents Chemother. 2017, 61, e00962-17. [Google Scholar] [CrossRef]
- Vaure, C.; Liu, Y. A comparative review of toll-like receptor 4 expression and functionality in different animal species. Front. Immunol. 2014, 5, 316. [Google Scholar] [CrossRef]
- Zandieh, M.; Kashi, T.; Taheri, T.; Zahedifard, F.; Taslimi, Y.; Doustdary, M.; Habibzadeh, S.; Eslamifar, A.; Shokri, F.; Rafati, S.; et al. Assessment of protection induced by DNA and live vaccine encoding Leishmania MHC class I restricted epitopes against L. major challenge in Balb/c mice model. Microb. Biochem. Technol. 2015, 7, 427–438. [Google Scholar] [CrossRef]
- Soto, M.; Alonso, C.; Requena, J.M. The Leishmania infantum acidic ribosomal protein LiP2a induces a prominent humoral response in vivo and stimulates cell proliferation in vitro and interferon-gamma (IFN-gamma) production by murine splenocytes. Clin. Exp. Immunol. 2000, 122, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Rafati, S.; Nakhaee, A.; Taheri, T.; Taslimi, Y.; Darabi, H.; Eravani, D.; Sanos, S.; Kaye, P.; Taghikhani, M.; Jamshidi, S.; et al. Protective vaccination against experimental canine visceral leishmaniasis using a combination of DNA and protein immunization with cysteine proteinases type I and II of L. infantum. Vaccine 2005, 23, 3716–3725. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.P.; Costa, M.M.S.; Coelho, E.A.F.; Michalick, M.S.M.; de Freitas, E.; Melo, M.N.; Tafuri, W.L.; Resende, D.M.; Hermont, V.; Abrantes, C.D.; et al. Protective immunity against challenge with Leishmania (Leishmania) chagasi in beagle dogs vaccinated with recombinant A2 protein. Vaccine 2008, 26, 5888–5895. [Google Scholar] [CrossRef]
- Khoshgoo, N.; Zahedifard, F.; Azizi, H.; Taslimi, Y.; Alonso, M.J.; Rafati, S. Cysteine proteinase type III is protective against Leishmania infantum infection in BALB/c mice and highly antigenic in visceral leishmaniasis individuals. Vaccine 2008, 26, 5822–5829. [Google Scholar] [CrossRef]
- Ramos, I.; Alonso, A.; Marcen, J.M.; Peris, A.; Castillo, J.A.; Colmenares, M.; Larraga, V. Heterologous prime-boost vaccination with a non-replicative vaccinia recombinant vector expressing LACK confers protection against canine visceral leishmaniasis with a predominant Th1-specific immune response. Vaccine 2008, 26, 333–344. [Google Scholar] [CrossRef]
- Baharia, R.K.; Tandon, R.; Sahasrabuddhe, A.A.; Sundar, S.; Dube, A. Nucleosomal histone proteins of L. donovani: A combination of recombinant H2A, H2B, H3 and H4 proteins were highly immunogenic and offered optimum prophylactic efficacy against Leishmania challenge in hamsters. PLoS ONE 2014, 9, e97911. [Google Scholar] [CrossRef]
- Oliva, G.; Nieto, J.; Foglia Manzillo, V.; Cappiello, S.; Fiorentino, E.; Di Muccio, T.; Scalone, A.; Moreno, J.; Chicharro, C.; Carrillo, E.; et al. A randomised, double-blind, controlled efficacy trial of the LiESP/QA-21 vaccine in naive dogs exposed to two leishmania infantum transmission seasons. PLoS Negl. Trop. Dis. 2014, 8, e3213. [Google Scholar] [CrossRef]
- Pereira, L.; Abbehusen, M.; Teixeira, C.; Cunha, J.; Nascimento, I.P.; Fukutani, K.; dos-Santos, W.; Barral, A.; de Oliveira, C.I.; Barral-Netto, M.; et al. Vaccination with Leishmania infantum acidic ribosomal P0 but not with nucleosomal histones proteins controls Leishmania infantum infection in hamsters. PLoS Negl. Trop. Dis. 2015, 9, e0003490. [Google Scholar] [CrossRef][Green Version]
- Nezafat, N.; Eslami, M.; Negahdaripour, M.; Rahbar, M.R.; Ghasemi, Y. Designing an efficient multi-epitope oral vaccine against Helicobacter pylori using immunoinformatics and structural vaccinology approaches. Mol. Biosyst. 2017, 13, 699–713. [Google Scholar] [CrossRef]
- Khatoon, N.; Pandey, R.K.; Prajapati, V.K. Exploring Leishmania secretory proteins to design B and T cell multi-epitope subunit vaccine using immunoinformatics approach. Sci. Rep. 2017, 7, 8285. [Google Scholar] [CrossRef] [PubMed]
- Kumar Pandey, R.; Ojha, R.; Mishra, A.; Kumar Prajapati, V. Designing B- and T-cell multi-epitope based subunit vaccine using immunoinformatics approach to control Zika virus infection. J. Cell. Biochem. 2018, 119, 7631–7642. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.K.; Bhatt, T.K.; Prajapati, V.K. Novel Immunoinformatics Approaches to Design Multi-epitope Subunit Vaccine for Malaria by Investigating Anopheles Salivary Protein. Sci. Rep. 2018, 8, 1125. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, V.; Rungta, T.; Goyal, K.; Singh, M.P. Designing a multi-epitope based vaccine to combat Kaposi Sarcoma utilizing immunoinformatics approach. Sci. Rep. 2019, 9, 2517. [Google Scholar] [CrossRef] [PubMed]
- Nezafat, N.; Karimi, Z.; Eslami, M.; Mohkam, M.; Zandian, S.; Ghasemi, Y. Designing an efficient multi-epitope peptide vaccine against Vibrio cholerae via combined immunoinformatics and protein interaction based approaches. Comput. Biol. Chem. 2016, 62, 82–95. [Google Scholar] [CrossRef]
- Apostolico, J.S.; Lunardelli, V.A.S.; Yamamoto, M.M.; Cunha-Neto, E.; Boscardin, S.B.; Rosa, D.S. Poly(I:C) Potentiates T Cell Immunity to a Dendritic Cell Targeted HIV-Multiepitope Vaccine. Front. Immunol. 2019, 10, 843. [Google Scholar] [CrossRef]
- Selvapandiyan, A.; Dey, R.; Nylen, S.; Duncan, R.; Sacks, D.; Nakhasi, H.L. Intracellular replication-deficient Leishmania donovani induces long lasting protective immunity against visceral leishmaniasis. J. Immunol. 2009, 183, 1813–1820. [Google Scholar] [CrossRef]
- Okwor, I.B.; Jia, P.; Mou, Z.; Onyilagha, C.; Uzonna, J.E. CD8+ T cells are preferentially activated during primary low dose leishmania major infection but are completely dispensable during secondary anti-Leishmania immunity. PLoS Negl. Trop. Dis. 2014, 8, e3300. [Google Scholar] [CrossRef]
- Reed, S.G.; Coler, R.N.; Mondal, D.; Kamhawi, S.; Valenzuela, J.G. Leishmania vaccine development: Exploiting the host-vector-parasite interface. Expert Rev. Vaccines 2016, 15, 81–90. [Google Scholar] [CrossRef]
- Passero, L.F.; Carvalho, A.K.; Bordon, M.L.; Bonfim-Melo, A.; Carvalho, K.; Kallas, E.G.; Santos, B.B.; Toyama, M.H.; Paes-Leme, A.; Corbett, C.E.; et al. Proteins of Leishmania (Viannia) shawi confer protection associated with Th1 immune response and memory generation. Parasites Vectors 2012, 5, 64. [Google Scholar] [CrossRef]
- Dey, R.; Dagur, P.K.; Selvapandiyan, A.; McCoy, J.P.; Salotra, P.; Duncan, R.; Nakhasi, H.L. Live attenuated Leishmania donovani p27 gene knockout parasites are nonpathogenic and elicit long-term protective immunity in BALB/c mice. J. Immunol. 2013, 190, 2138–2149. [Google Scholar] [CrossRef] [PubMed]
- Uzonna, J.E.; Spath, G.F.; Beverley, S.M.; Scott, P. Vaccination with phosphoglycan-deficient Leishmania major protects highly susceptible mice from virulent challenge without inducing a strong Th1 response. J. Immunol. 2004, 172, 3793–3797. [Google Scholar] [CrossRef] [PubMed]
- Martins, V.T.; Lage, D.P.; Duarte, M.C.; Carvalho, A.M.; Costa, L.E.; Mendes, T.A.; Vale, D.L.; Menezes-Souza, D.; Roatt, B.M.; Tavares, C.A.; et al. A recombinant fusion protein displaying murine and human MHC class I- and II-specific epitopes protects against Leishmania amazonensis infection. Cell. Immunol. 2017, 313, 32–42. [Google Scholar] [CrossRef]
- Bogdan, C.; Schroppel, K.; Lohoff, M.; Rollinghoff, M.; Solbach, W. Immunization of susceptible hosts with a soluble antigen fraction from Leishmania major leads to aggravation of murine leishmaniasis mediated by CD4+ T cells. Eur. J. Immunol. 1990, 20, 2533–2540. [Google Scholar] [CrossRef] [PubMed]
- Manna, L.; Reale, S.; Vitale, F.; Gravino, A.E. Evidence for a relationship between Leishmania load and clinical manifestations. Res. Vet. Sci. 2009, 87, 76–78. [Google Scholar] [CrossRef] [PubMed]
- Blackwell, J.M. Genetic susceptibility to leishmanial infections: Studies in mice and man. Parasitology 1996, 112 (Suppl. S1), S67–S74. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Bhattacharya, P.; Dagur, P.K.; Karmakar, S.; Ismail, N.; Joshi, A.B.; Akue, A.D.; KuKuruga, M.; McCoy, J.P., Jr.; Dey, R.; et al. Live Attenuated Leishmania donovani Centrin Gene-Deleted Parasites Induce IL-23-Dependent IL-17-Protective Immune Response against Visceral Leishmaniasis in a Murine Model. J. Immunol. 2018, 200, 163–176. [Google Scholar] [CrossRef]
- Rodrigues, A.; Claro, M.; Alexandre-Pires, G.; Santos-Mateus, D.; Martins, C.; Valerio-Bolas, A.; Rafael-Fernandes, M.; Pereira, M.A.; Pereira da Fonseca, I.; Tomas, A.M.; et al. Leishmania infantum antigens modulate memory cell subsets of liver resident T lymphocyte. Immunobiology 2017, 222, 409–422. [Google Scholar] [CrossRef]
- Farber, D.L.; Yudanin, N.A.; Restifo, N.P. Human memory T cells: Generation, compartmentalization and homeostasis. Nat. Rev. Immunol. 2014, 14, 24–35. [Google Scholar] [CrossRef]
- Sprent, J. T memory cells: Quality not quantity. Curr. Biol. 2002, 12, R174–R176. [Google Scholar] [CrossRef]
- Loeuillet, C.; Banuls, A.L.; Hide, M. Study of Leishmania pathogenesis in mice: Experimental considerations. Parasites Vectors 2016, 9, 144. [Google Scholar] [CrossRef] [PubMed]
- Martins, V.T.; Duarte, M.C.; Lage, D.P.; Costa, L.E.; Carvalho, A.M.; Mendes, T.A.; Roatt, B.M.; Menezes-Souza, D.; Soto, M.; Coelho, E.A. A recombinant chimeric protein composed of human and mice-specific CD4(+) and CD8(+) T-cell epitopes protects against visceral leishmaniasis. Parasite Immunol. 2017, 39, e12359. [Google Scholar] [CrossRef] [PubMed]
| Chimera | Protein | Epitope Sequence | MHC Class I and II Alleles | Conservancy of the Selected Epitope across Leishmania Species (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| L. donovani | L. major | L. amazonensis | L. braziliensis | ||||||||
| Chimera A | Histone protein (H2A) | KKRCRLNPR | H2-Dk | 100 | 89 | 100 | 66 | ||||
| DDISSLLKNVTLSHS | HLA-DRB1*1302 | HLA-DRB1*0101 | HLA-DRB1*0401 | HLA-DRB1*0404 | HLA-DRB1*1501 | 100 | 87 | 100 | 66 | ||
| Acid ribosomal protein P2 (LiP2a) | AAKMSAMPAASSGAA | HLA-DRB1*0901 | HLA-DRB1*0101 | 100 | 100 | 100 | 93 | ||||
| MSTKYLAAY | HLA-A*01 | HLA-A*26 | HLA-B*62 | HLA-A*03 | HLA-B*58 | 100 | 100 | 100 | 100 | ||
| Acid ribosomal protein P0 (LiP0) | VDAFKNLLAVSVATSYEF | HLA-DRB1*0101 | HLA-DRB1*0701 | HLA-DRB1*1501 | HLA-DRB1*1302 | 100 | 100 | 100 | 94 | ||
| AHRVKAPAR | H2-Dk | 100 | 100 | 100 | 89 | ||||||
| Leishmania homologue of activated C kinase (LACK) | WSADGNTLY | HLA-A*01 | HLA-A*26 | HLA-B*62 | HLA-B*58 | 100 | 100 | 100 | 100 | ||
| DRSIRMWDLRNGQCQ | HLA-DRB4*0101 | HLA-DRB1*1501 | 100 | 100 | 100 | 93 | |||||
| ATERSLSVY | HLA-A*01 | HLA-A*26 | HLA-B*62 | 100 | 100 | 100 | 78 | ||||
| Cysteine peptidase C (CPC) | GYLVSGKSL | HLA-A*24 | H2-Kd | 100 | 100 | 100 | 78 | ||||
| WTASADNGY | HLA-A*01 | HLA-A*26 | HLA-B*62 | HLA-B*58 | 100 | 100 | 100 | 89 | |||
| LVKYKGGTSYSVKGE | HLA-DRB1*0101 | HLA-DRB1*0701 | HLA-DRB1*1501 | 100 | 100 | 100 | 60 | ||||
| Chimera B | Cysteine peptidase A (CPA) | MTEDYMGMY | HLA-A*01 | HLA-A*26 | B62 | 89 | 100 | 67 | 100 | ||
| AKRRRLPTT | H2-Dk | 100 | 100 | 78 | 78 | ||||||
| RPDFMNMTPRGVPLE | HLA-DRB1*0101 | HLA-DRB1*0701 | HLA-DRB1*1501 | HLA-DRB1*1302 | HLA-DRB5*0101 | 100 | 87 | 93 | 67 | ||
| Cysteine peptidase B (CPB) | SKKFSHPSL | HLA-B*39 | 100 | 66 | 89 | 56 | |||||
| AGALVMGTALLTESA | HLA-DRB1*0101 | HLA-DRB1*0404 | HLA-DRB1*1302 | 100 | 60 | 80 | 67 | ||||
| RTDRQSCLY | HLA-A*01 | HLA-B*58 | HLA-A*03 | 100 | 78 | 89 | 78 | ||||
| Surface antigenic protein (PSA-50S) | DSWSRLQGLTSLTLS | HLA-DRB1*0101 | HLA-DRB1*1501 | 53 | 87 | 53 | 53 | ||||
| LPPEWAAMP | H2-Dd | 78 | 89 | 78 | 78 | ||||||
| LTDERTCLV | HLA-A*01 | HLA-A*02 | 67 | 100 | 78 | 67 | |||||
| Amastigote protein A2 (A2) | GKGLRAPPL | H2-Dk | 100 | 100 | 78 | 56 | |||||
| SQAGDVFAL | HLA-B*39 | HLA-B*62 | HLA-B*44 | HLA-A*02 | HLA-B*27 | 100 | 89 | 78 | 56 | ||
| GPHLRGGAVTSSVVT | HLA-DRB1*0101 | HLA-DRB1*0701 | 100 | 87 | 67 | 60 | |||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brito, R.C.F.D.; Ruiz, J.C.; Cardoso, J.M.d.O.; Ostolin, T.L.V.D.P.; Reis, L.E.S.; Mathias, F.A.S.; Aguiar-Soares, R.D.d.O.; Roatt, B.M.; Corrêa-Oliveira, R.; Resende, D.d.M.; et al. Chimeric Vaccines Designed by Immunoinformatics-Activated Polyfunctional and Memory T Cells That Trigger Protection against Experimental Visceral Leishmaniasis. Vaccines 2020, 8, 252. https://doi.org/10.3390/vaccines8020252
Brito RCFD, Ruiz JC, Cardoso JMdO, Ostolin TLVDP, Reis LES, Mathias FAS, Aguiar-Soares RDdO, Roatt BM, Corrêa-Oliveira R, Resende DdM, et al. Chimeric Vaccines Designed by Immunoinformatics-Activated Polyfunctional and Memory T Cells That Trigger Protection against Experimental Visceral Leishmaniasis. Vaccines. 2020; 8(2):252. https://doi.org/10.3390/vaccines8020252
Chicago/Turabian StyleBrito, Rory Cristiane Fortes De, Jeronimo Conceição Ruiz, Jamille Mirelle de Oliveira Cardoso, Thais Lopes Valentim Di Paschoale Ostolin, Levi Eduardo Soares Reis, Fernando Augusto Siqueira Mathias, Rodrigo Dian de Oliveira Aguiar-Soares, Bruno Mendes Roatt, Rodrigo Corrêa-Oliveira, Daniela de Melo Resende, and et al. 2020. "Chimeric Vaccines Designed by Immunoinformatics-Activated Polyfunctional and Memory T Cells That Trigger Protection against Experimental Visceral Leishmaniasis" Vaccines 8, no. 2: 252. https://doi.org/10.3390/vaccines8020252
APA StyleBrito, R. C. F. D., Ruiz, J. C., Cardoso, J. M. d. O., Ostolin, T. L. V. D. P., Reis, L. E. S., Mathias, F. A. S., Aguiar-Soares, R. D. d. O., Roatt, B. M., Corrêa-Oliveira, R., Resende, D. d. M., & Reis, A. B. (2020). Chimeric Vaccines Designed by Immunoinformatics-Activated Polyfunctional and Memory T Cells That Trigger Protection against Experimental Visceral Leishmaniasis. Vaccines, 8(2), 252. https://doi.org/10.3390/vaccines8020252






