Severe Multiorgan Failure Following Yellow Fever Vaccination
Abstract
1. Background
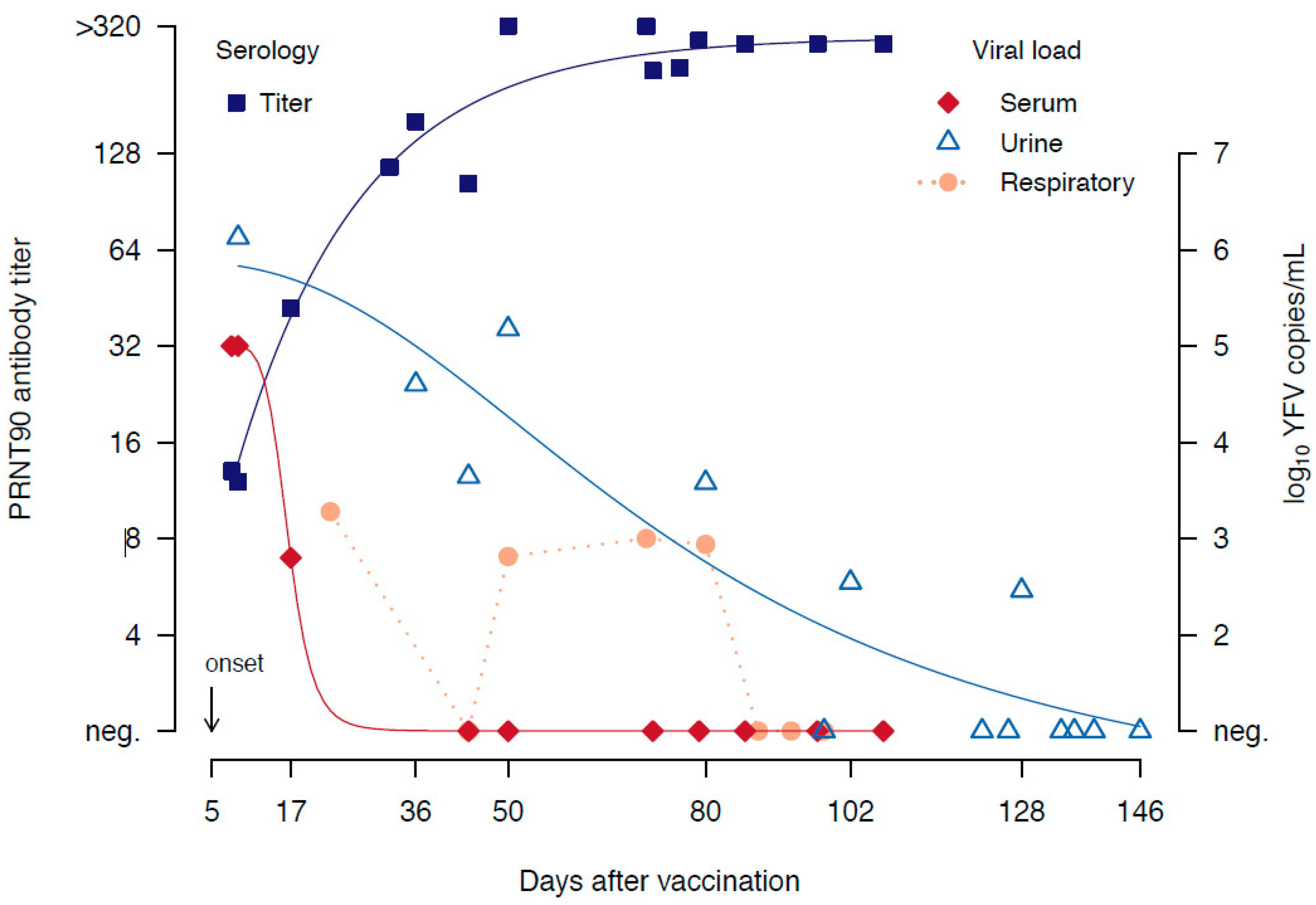
2. Case Presentation
3. Discussion and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Ethics Approval and Consent to Participate
Consent for Publication
Availability of Data and Materials
Abbreviations
References
- Tuboi, S.H.; Costa, Z.G.A.; Vasconcelos, P.F.C.; Hatch, D. Clinical and epidemiological characteristics of yellow fever in Brazil: Analysis of reported cases 1998–2002. Trans. R. Soc. Trop. Med. Hyg. 2007, 101, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, N.P.; Rabe, I.B.; Miller, E.R.; Fischer, M.; Staples, J.E. Adverse event reports following yellow fever vaccination, 2007–2013. J. Travel Med. 2016, 23, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.; Weld, L.; Tsai, T.F.; Mootrey, G.T.; Chen, R.T.; Niu, M.; Cetron, M.S. Advanced age a risk factor for illness temporally associated with yellow fever vaccination. Emerg. Infect. Dis. 2001, 7, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Khromava, A.Y.; Eidex, R.B.; Weld, L.; Kohl, K.S.; Bradshaw, R.D.; Chen, R.T.; Cetron, M.S. Yellow fever vaccine: An updated assessment of advanced age as a risk factor for serious adverse events. Vaccine 2005, 23, 3256–3263. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, B.; Jaspert, R.; Niedrig, M.; Kostner, C.; L’Age-Stehr, J. Development of viremia and humoral and cellular parameters of immune activation after vaccination with yellow fever virus strain 17D: A model of human flavivirus infection. J. Med. Virol. 1998, 56, 159–167. [Google Scholar] [CrossRef]
- Monath, T.P.; Cetron, M.S.; McCarthy, K.; Nichols, R.; Archambault, W.T.; Weld, L.; Bedford, P. Yellow fever 17D vaccine safety and immunogenicity in the elderly. Hum. Vaccines 2005, 1, 207–214. [Google Scholar] [CrossRef]
- Belsher, J.L.; Gay, P.; Brinton, M.; DellaValla, J.; Ridenour, R.; Lanciotti, R.; Perelygin, A.; Zaki, S.; Paddock, C.; Querec, T.; et al. Fatal multiorgan failure due to yellow fever vaccine-associated viscerotropic disease. Vaccine 2007, 25, 8480–8485. [Google Scholar] [CrossRef]
- Muñoz, J.; Vilella, A.; Domingo, C.; Nicolas, J.M.; De Ory, F.; Corachan, M.; Tenorio, A.; Gascon, J.; Gutierrez, J.M. Yellow Fever–Associated Viscerotropic Disease in Barcelona, Spain. J. Travel Med. 2008, 15, 202–205. [Google Scholar] [CrossRef][Green Version]
- Niedrig, M.; Patel, P.; El Wahed, A.A.; Schädler, R.; Yactayo, S. Find the right sample: A study on the versatility of saliva and urine samples for the diagnosis of emerging viruses. BMC Infect. Dis. 2018, 18, 707. [Google Scholar] [CrossRef]
- Bae, H.-G.; Drosten, C.; Emmerich, P.; Colebunders, R.; Hantson, P.; Pest, S.; Parent, M.; Schmitz, H.; Warnat, M.-A.; Niedrig, M. Analysis of two imported cases of yellow fever infection from Ivory Coast and The Gambia to Germany and Belgium. J. Clin. Virol. 2005, 33, 274–280. [Google Scholar] [CrossRef]
- Domingo, C.; Yactayo, S.; Agbenu, E.; Demanou, M.; Schulz, A.R.; Daskalow, K.; Niedrig, M. Detection of Yellow Fever 17D Genome in Urine. J. Clin. Microbiol. 2010, 49, 760–762. [Google Scholar] [CrossRef] [PubMed]
- Campos, W.R.; Cenachi, S.P.F.; Soares, M.S.; Gonçalves, P.F.; Vasconcelos-Santos, D.V. Vogt-Koyanagi-Harada-like Disease following Yellow Fever Vaccination. Ocul. Immunol. Inflamm. 2019, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Cottin, P.; Niedrig, M.; Domingo, C. Safety profile of the yellow fever vaccine Stamaril®: A 17-year review. Expert Rev. Vaccines 2013, 12, 1351–1368. [Google Scholar] [CrossRef] [PubMed]
- Jääskeläinen, A.J.; Huhtamo, E.; Kivioja, R.; Domingo, C.; Vene, S.; Kallio-Kokko, H.; Niedrig, M.; Tienari, P.J.; Vapalahti, O.P. Suspected YF-AND after yellow fever vaccination in Finland. J. Clin. Virol. 2014, 61, 444–447. [Google Scholar] [CrossRef]
- Thomas, R.E. Yellow fever vaccine-associated viscerotropic disease: Current perspectives. Drug Des. Dev. Ther. 2016, 10, 3345–3353. [Google Scholar] [CrossRef]
- Slesak, G.; Gabriel, M.; Domingo, C.; Schäfer, J. Severe yellow fever vaccine-associated disease: A case report and current overview. Dtsch. Med. Wochenschr. 2017, 142, 1219–1222. [Google Scholar]
- El Nawar, R.; Bayle, P.; Girbovan, A.; Marque, S.J.; Servan, J.; Pico, F. Meningomyeloradiculitis following yellow fever 17D vaccination: A case report. J. Neurovirol. 2018, 24, 642–646. [Google Scholar] [CrossRef]
- Engel, A.R.; Vasconcelos, P.F.C.; McArthur, M.A.; Barrett, A. Characterization of a viscerotropic yellow fever vaccine variant from a patient in Brazil. Vaccine 2006, 24, 2803–2809. [Google Scholar] [CrossRef]
- Rafferty, E.; Duclos, P.; Yactayo, S.; Schuster, M. Risk of yellow fever vaccine-associated viscerotropic disease among the elderly: A systematic review. Vaccine 2013, 31, 5798–5805. [Google Scholar] [CrossRef]
- Roukens, A.H.; Soonawala, D.; Joosten, S.A.; De Visser, A.W.; Jiang, X.; Dirksen, K.; De Gruijter, M.; Van Dissel, J.T.; Bredenbeek, P.J.; Visser, L.G. Elderly Subjects Have a Delayed Antibody Response and Prolonged Viraemia following Yellow Fever Vaccination: A Prospective Controlled Cohort Study. PLoS ONE 2011, 6, e27753. [Google Scholar] [CrossRef]
- Jonker, E.F.F.; Visser, L.; Roukens, A.H. Advances and controversies in yellow fever vaccination. Ther. Adv. Vaccines 2013, 1, 144–152. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Normal Range | Days after Yellow Fever Vaccination | |||||
|---|---|---|---|---|---|---|---|
| Day 8 | Day 13 | Day 15 | Day 22 | Day 40 | Day 134 | ||
| creatinine (mg/dL) | 0.6–1.4 mg/dL | 4.77 | 3.7 | 3.2 | 2.45 | 6.03 | 1.09 |
| GFR (MDRD, mL/min/1.73 qm) | >60 | 11.8 | - | - | - | - | - |
| GOT (U/L) | <46 U/L | 607 | 2056 | 1747 | 255 | 75 | 45 |
| GPT (U/L) | <50 U/L | 473 | - | 286 | 87 | 45 | |
| gGT (U/L) | <60 U/L | 131 | - | 152 | 241 | 152 | 46 |
| AP (U/L) | 40–130 U/L | 119 | - | 130 | 235 | - | 115 |
| bilirubin (mg/dL) | <1.0 mg/dL | 1.1 | - | 6.3 | 4.3 | - | - |
| LDH (U/L) | <342 U/L | 853 | - | - | - | 454 | - |
| CRP (mg/L) | <5 mg/L | 143.5 | 146 | 149 | 67.5 | 39.8 | 3.7 |
| PCT (ng/mL) | <0.05 | 41.08 | 55.76 | 28.11 | - | - | - |
| leucocytes (/nL) | 4–10/nL | 11.5 | 11.9 | 10.8 | 14 | 14 | 9.4 |
| quick (%) | 70–125% | 61.6 | 68.2 | 69 | 81 | 95.5 | 112 |
| INR | <1.2 | 1.25 | 1.18 | 1.18 | 1.09 | 1.03 | 0.96 |
| thrombocytes (/nL) | 150–440/nL | 35 | 13 | 33 | 153 | 461 | 358 |
| lactate (mg/dL) | <16 mg/dL | 69.6 | - | - | - | - | - |
| CK (U/L) | <190 U/L | 68,329 | 33,753 | 1261 | 268 | - | |
| pH | 7.37–7.45 | 7.42 | - | - | - | - | - |
| pO2 (mmHg) | - | 76 | - | - | - | - | - |
| pCO2 (mmHg) | 35–45 mmHg | 27 | - | - | - | - | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domingo, C.; Lamerz, J.; Cadar, D.; Stojkovic, M.; Eisermann, P.; Merle, U.; Nitsche, A.; Schnitzler, P. Severe Multiorgan Failure Following Yellow Fever Vaccination. Vaccines 2020, 8, 249. https://doi.org/10.3390/vaccines8020249
Domingo C, Lamerz J, Cadar D, Stojkovic M, Eisermann P, Merle U, Nitsche A, Schnitzler P. Severe Multiorgan Failure Following Yellow Fever Vaccination. Vaccines. 2020; 8(2):249. https://doi.org/10.3390/vaccines8020249
Chicago/Turabian StyleDomingo, Cristina, Judith Lamerz, Daniel Cadar, Marija Stojkovic, Philip Eisermann, Uta Merle, Andreas Nitsche, and Paul Schnitzler. 2020. "Severe Multiorgan Failure Following Yellow Fever Vaccination" Vaccines 8, no. 2: 249. https://doi.org/10.3390/vaccines8020249
APA StyleDomingo, C., Lamerz, J., Cadar, D., Stojkovic, M., Eisermann, P., Merle, U., Nitsche, A., & Schnitzler, P. (2020). Severe Multiorgan Failure Following Yellow Fever Vaccination. Vaccines, 8(2), 249. https://doi.org/10.3390/vaccines8020249






