Pustulan Activates Chicken Bone Marrow-Derived Dendritic Cells In Vitro and Promotes Ex Vivo CD4+ T Cell Recall Response to Infectious Bronchitis Virus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Ethics
2.2. Cell Culture
2.3. Glycan-Based Ligand Stimulation of BM-DCs
2.4. Flow Cytometry
2.5. Endocytosis Assay
2.6. Confocal Microscopy
2.7. RT-qPCR
2.8. Recombinant IBV N Protein
2.9. BM-DC Antigen Pulsing and Ex Vivo Antigen Stimulation of PBMCs
2.10. Statistical Analysis
3. Results
3.1. BM-DC MHCII Surface Expression Is Up Regulated by Pustulan, Mannan, and Chitosan
3.2. Pustulan and Mannan Induce a Gene Expression of Proinflammatory Cytokines
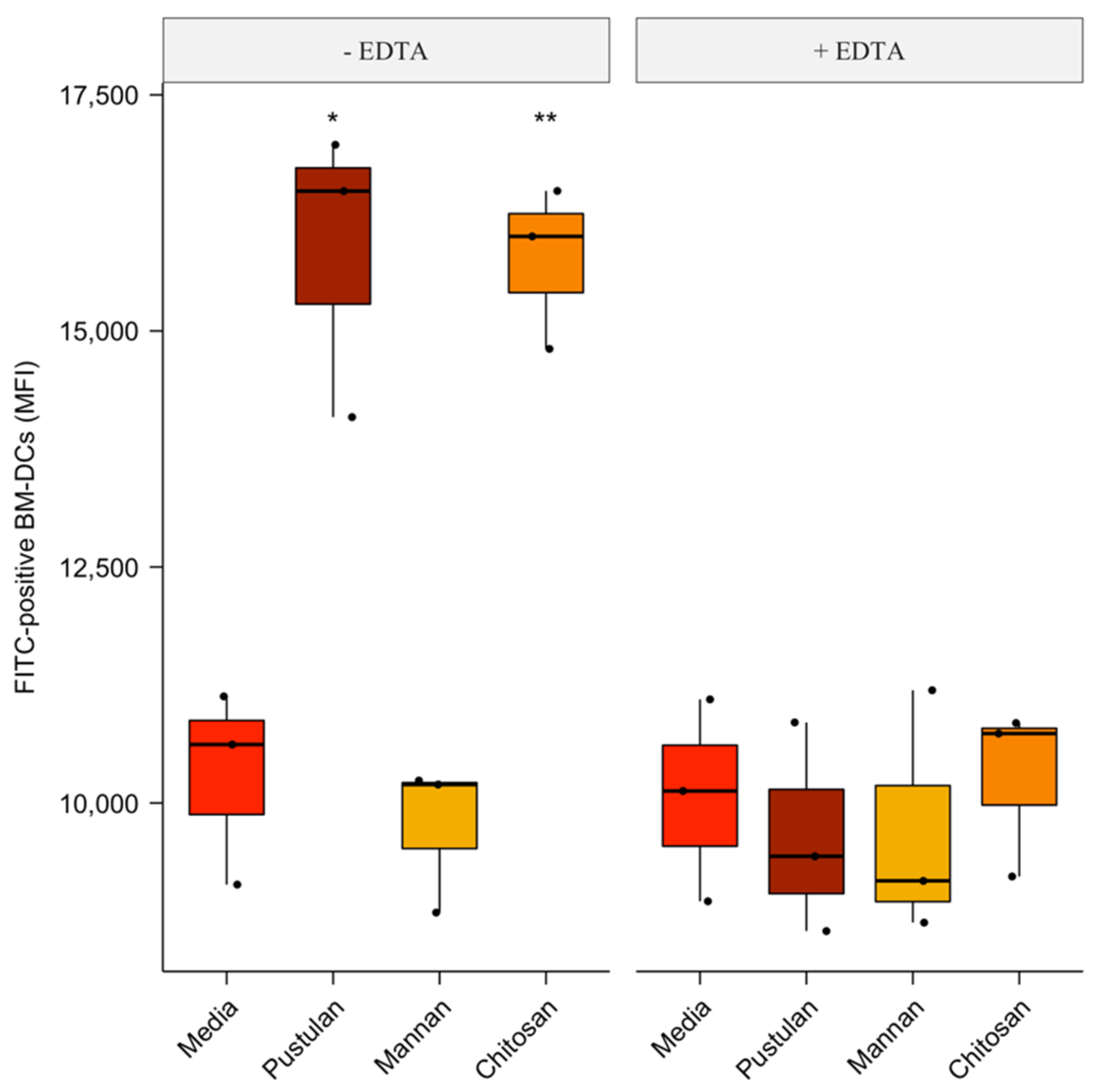
3.3. Cation-Dependent Increase in Endocytosis of FITC-Labelled BSA by Pustulan and Chitosan
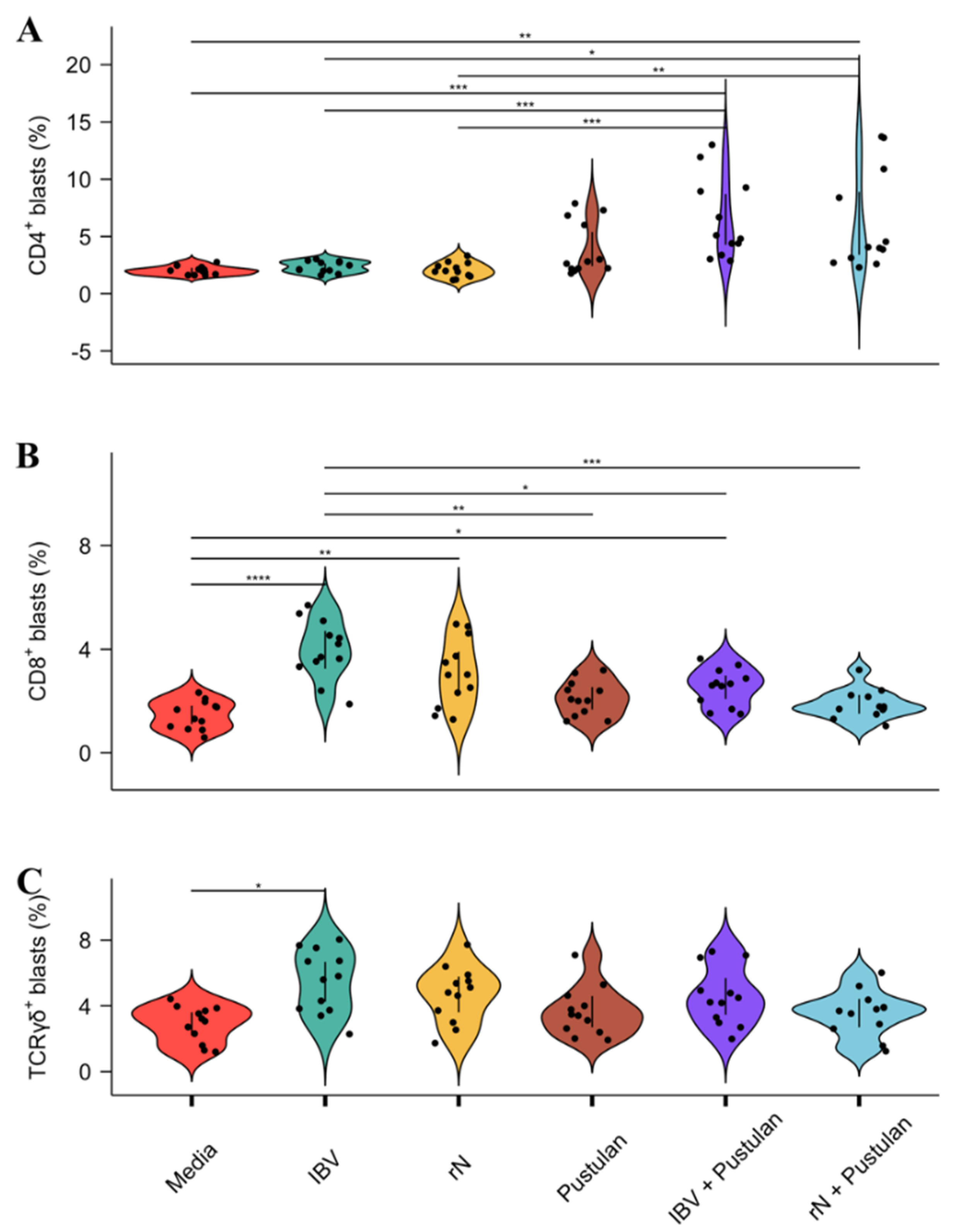
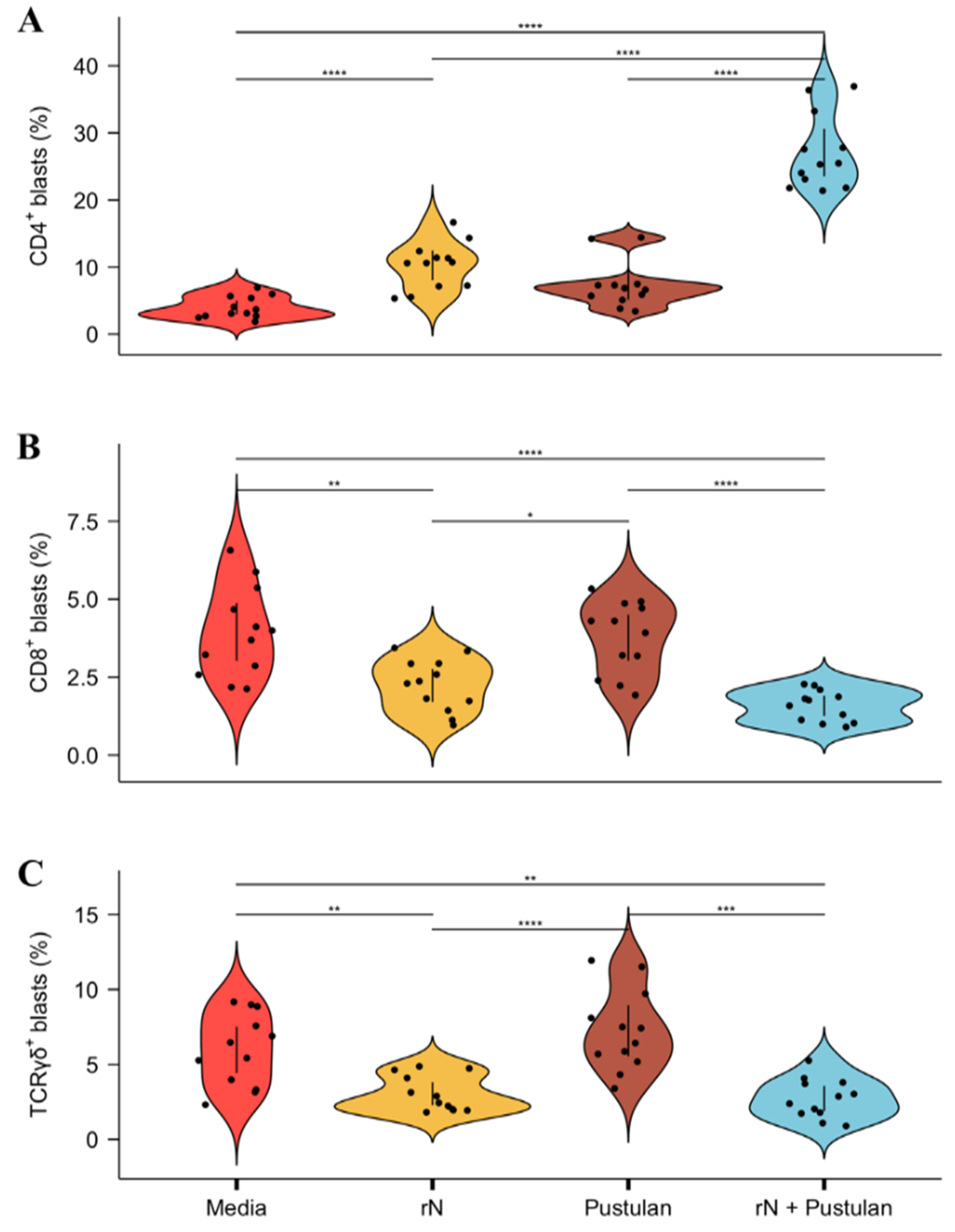
3.4. Pustulan Enhances Activation of Recall Antigen-Specific CD4+ But Not CD8+ T Cells in PBMC Cultures
3.5. Pustulan Augments BM-DC-Induced Activation of Recall Antigen-Specific CD4+ T Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jordan, B. Vaccination against infectious bronchitis virus: A continuous challenge. Vet. Microbiol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.K.A.; Davison, T.F.; Huggins, M.B.; McLaughlan, P. Effect of in ovo bursectomy on the course of an infectious-bronchitis virus-infection in line-c white leghorn chickens. Arch. Virol. 1991, 118, 225–234. [Google Scholar] [CrossRef] [PubMed]
- da Silva, A.P.; Hauck, R.; Zhou, H.; Gallardo, R.A. Understanding Immune Resistance to Infectious Bronchitis Using Major Histocompatibility Complex Chicken Lines. Avian Dis. 2017, 61, 358–365. [Google Scholar] [CrossRef]
- Gelb, J.; Nix, W.A.; Gellman, S.D. Infectious bronchitis virus antibodies in tears and their relationship to immunity. Avian Dis. 1998, 42, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Gough, R.E.; Alexander, D.J. Comparison of duration of immunity in chickens infected with a live infectious-bronchitis vaccine by 3 different routes. Res. Vet. Sci. 1979, 26, 329–332. [Google Scholar] [CrossRef]
- Hanley, K.A. The double-edged sword: How evolution can make or break a live-attenuated virus vaccine. Evol. Educ. Outreach 2011, 4, 635–643. [Google Scholar] [CrossRef] [Green Version]
- Ladman, B.S.; Pope, C.R.; Ziegler, A.F.; Swieczkowski, T.; Callahan, J.M.; Davison, S.; Gelb, J. Protection of chickens after live and inactivated virus vaccination against Challenge with nephropathogenic infectious bronchitis virus PA/Wolgemuth/98. Avian Dis. 2002, 46, 938–944. [Google Scholar] [CrossRef] [Green Version]
- Collisson, E.W.; Pei, J.W.; Dzielawa, J.; Seo, S.H. Cytotoxic T lymphocytes are critical in the control of infectious bronchitis virus in poultry. Dev. Comp. Immunol. 2000, 24, 187–200. [Google Scholar] [CrossRef]
- Carvalho, A.; Giovannini, G.; De Luca, A.; D’Angelo, C.; Casagrande, A.; Iannitti, R.G.; Ricci, G.; Cunha, C.; Romani, L. Dectin-1 isoforms contribute to distinct Th1/Th17 cell activation in mucosal candidiasis. Cell Mol. Immunol. 2012, 9, 276–286. [Google Scholar] [CrossRef] [Green Version]
- Petrovsky, N.; Cooper, P.D. Carbohydrate-based immune adjuvants. Expert Rev. Vaccines 2011, 10, 523–537. [Google Scholar] [CrossRef]
- Singh, S.K.; Streng-Ouwehand, I.; Litjens, M.; Kalay, H.; Saeland, E.; van Kooyk, Y. Tumour-associated glycan modifications of antigen enhance MGL2 dependent uptake and MHC class I restricted CD8 T cell responses. Int. J. Cancer 2011, 128, 1371–1383. [Google Scholar] [CrossRef] [PubMed]
- Tacken, P.J.; Figdor, C.G. Targeted antigen delivery and activation of dendritic cells in vivo: Steps towards cost effective vaccines. Semin. Immunol. 2011, 23, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Kopp, E.B.; Medzhitov, R. The Toll-receptor family and control of innate immunity. Curr. Opin. Immunol. 1999, 11, 13–18. [Google Scholar] [CrossRef]
- Zhong, Y.; Kinio, A.; Saleh, M. Functions of NOD-like receptors in human diseases. Front. Immunol. 2013, 4, 333. [Google Scholar] [CrossRef] [Green Version]
- Weber, F. The catcher in the RIG-I. Cytokine 2015, 76, 38–41. [Google Scholar] [CrossRef]
- Cai, X.; Chiu, Y.H.; Chen, Z.J. The cGAS-cGAMP-STING pathway of cytosolic DNA sensing and signaling. Mol. Cell 2014, 54, 289–296. [Google Scholar] [CrossRef] [Green Version]
- Osorio, F.; Reis e Sousa, G. Myeloid C-type Lectin Receptors in Pathogen Recognition and Host Defense. Immunity 2011, 34, 651–664. [Google Scholar] [CrossRef] [Green Version]
- Weis, W.I.; Taylor, M.E.; Drickamer, K. The C-type lectin superfamily in the immune system. Immunol. Rev. 1998, 163, 19–34. [Google Scholar] [CrossRef]
- Johannssen, T.; Lepenies, B. Glycan-Based Cell Targeting To Modulate Immune Responses. Trends Biotechnol. 2017, 35, 334–346. [Google Scholar] [CrossRef] [Green Version]
- Lehmann, C.H.K.; Heger, L.; Heidkamp, G.F.; Baranska, A.; Luhr, J.J.; Hoffmann, A.; Dudziak, D. Direct Delivery of Antigens to Dendritic Cells via Antibodies Specific for Endocytic Receptors as a Promising Strategy for Future Therapies. Vaccines 2016, 4, 8. [Google Scholar] [CrossRef]
- Chari, R.V. Targeted cancer therapy: Conferring specificity to cytotoxic drugs. Acc. Chem. Res. 2008, 41, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Fasting, C.; Schalley, C.A.; Weber, M.; Seitz, O.; Hecht, S.; Koksch, B.; Dernedde, J.; Graf, C.; Knapp, E.W.; Haag, R. Multivalency as a chemical organization and action principle. Angew. Chem. Int. Ed. 2012, 51, 10472–10498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, S.K.; Stephani, J.; Schaefer, M.; Kalay, H.; Garcia-Vallejo, J.J.; den Haan, J.; Saeland, E.; Sparwasser, T.; van Kooyk, Y. Targeting glycan modified OVA to murine DC-SIGN transgenic dendritic cells enhances MHC class I and II presentation. Mol. Immunol. 2009, 47, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Vallejo, J.J.; Ambrosini, M.; Overbeek, A.; van Riel, W.E.; Bloem, K.; Unger, W.W.; Chiodo, F.; Bolscher, J.G.; Nazmi, K.; Kalay, H.; et al. Multivalent glycopeptide dendrimers for the targeted delivery of antigens to dendritic cells. Mol. Immunol. 2013, 53, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Unger, W.W.; van Beelen, A.J.; Bruijns, S.C.; Joshi, M.; Fehres, C.M.; van Bloois, L.; Verstege, M.I.; Ambrosini, M.; Kalay, H.; Nazmi, K.; et al. Glycan-modified liposomes boost CD4+ and CD8+ T-cell responses by targeting DC-SIGN on dendritic cells. J. Control. Release 2012, 160, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Brzezicka, K.; Vogel, U.; Serna, S.; Johannssen, T.; Lepenies, B.; Reichardt, N.C. Influence of Core beta-1,2-Xylosylation on Glycoprotein Recognition by Murine C-type Lectin Receptors and Its Impact on Dendritic Cell Targeting. ACS Chem. Biol. 2016, 11, 2347–2356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jauregui-Zuniga, D.; Pedraza-Escalona, M.; Espino-Solis, G.P.; Quintero-Hernandez, V.; Olvera-Rodriguez, A.; Diaz-Salinas, M.A.; Lopez, S.; Possani, L.D. Targeting antigens to Dec-205 on dendritic cells induces a higher immune response in chickens: Hemagglutinin of avian influenza virus example. Res. Vet. Sci. 2017, 111, 55–62. [Google Scholar] [CrossRef]
- Jauregui-Zuniga, D.; Pedraza-Escalona, M.; Merino-Guzman, R.; Possani, L.D. Construction and expression of a single-chain variable fragment antibody against chicken DEC 205 for targeting the bacterial expressed hemagglutinin-neuraminidase of Newcastle disease virus. Vet. Immunol. Immunopathol. 2019, 212, 9–14. [Google Scholar] [CrossRef]
- de Geus, E.D.; Tefsen, B.; van Haarlem, D.A.; van Eden, W.; van Die, I.; Vervelde, L. Glycans from avian influenza virus are recognized by chicken dendritic cells and are targets for the humoral immune response in chicken. Mol. Immunol. 2013, 56, 452–462. [Google Scholar] [CrossRef]
- Andersen, C.S.; Agger, E.M.; Rosenkrands, I.; Gomes, J.M.; Bhowruth, V.; Gibson, K.J.C.; Petersen, R.V.; Minnikin, D.E.; Besra, G.S.; Andersen, P. A Simple Mycobacterial Monomycolated Glycerol Lipid Has Potent Immunostimulatory Activity. J. Immunol. 2009, 182, 424–432. [Google Scholar] [CrossRef]
- Ishikawa, T.; Itoh, F.; Yoshida, S.; Saijo, S.; Matsuzawa, T.; Gonoi, T.; Saito, T.; Okawa, Y.; Shibata, N.; Miyamoto, T.; et al. Identification of Distinct Ligands for the C-type Lectin Receptors Mincle and Dectin-2 in the Pathogenic Fungus Malassezia. Cell Host Microbe 2013, 13, 477–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, K.; Yang, X.-l.; Yudate, T.; Chung, J.-S.; Wu, J.; Luby-Phelps, K.; Kimberly, R.P.; Underhill, D.; Cruz, P.D., Jr.; Ariizumi, K. Dectin-2 is a pattern recognition receptor for fungi that couples with the Fc receptor gamma chain to induce innate immune responses. J. Biol. Chem. 2006, 281, 38854–38866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noss, I.; Doekes, G.; Thorne, P.S.; Heederik, D.J.; Wouters, I.M. Comparison of the potency of a variety of beta-glucans to induce cytokine production in human whole blood. Innate Immun. 2013, 19, 10–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheng, K.C.; Pouniotis, D.S.; Wright, M.D.; Tang, C.K.; Lazoura, E.; Pietersz, G.A.; Apostolopoulos, V. Mannan derivatives induce phenotypic and functional maturation of mouse dendritic cells. Immunology 2006, 118, 372–383. [Google Scholar] [CrossRef] [PubMed]
- McConnell, S.K.J.; Dawson, D.A.; Wardle, A.; Burke, T. The isolation and mapping of 19 tetranucleotide microsatellite markers in the chicken. Anim. Genet. 1999, 30, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Dalgaard, T.S.; Vitved, L.; Skjodt, K.; Thomsen, B.; Labouriau, R.; Jensen, K.H.; Juul-Madsen, H.R. Molecular characterization of major histocompatibility complex class I (B-F) mRNA variants from chickens differing in resistance to Marek’s disease. Scand J. Immunol. 2005, 62, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Ravindranathan, S.; Koppolu, B.P.; Smith, S.G.; Zaharoff, D.A. Effect of Chitosan Properties on Immunoreactivity. Mar. Drugs 2016, 14, 91. [Google Scholar] [CrossRef] [Green Version]
- Lew, D.B.; LeMessurier, K.S.; Palipane, M.; Lin, Y.; Samarasinghe, A.E. Saccharomyces cerevisiae-Derived Mannan Does Not Alter Immune Responses to Aspergillus Allergens. Biomed Res. Int. 2018, 2018, 3298378. [Google Scholar] [CrossRef] [Green Version]
- Andersen, C.L.; Jensen, J.L.; Orntoft, T.F. Normalization of real-time quantitative reverse transcription-PCR data: A model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 2004, 64, 5245–5250. [Google Scholar] [CrossRef] [Green Version]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Meir, R.; Krispel, S.; Simanov, L.; Eliahu, D.; Maharat, O.; Pitcovski, J. Immune Responses to Mucosal Vaccination by the Recombinant S1 and N Proteins of Infectious Bronchitis Virus. Viral Immunol. 2012, 25, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Andersen, S.H.; Vervelde, L.; Sutton, K.; Norup, L.R.; Wattrang, E.; Juul-Madsen, H.R.; Dalgaard, T.S. Quantification and phenotypic characterisation of peripheral IFN-gamma producing leucocytes in chickens vaccinated against Newcastle disease. Vet. Immunol. Immunopathol. 2017, 193, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Carrio, R.; Zhang, G.; Drake, D.R.; Schanen, B.C. A novel dendritic cell-based direct ex vivo assay for detection and enumeration of circulating antigen-specific human T cells. Cytotechnology 2018, 70, 1325–1335. [Google Scholar] [CrossRef] [PubMed]
- Kassambara, A. Ggpubr: ‘ggplot2’ Based Publication Ready Plots. 2017. Available online: https://github.com/kassambara/ggpubr (accessed on 17 February 2020).
- van den Biggelaar, R.H.G.A.; Arkesteijn, G.J.A.; Rutten, V.P.M.G.; van Eden, W.; Jansen, C.A. In vitro Chicken Bone Marrow-Derived Dendritic Cells Comprise Subsets at Different States of Maturation. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef]
- Helft, J.; Bottcher, J.; Chakravarty, P.; Zelenay, S.; Huotari, J.; Schraml, B.U.; Goubau, D.; Sousa, C.R.E. GM-CSF Mouse Bone Marrow Cultures Comprise a Heterogeneous Population of CD11c(+)MHCII(+) Macrophages and Dendritic Cells. Immunity 2015, 42, 1197–1211. [Google Scholar] [CrossRef] [Green Version]
- Yao, L.L.; Bai, L.; Tan, Y.; Sun, J.Q.; Qu, Q.; Shi, D.Y.; Guo, S.N.; Liu, C. The immunoregulatory effect of sulfated Echinacea purpurea polysaccharide on chicken bone marrow-derived dendritic cells. Int. J. Biol. Macromol. 2019, 139, 1123–1132. [Google Scholar] [CrossRef]
- Liang, J.F.; Yin, Y.Y.; Qin, T.; Yang, Q. Chicken bone marrow-derived dendritic cells maturation in response to infectious bursal disease virus. Vet. Immunol. Immunopathol. 2015, 164, 51–55. [Google Scholar] [CrossRef]
- Chakraborty, P.; Vervelde, L.; Dalziel, R.G.; Wasson, P.S.; Nair, V.; Dutia, B.M.; Kaiser, P. Marek’s disease virus infection of phagocytes: A de novo in vitro infection model. J. Gen. Virol. 2017, 98, 1080–1088. [Google Scholar] [CrossRef]
- Wu, Z.; Rothwell, L.; Young, J.R.; Kaufman, J.; Butter, C.; Kaiser, P. Generation and characterization of chicken bone marrow-derived dendritic cells. Immunology 2010, 129, 133–145. [Google Scholar] [CrossRef]
- Yasmin, A.R.; Yeap, S.K.; Tan, S.W.; Hair-Bejo, M.; Fakurazi, S.; Kaiser, P.; Omar, A.R. In vitro characterization of chicken bone marrow-derived dendritic cells following infection with very virulent infectious bursal disease virus. Avian Pathol. 2015, 44, 452–462. [Google Scholar] [CrossRef] [Green Version]
- Morelli, A.E.; Zahorchak, A.F.; Larregina, A.T.; Colvin, B.L.; Logar, A.J.; Takayama, T.; Falo, L.D.; Thomson, A.W. Cytokine production by mouse myeloid dendritic cells in relation to differentiation and terminal maturation induced by lipopolysaccharide or CD40 ligation. Blood 2001, 98, 1512–1523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geijtenbeek, T.B.; Gringhuis, S.I. Signalling through C-type lectin receptors: Shaping immune responses. Nat. Rev. Immunol. 2009, 9, 465–479. [Google Scholar] [CrossRef] [PubMed]
- Larsen, F.T.; Bed’Hom, B.; Guldbrandtsen, B.; Dalgaard, T.S. Identification and tissue-expression profiling of novel chicken c-type lectin-like domain containing proteins as potential targets for carbohydrate-based vaccine strategies. Mol. Immunol. 2019, 114, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Mathiesen, R.; Eld, H.M.S.; Sorensen, J.; Fuglsang, E.; Lund, L.D.; Taverniti, V.; Frokiaer, H. Mannan Enhances IL-12 Production by Increasing Bacterial Uptake and Endosomal Degradation in L. acidophilus and S. aureus Stimulated Dendritic Cells. Front. Immunol. 2019, 10, 2646. [Google Scholar] [CrossRef] [Green Version]
- Abel, G.; Szollosi, J.; Chihara, G.; Fachet, J. Effect of lentinan and mannan on phagocytosis of fluorescent latex microbeads by mouse peritoneal macrophages: A flow cytometric study. Int. J. Immunopharmacol. 1989, 11, 615–621. [Google Scholar] [CrossRef]
- Willment, J.A.; Gordon, S.; Brown, G.D. Characterization of the human beta-glucan receptor and its alternatively spliced isoforms. J. Biol. Chem. 2001, 276, 43818–43823. [Google Scholar] [CrossRef] [Green Version]
- Drummond, R.A.; Brown, G.D. The role of Dectin-1 in the host defence against fungal infections. Curr. Opin. Microbiol. 2011, 14, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Rogers, N.C.; Slack, E.C.; Edwards, A.D.; Nolte, M.A.; Schulz, O.; Schweighoffer, E.; Williams, D.L.; Gordon, S.; Tybulewicz, V.L.; Brown, G.D.; et al. Syk-dependent cytokine induction by Dectin-1 reveals a novel pattern recognition pathway for C type lectins. Immunity 2005, 22, 507–517. [Google Scholar] [CrossRef] [Green Version]
- LeibundGut-Landmann, S.; Gross, O.; Robinson, M.J.; Osorio, F.; Slack, E.C.; Tsoni, S.V.; Schweighoffer, E.; Tybulewicz, V.; Brown, G.D.; Ruland, J.; et al. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat. Immunol. 2007, 8, 630–638. [Google Scholar] [CrossRef]
- Rubin-Bejerano, I.; Abeijon, C.; Magnelli, P.; Grisafi, P.; Fink, G.R. Phagocytosis by human neutrophils is stimulated by a unique fungal cell wall component. Cell Host Microbe 2007, 2, 55–67. [Google Scholar] [CrossRef] [Green Version]
- Ganesan, S.; Rathinam, V.A.K.; Bossaller, L.; Army, K.; Kaiser, W.J.; Mocarski, E.S.; Dillon, C.P.; Green, D.R.; Mayadas, T.N.; Levitz, S.M.; et al. Caspase-8 Modulates Dectin-1 and Complement Receptor 3-Driven IL-1 beta Production in Response to beta-Glucans and the Fungal Pathogen, Candida albicans. J. Immunol. 2014, 193, 2519–2530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lebron, F.; Vassallo, R.; Puri, V.; Limper, A.H. Pneumocystis carinii cell wall beta-glucans initiate macrophage inflammatory responses through NF-kappa B activation. J. Biol. Chem. 2003, 278, 25001–25008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, J.; O’Callaghan, C.A.; Marshall, A.S.; Gilbert, R.J.; Siebold, C.; Gordon, S.; Brown, G.D.; Jones, E.Y. Structure of the fungal beta-glucan-binding immune receptor dectin-1: Implications for function. Protein Sci. 2007, 16, 1042–1052. [Google Scholar] [CrossRef]
- Botos, I.; Segal, D.M.; Davies, D.R. The structural biology of Toll-like receptors. Structure 2011, 19, 447–459. [Google Scholar] [CrossRef] [Green Version]
- Thornton, B.P.; Vetvicka, V.; Pitman, M.; Goldman, R.C.; Ross, G.D. Analysis of the sugar specificity and molecular location of the beta-glucan-binding lectin site of complement receptor type 3 (CD11b/CD18). J. Immunol. 1996, 156, 1235–1246. [Google Scholar] [PubMed]
- Bornelov, S.; Seroussi, E.; Yosefi, S.; Pendavis, K.; Burgess, S.C.; Grabherr, M.; Friedman-Einat, M.; Andersson, L. Correspondence on Lovell et al.: Identification of chicken genes previously assumed to be evolutionarily lost. Genome Biol. 2017, 18, 112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norup, L.R.; Dalgaard, T.S.; Pedersen, A.R.; Juul-Madsen, H.R. Assessment of Newcastle Disease-Specific T Cell Proliferation in Different Inbred MHC Chicken Lines. Scand. J. Immunol. 2011, 74, 23–30. [Google Scholar] [CrossRef]
- Pei, J.W.; Collisson, E.W. Specific antibody secreting cells from chickens can be detected by three days and memory B cells by three weeks post-infection with the avian respiratory coronavirus. Dev. Comp. Immunol. 2005, 29, 153–160. [Google Scholar] [CrossRef]
- Embgenbroich, M.; Burgdorf, S. Current Concepts of Antigen Cross-Presentation. Front. Immunol. 2018, 9, 1643. [Google Scholar] [CrossRef] [Green Version]
- Fehres, C.M.; Kalay, H.; Bruijns, S.C.M.; Musaafir, S.A.M.; Ambrosini, M.; van Bloois, L.; van Vliet, S.J.; Storm, G.; Garcia-Vallejo, J.J.; van Kooyk, Y. Cross-presentation through langerin and DC-SIGN targeting requires different formulations of glycan-modified antigens. J. Control. Release 2015, 203, 67–76. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Larsen, F.T.; Guldbrandtsen, B.; Christensen, D.; Pitcovski, J.; Kjærup, R.B.; Dalgaard, T.S. Pustulan Activates Chicken Bone Marrow-Derived Dendritic Cells In Vitro and Promotes Ex Vivo CD4+ T Cell Recall Response to Infectious Bronchitis Virus. Vaccines 2020, 8, 226. https://doi.org/10.3390/vaccines8020226
Larsen FT, Guldbrandtsen B, Christensen D, Pitcovski J, Kjærup RB, Dalgaard TS. Pustulan Activates Chicken Bone Marrow-Derived Dendritic Cells In Vitro and Promotes Ex Vivo CD4+ T Cell Recall Response to Infectious Bronchitis Virus. Vaccines. 2020; 8(2):226. https://doi.org/10.3390/vaccines8020226
Chicago/Turabian StyleLarsen, Frederik T., Bernt Guldbrandtsen, Dennis Christensen, Jacob Pitcovski, Rikke B. Kjærup, and Tina S. Dalgaard. 2020. "Pustulan Activates Chicken Bone Marrow-Derived Dendritic Cells In Vitro and Promotes Ex Vivo CD4+ T Cell Recall Response to Infectious Bronchitis Virus" Vaccines 8, no. 2: 226. https://doi.org/10.3390/vaccines8020226
APA StyleLarsen, F. T., Guldbrandtsen, B., Christensen, D., Pitcovski, J., Kjærup, R. B., & Dalgaard, T. S. (2020). Pustulan Activates Chicken Bone Marrow-Derived Dendritic Cells In Vitro and Promotes Ex Vivo CD4+ T Cell Recall Response to Infectious Bronchitis Virus. Vaccines, 8(2), 226. https://doi.org/10.3390/vaccines8020226





