Uncovering Distinct Primary Vaccination-Dependent Profiles in Human Bordetella pertussis Specific CD4+ T-Cell Responses Using a Novel Whole Blood Assay
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Study Population and Booster Vaccination
2.3. Whole Blood Sampling
2.4. Antigens, Peptides and Co-Stimulants
2.5. PerfectCount and CD4+ Euroflow T-Cell Tubes
2.6. Whole Blood Stimulation
2.7. Processing Whole Blood
2.8. Staining of the Cells for Flow Cytometry and Acquisition of the Data
2.9. Multiplex Cytokine Assay
2.10. Data Analysis
3. Results
3.1. Study Design to Evaluate Bp Specific CD4+ T-Cell Immunity in a Whole Blood Assay
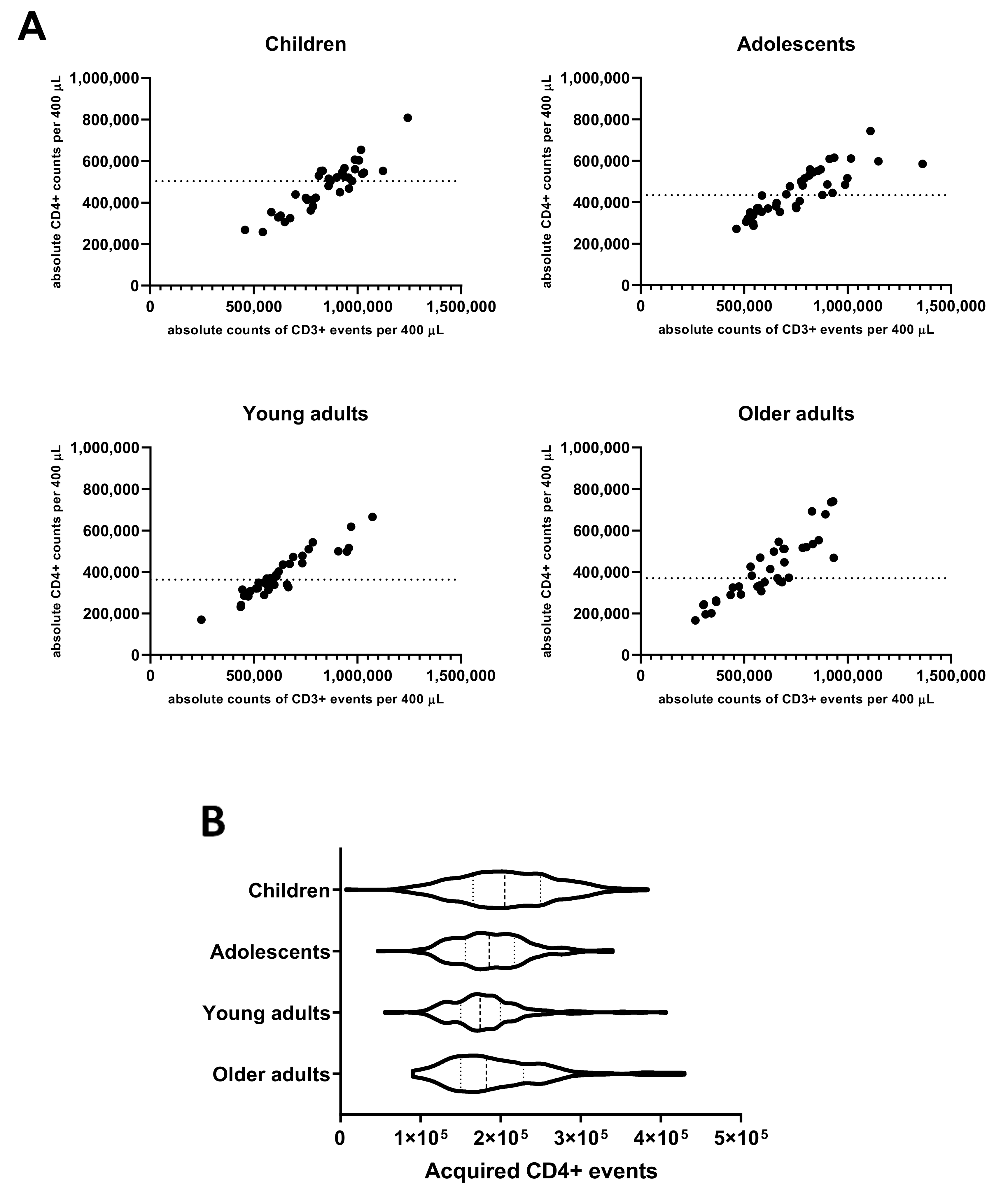
3.2. CD4+ T-Cell Numbers in Cultured Whole Blood Samples Are Adequate for a Reliable Analysis of Rare Events in All Age Cohorts
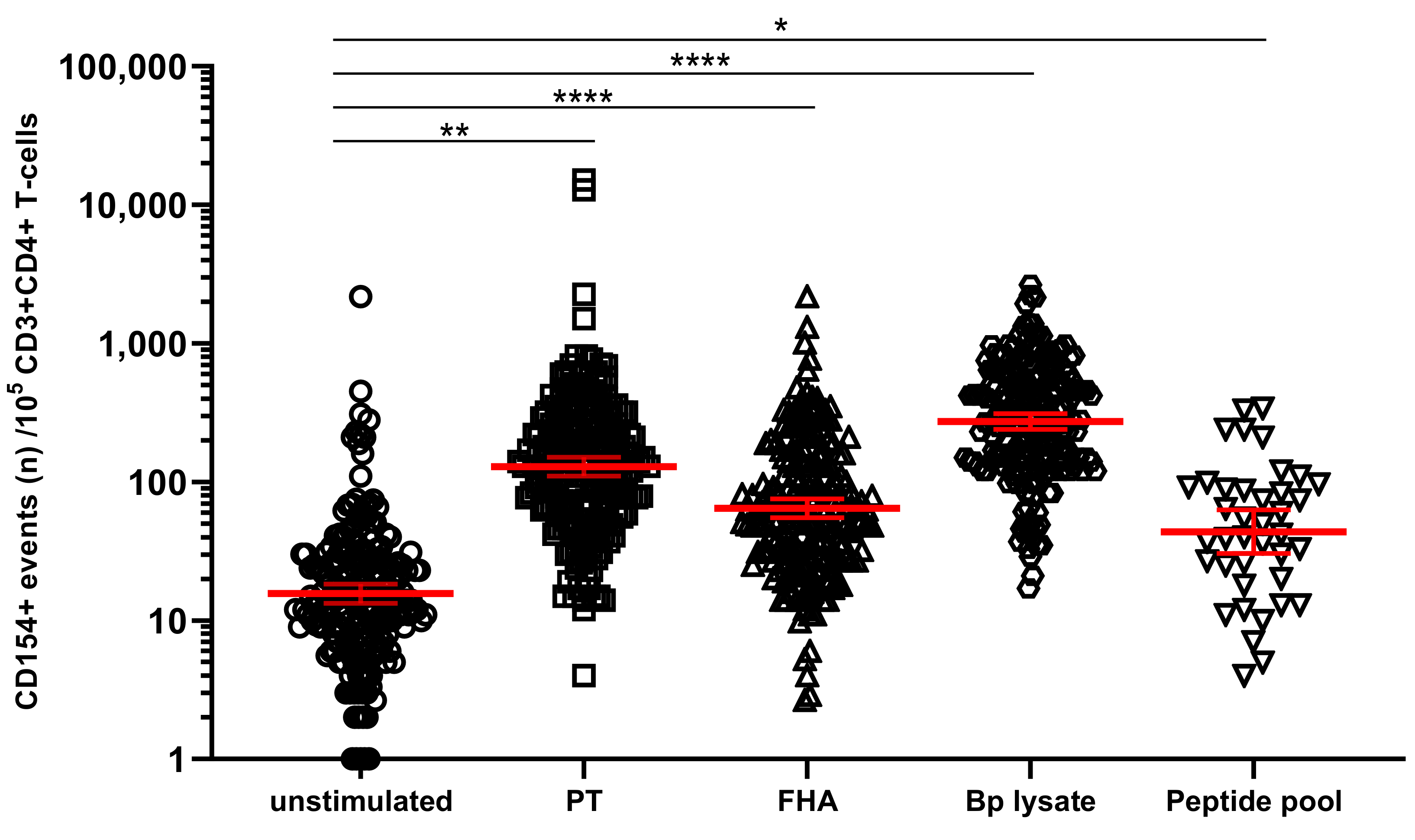
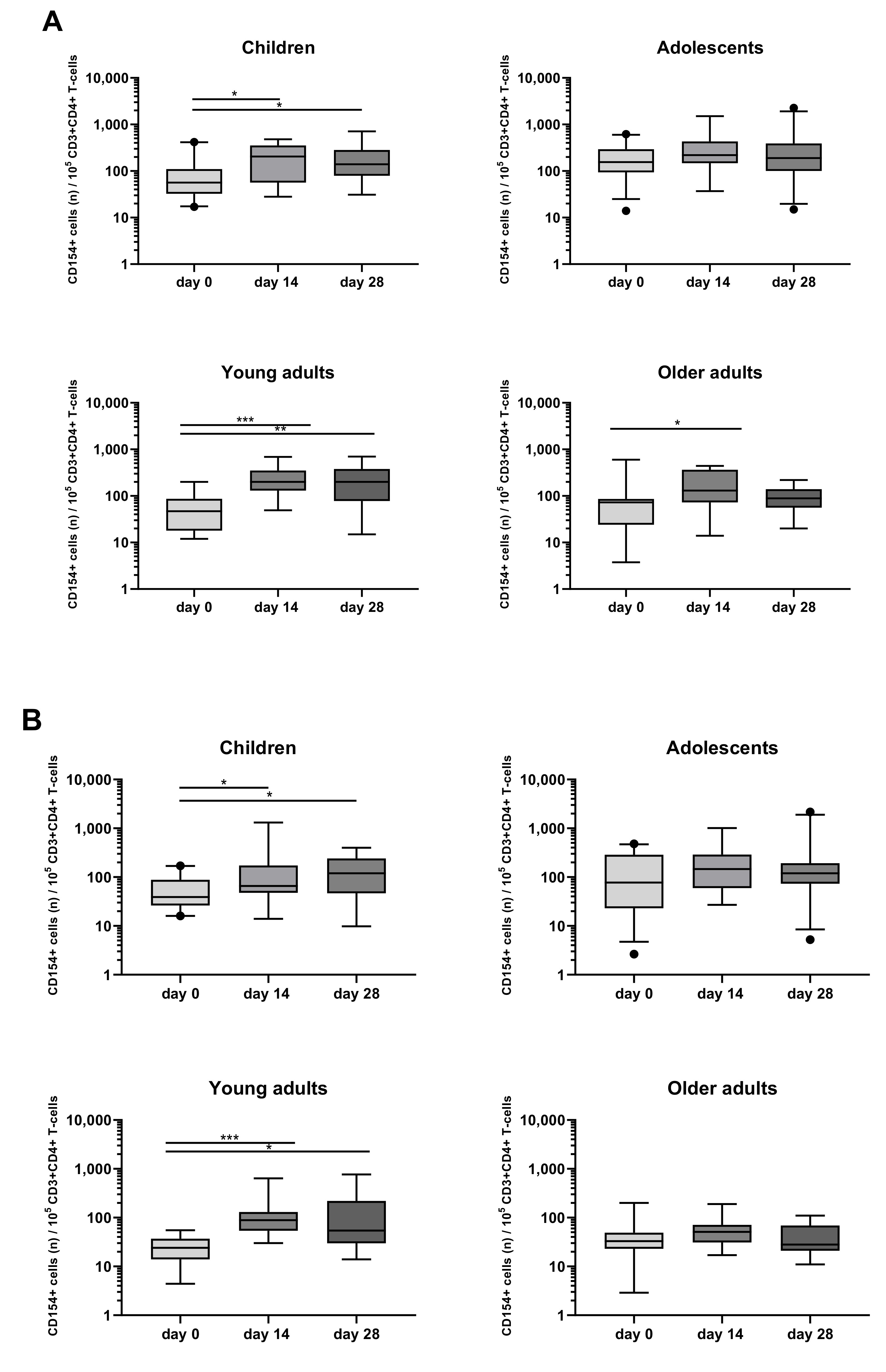
3.3. Detection and Monitoring of Bp Specific CD4+ T-Cell Responses Based on CD154 Expression
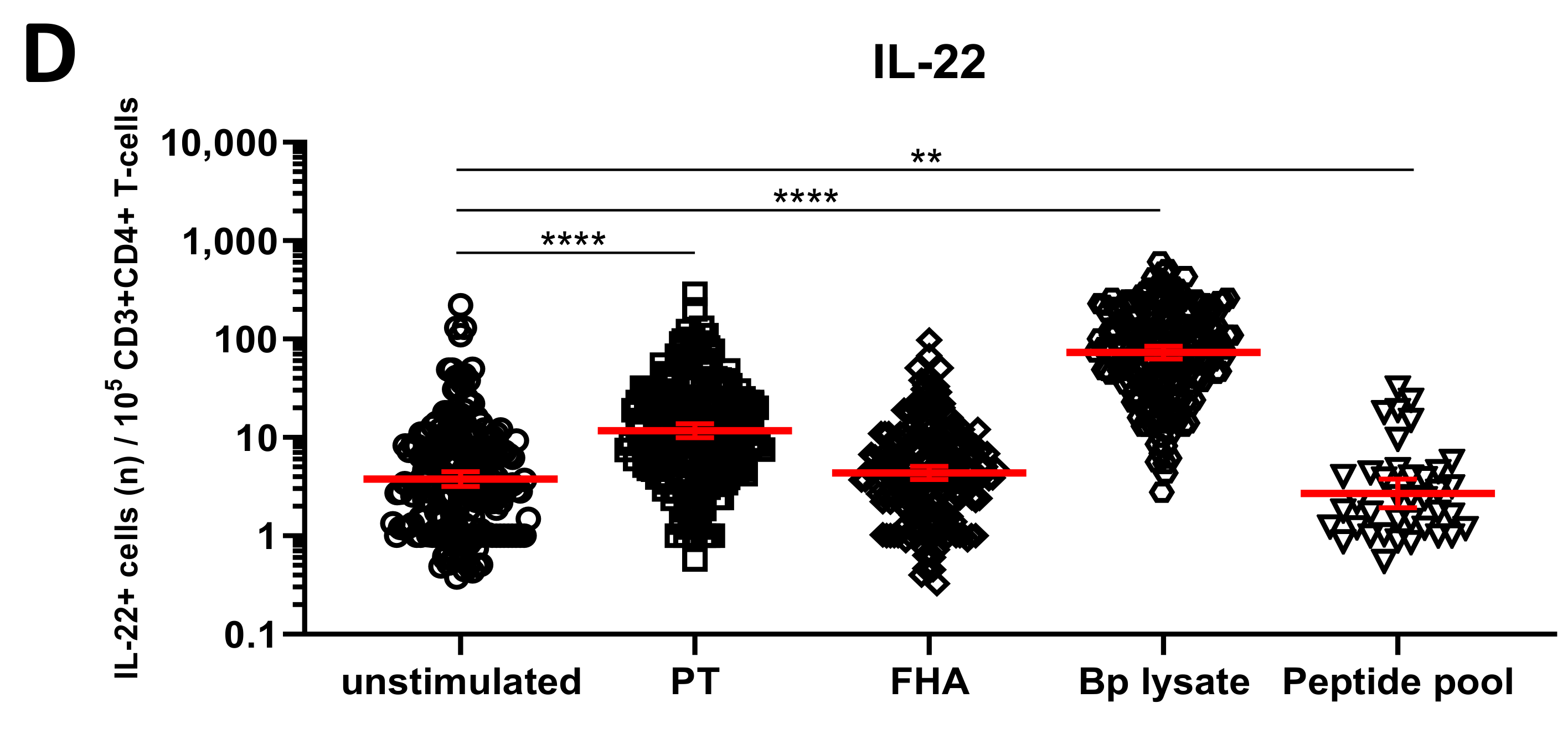
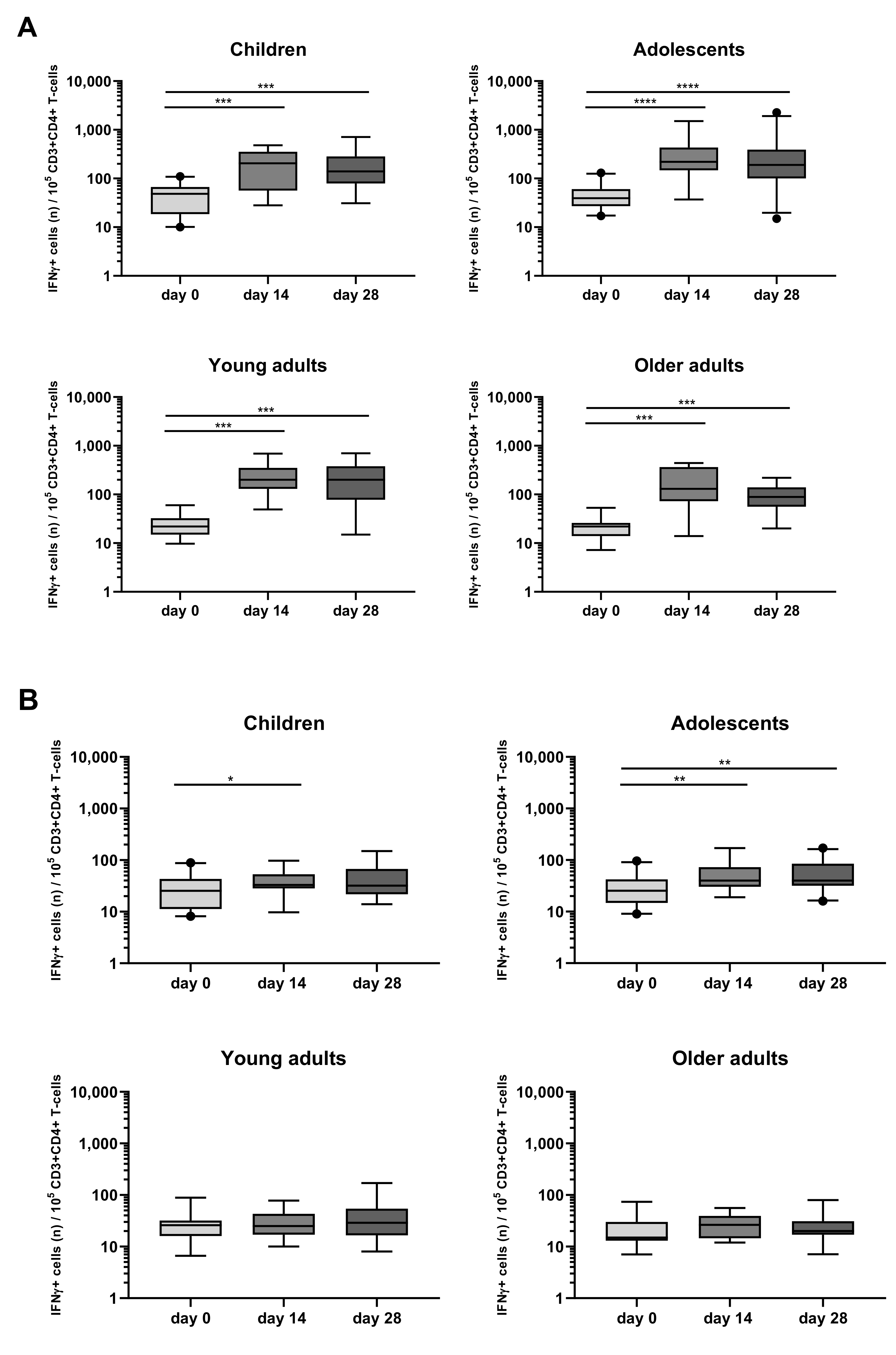
3.4. Detection of Bp Specific CD4+ T-Cells by Their Intracellular Cytokine Profile
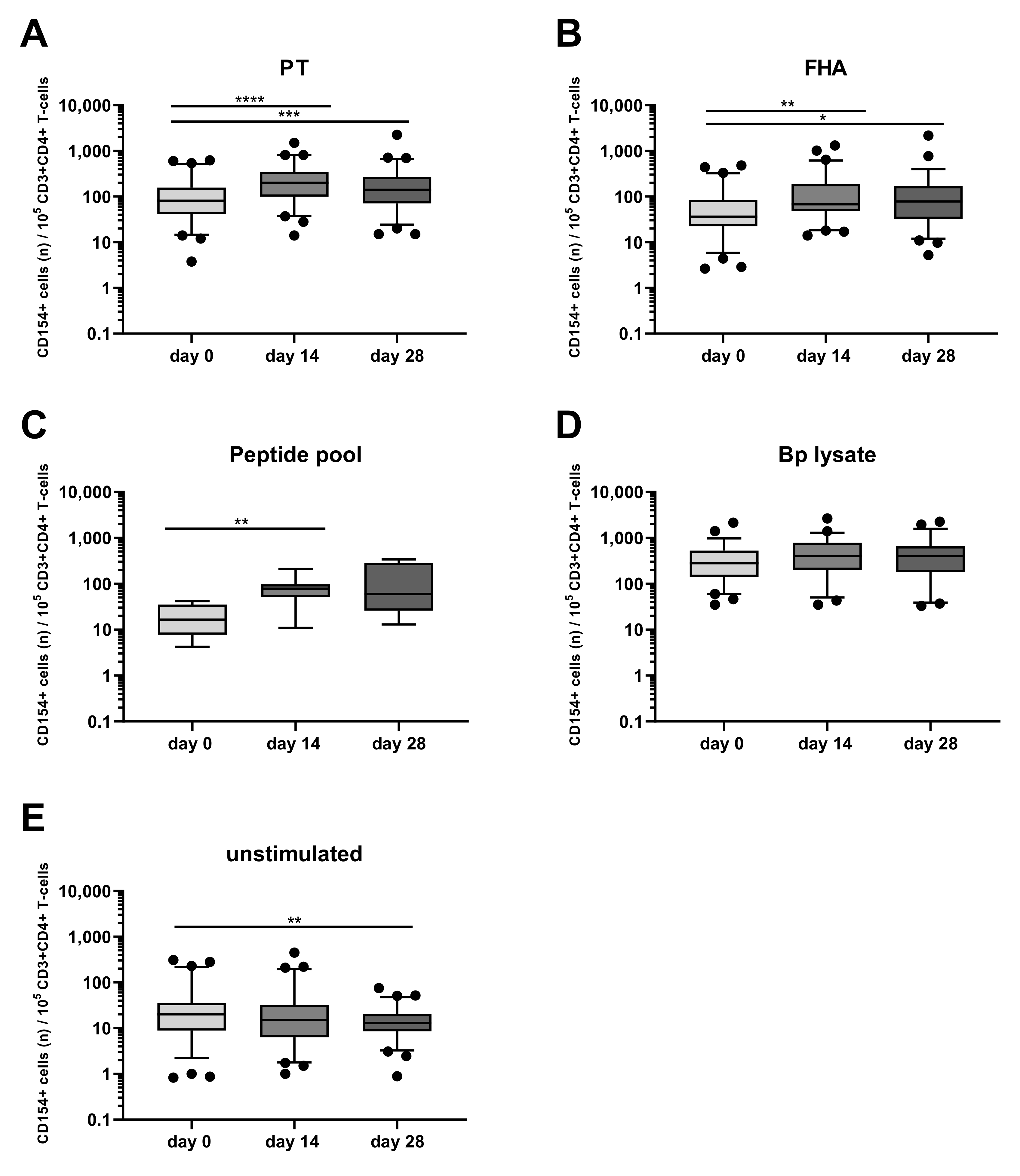
3.5. Kinetics of the Bp Specific CD4+ T-Cells after an aP Booster Vaccination Per Age Cohort
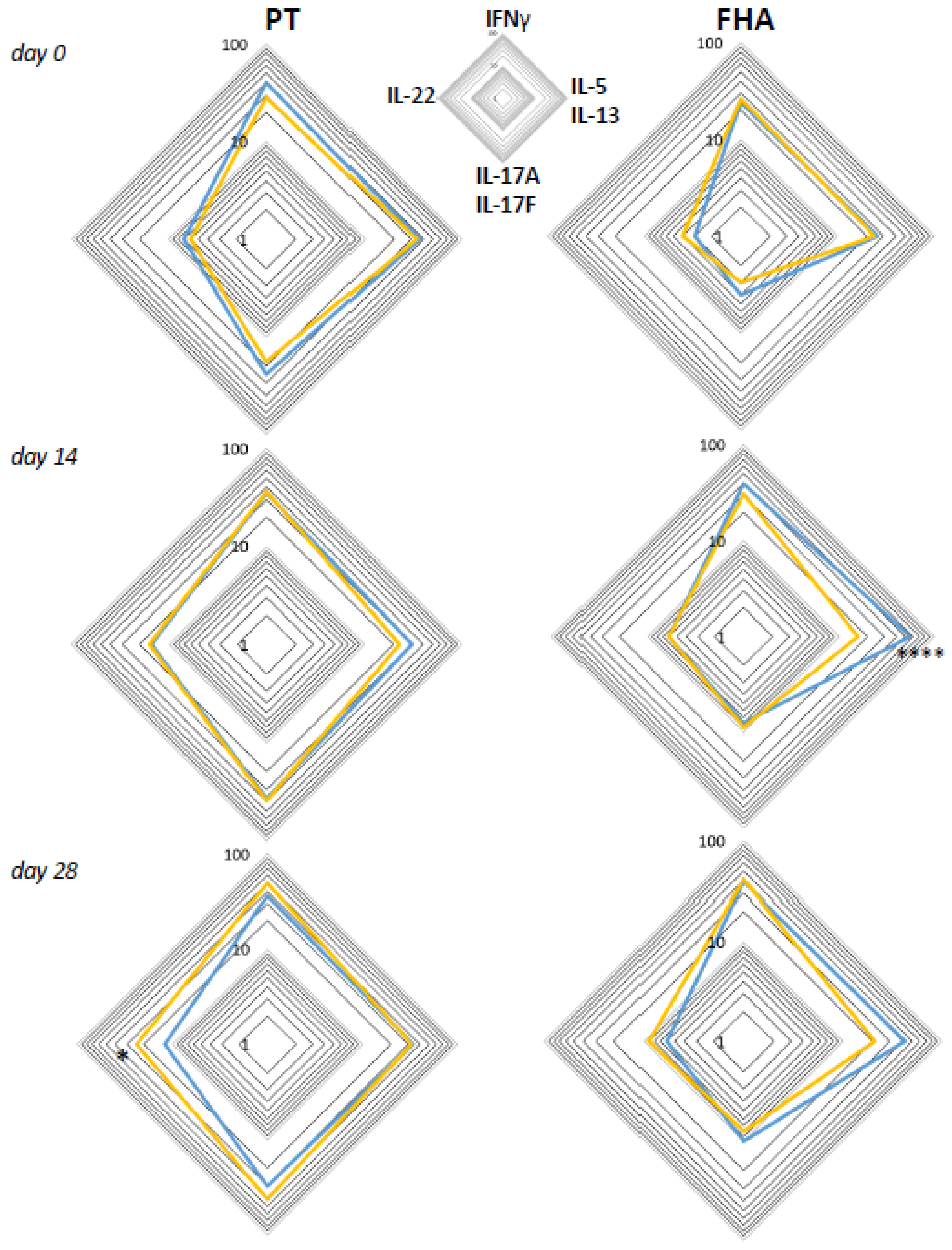
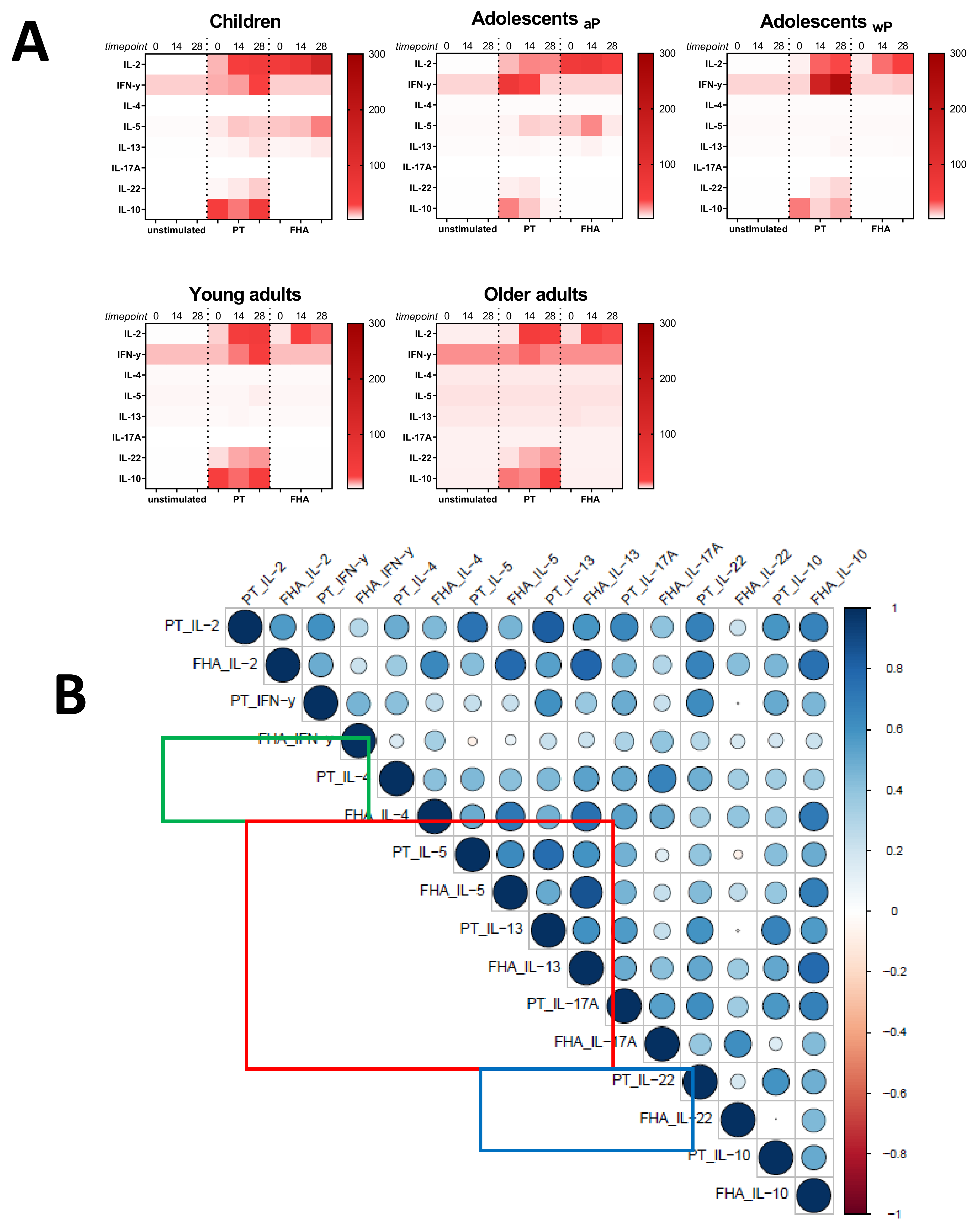
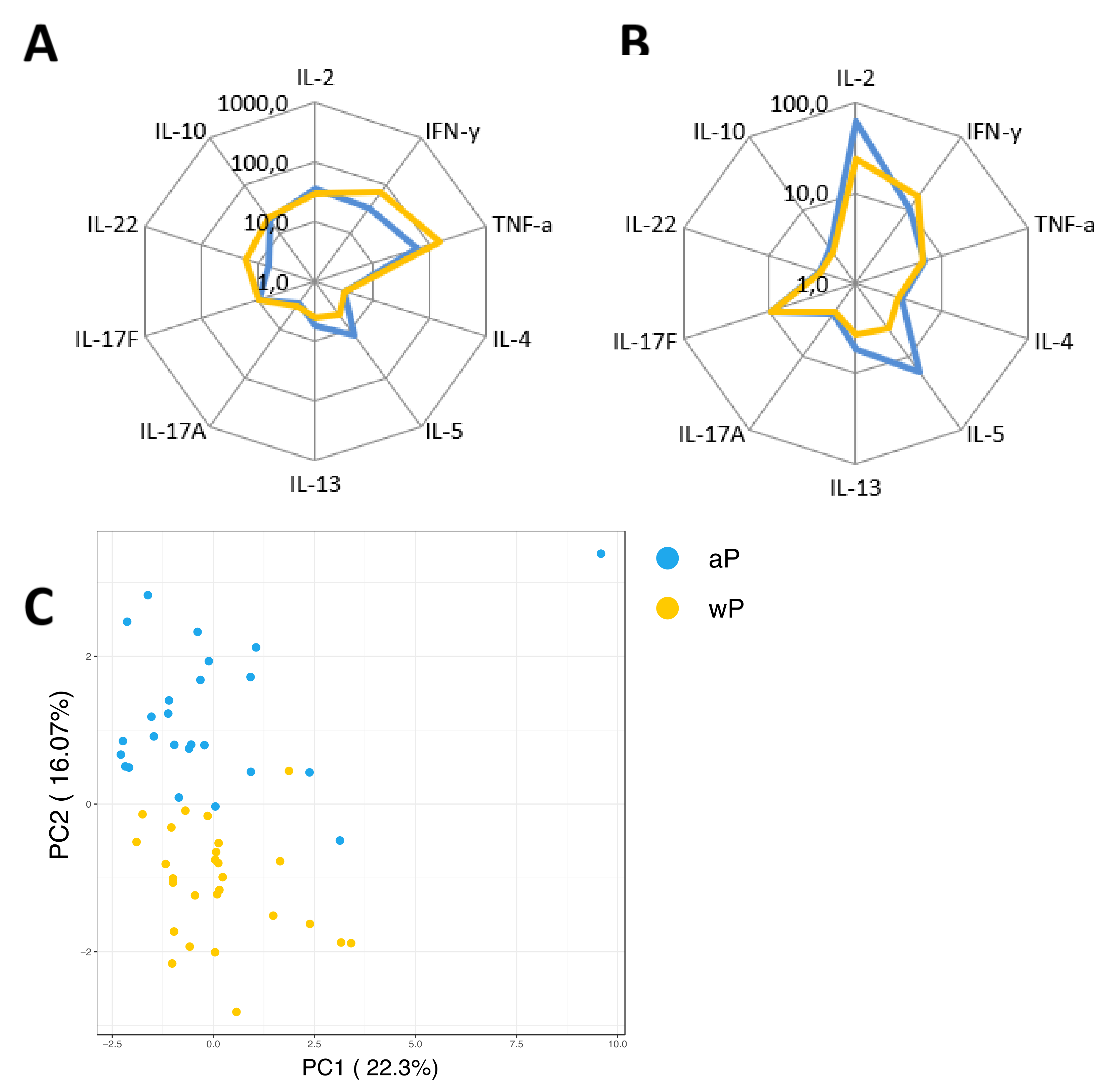
3.6. Primary Vaccination Background Impacts Booster-Induced Bp Specific CD4+ T-Cell Cytokine Profile Later in Life
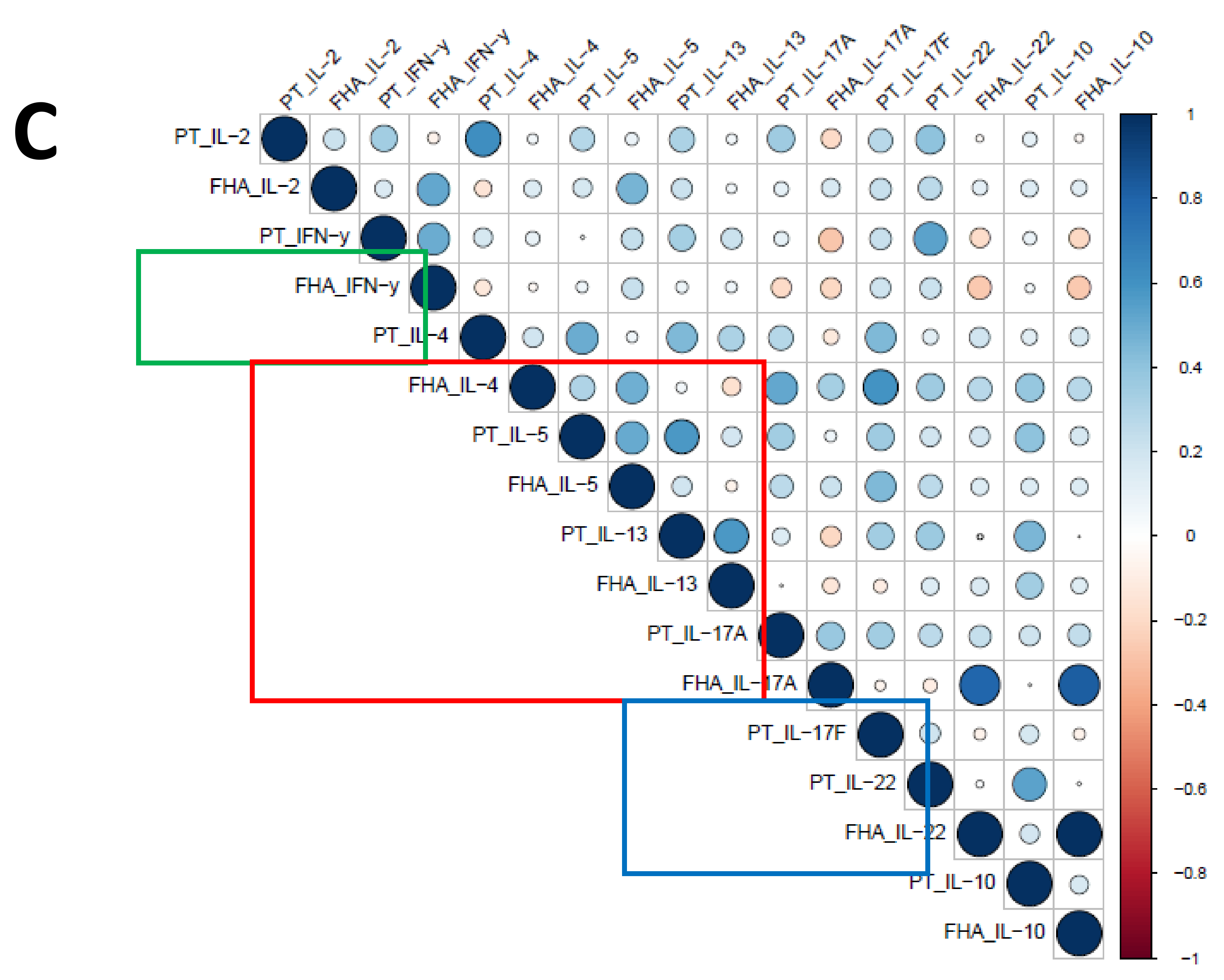
3.7. Influence of the Primary Vaccination Background on the Cytokine Responses in Supernatants
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Marcinak, J.F.; Ward, M.; Frank, A.L.; Boyer, K.M.; Froeschle, J.E.; Hosbach, P.H., IV. Comparison of the safety and immunogenicity of acellular (BIKEN) and whole-cell pertussis vaccines in 15-to 20-month-old children. Am. J. Dis. Child. 1993, 147, 290–294. [Google Scholar] [CrossRef]
- David, S.; Vermeer-de Bondt, P.E.; van der Maas, N.A.J.V. Reactogenicity of infant whole cell pertussis combination vaccine compared with acellular pertussis vaccines with or without simultaneous pneumococcal vaccine in the Netherlands. Vaccine 2008, 26, 5883–5887. [Google Scholar] [CrossRef] [PubMed]
- Kitchin, N.; Southern, J.; Morris, R.; Hemme, F.; Cartwright, K.; Watson, M.; Miller, E.J.V. A randomised controlled study of the reactogenicity of an acellular pertussis-containing pentavalent infant vaccine compared to a quadrivalent whole cell pertussis-containing vaccine and oral poliomyelitis vaccine, when given concurrently with meningococcal group C conjugate vaccine to healthy UK infants at 2, 3 and 4 months of age. Vaccine 2006, 24, 3964–3970. [Google Scholar]
- Vickers, D.; Ross, A.G.; Mainar-Jaime, R.C.; Neudorf, C.; Shah, S.J.C. Whole-cell and acellular pertussis vaccination programs and rates of pertussis among infants and young children. Can. Med. Assoc. J. 2006, 175, 1213–1217. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, S.L.; Frith, K.; Snelling, T.L.; Grimwood, K.; McIntyre, P.B.; Lambert, S.B. Waning vaccine immunity in teenagers primed with whole cell and acellular pertussis vaccine: Recent epidemiology. Expert Rev. Vaccines 2014, 13, 1081–1106. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, S.L.; Ware, R.S.; Grimwood, K.; Lambert, S.B.J.J. Number and order of whole cell pertussis vaccines in infancy and disease protection. J. Am. Med. Assoc. 2012, 308, 454–456. [Google Scholar] [CrossRef]
- Klein, N.P.; Bartlett, J.; Fireman, B.; Rowhani-Rahbar, A.; Baxter, R.J.P. Comparative effectiveness of acellular versus whole-cell pertussis vaccines in teenagers. Pediatr. 2013, 131, e1716–e1722. [Google Scholar] [CrossRef]
- Ausiello, C.M.; Urbani, F.; la Sala, A.; Lande, R.; Cassone, A. Vaccine- and antigen-dependent type 1 and type 2 cytokine induction after primary vaccination of infants with whole-cell or acellular pertussis vaccines. Infect. Immun. 1997, 65, 2168–2174. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.; Murphy, G.; Ryan, E.; Nilsson, L.; Shackley, F.; Gothefors, L.; Oymar, K.; Miller, E.; Storsaeter, J.; Mills, K.H. Distinct T–cell subtypes induced with whole cell and acellular pertussis vaccines in children. Immunol. 1998, 93, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mascart, F.; Hainaut, M.; Peltier, A.; Verscheure, V.; Levy, J.; Locht, C. Modulation of the infant immune responses by the first pertussis vaccine administrations. Vaccine 2007, 25, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Schure, R.M.; Hendrikx, L.H.; de Rond, L.G.; Ozturk, K.; Sanders, E.A.; Berbers, G.A.; Buisman, A.M. Differential T- and B-cell responses to pertussis in acellular vaccine–primed versus whole–cell vaccine–primed children 2 years after preschool acellular booster vaccination. Clin. Vaccine Immunol. 2013, 20, 1388–1395. [Google Scholar] [CrossRef] [PubMed]
- Smits, K.; Pottier, G.; Smet, J.; Dirix, V.; Vermeulen, F.; De Schutter, I.; Carollo, M.; Locht, C.; Ausiello, C.M.; Mascart, F. Different T cell memory in preadolescents after whole–cell or acellular pertussis vaccination. Vaccine 2013, 32, 111–118. [Google Scholar] [CrossRef] [PubMed]
- van der Lee, S.; Hendrikx, L.H.; Sanders, E.A.M.; Berbers, G.A.M.; Buisman, A.M. Whole–Cell or Acellular Pertussis Primary Immunizations in Infancy Determines Adolescent Cellular Immune Profiles. Front. Immunol. 2018, 9, 51. [Google Scholar] [CrossRef] [PubMed]
- da Silva Antunes, R.; Babor, M.; Carpenter, C.; Khalil, N.; Cortese, M.; Mentzer, A.J.; Seumois, G.; Petro, C.D.; Purcell, L.A.; Vijayanand, P.; et al. Th1/Th17 polarization persists following whole–cell pertussis vaccination despite repeated acellular boosters. J. Clin. Invest. 2018, 128, 3853–3865. [Google Scholar] [CrossRef] [PubMed]
- Bancroft, T.; Dillon, M.B.; da Silva Antunes, R.; Paul, S.; Peters, B.; Crotty, S.; Arlehamn, C.S.L.; Sette, A. Th1 versus Th2 T cell polarization by whole–cell and acellular childhood pertussis vaccines persists upon re–immunization in adolescence and adulthood. Cell. Immunol. 2016, 304, 35–43. [Google Scholar] [CrossRef]
- Ryan, M.; Murphy, G.; Gothefors, L.; Nilsson, L.; Storsaeter, J.; Mills, K.H. Bordetella pertussis respiratory infection in children is associated with preferential activation of type 1 T helper cells. J. Infect. Dis. 1997, 175, 1246–1250. [Google Scholar] [CrossRef]
- Mascart, F.; Verscheure, V.; Malfroot, A.; Hainaut, M.; Pierard, D.; Temerman, S.; Peltier, A.; Debrie, A.S.; Levy, J.; Del Giudice, G.; et al. Bordetella pertussis infection in 2-month-old infants promotes type 1 T cell responses. J. Immunol. 2003, 170, 1504–1509. [Google Scholar] [CrossRef]
- Lambert, E.E.; Buisman, A.M.; van Els, C. Superior B. pertussis Specific CD4+ T–Cell Immunity Imprinted by Natural Infection. Adv. Exp. Med. Biol. 2019, 1183, 81–98. [Google Scholar] [CrossRef]
- Warfel, J.M.; Merkel, T.J. Bordetella pertussis infection induces a mucosal IL-17 response and long–lived Th17 and Th1 immune memory cells in nonhuman primates. Mucosal. Immunol. 2013, 6, 787–796. [Google Scholar] [CrossRef]
- Warfel, J.M.; Zimmerman, L.I.; Merkel, T.J. Acellular pertussis vaccines protect against disease but fail to prevent infection and transmission in a nonhuman primate model. Proc. Natl. Acad. Sci. USA 2014, 111, 787–792. [Google Scholar] [CrossRef]
- Warfel, J.M.; Zimmerman, L.I.; Merkel, T.J. Comparison of Three Whole-Cell Pertussis Vaccines in the Baboon Model of Pertussis. Clin. Vaccine Immunol. 2016, 23, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Wilk, M.M.; Misiak, A.; McManus, R.M.; Allen, A.C.; Lynch, M.A.; Mills, K.H.G. Lung CD4 Tissue–Resident Memory T Cells Mediate Adaptive Immunity Induced by Previous Infection of Mice with Bordetella pertussis. J. Immunol. 2017, 199, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Wilk, M.M.; Borkner, L.; Misiak, A.; Curham, L.; Allen, A.C.; Mills, K.H.G. Immunization with whole cell but not acellular pertussis vaccines primes CD4 TRM cells that sustain protective immunity against nasal colonization with Bordetella pertussis. Emerging Microbes Infect. 2019, 8, 169–185. [Google Scholar] [CrossRef] [PubMed]
- Diavatopoulos, D.A.; Mills, K.H.; Kester, K.E.; Kampmann, B.; Silerova, M.; Heininger, U.; van Dongen, J.J.; van der Most, R.G.; Huijnen, M.A.; Siena, E.J.T.L.I.D. PERISCOPE: Road towards effective control of pertussis. Lancet Infect. Dis. 2018, 16, E179–E186. [Google Scholar] [CrossRef]
- Penn-Nicholson, A.; Nemes, E.; Hanekom, W.A.; Hatherill, M.; Scriba, T.J.J.T. Mycobacterium tuberculosis-specific CD4 T cells are the principal source of IFN-γ in QuantiFERON assays in healthy persons. Tuberc. 2015, 95, 350–351. [Google Scholar] [CrossRef]
- Hanekom, W.A.; Hughes, J.; Mavinkurve, M.; Mendillo, M.; Watkins, M.; Gamieldien, H.; Gelderbloem, S.J.; Sidibana, M.; Mansoor, N.; Davids, V. Novel application of a whole blood intracellular cytokine detection assay to quantitate specific T–cell frequency in field studies. J. Immunol. Methods 2004, 291, 185–195. [Google Scholar] [CrossRef]
- Suni, M.A.; Picker, L.J.; Maino, V.C. Detection of antigen–specific T cell cytokine expression in whole blood by flow cytometry. J. Immunol. Methods 1998, 212, 89–98. [Google Scholar] [CrossRef]
- Harenberg, A.; Begue, S.; Mamessier, A.; Gimenez–Fourage, S.; Ching Seah, C.; Wei Liang, A.; Li Ng, J.; Yun Toh, X.; Archuleta, S.; Wilder–Smith, A. Persistence of Th1/Tc1 responses one year after tetravalent dengue vaccination in adults and adolescents in Singapore. Hu. Vaccin. Immunother. 2013, 9, 2317–2325. [Google Scholar] [CrossRef]
- Geluk, A.; van der Ploeg-van, J.J.; van Meijgaarden, K.E.; Commandeur, S.; Drijfhout, J.W.; Benckhuijsen, W.E.; Franken, K.L.; Naafs, B.; Ottenhoff, T.H. Enhancing sensitivity of detection of immune responses to Mycobacterium leprae peptides in whole–blood assays. Clin. Vaccine Immunol. 2010, 17, 993–1004. [Google Scholar] [CrossRef][Green Version]
- Bart, M.J.; Zeddeman, A.; van der Heide, H.G.; Heuvelman, K.; van Gent, M.; Mooi, F.R.J.G.A. Complete genome sequences of Bordetella pertussis isolates B1917 and B1920, representing two predominant global lineages. Microbiol. Resour. Announc. 2014, 2, e01301–e01314. [Google Scholar] [CrossRef]
- Botafogo, V.; Pérez-Andres, M.; Jara-Acevedo, M.; Bárcena, P.; Grigore, G.; Hernández–Delgado, A.; Damasceno, D.; Comans, S.; Blanco, E.; Romero, A. Age Distribution of Multiple Functionally Relevant Subsets of CD4+ T Cells in Human Blood Using a Standardized and Validated 14–Color EuroFlow Immune Monitoring Tube. Front. Immunol. 2020, 11, 166. [Google Scholar] [CrossRef]
- Candia, J.; Tsang, J.S. ENetXplorer: An R package for the quantitative exploration of elastic net families for generalized linear models. BMC Bioinform. 2019, 20, 189. [Google Scholar] [CrossRef]
- Package “corrplot”: Visualization of a Correlation Matrix (Version 0.84). Available online: https://github.com/taiyun/corrplot (accessed on 4 February 2020).
- Friedman, J.; Hastie, T.; Tibshirani, R. Regularization paths for generalized linear models via coordinate descent. J. Stat. Softw. 2010, 33, 1. [Google Scholar] [CrossRef]
- Hedley, B.; Keeney, M. Technical issues: flow cytometry and rare event analysis. Int. J. lab. Hematol. 2013, 35, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Kirchhoff, D.; Frentsch, M.; Leclerk, P.; Bumann, D.; Rausch, S.; Hartmann, S.; Thiel, A.; Scheffold, A. Identification and isolation of murine antigen–reactive T cells according to CD154 expression. Eur. J. Immunol. 2007, 37, 2370–2377. [Google Scholar] [CrossRef] [PubMed]
- Frentsch, M.; Arbach, O.; Kirchhoff, D.; Moewes, B.; Worm, M.; Rothe, M.; Scheffold, A.; Thiel, A. Direct access to CD4+ T cells specific for defined antigens according to CD154 expression. Nat. Med. 2005, 11, 1118–1124. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Hastie, T. Regularization and variable selection via the elastic net. J. R. Stat. Soc. Ser. B Stat. Methodol. 2005, 67, 301–320. [Google Scholar] [CrossRef]
- Breinig, T.; Sester, M.; Sester, U.; Meyerhans, A. Antigen–specific T cell responses: Determination of their frequencies, homing properties, and effector functions in human whole blood. Methods 2006, 38, 77–83. [Google Scholar] [CrossRef]
- Causi, E.L.; Parikh, S.C.; Chudley, L.; Layfield, D.M.; Ottensmeier, C.H.; Stevenson, F.K.; Di Genova, G. Vaccination expands antigen-specific CD4+ memory T cells and mobilizes bystander central memory T cells. PLoS ONE 2015, 10, e0136717. [Google Scholar] [CrossRef]
- Mangada, M.M.; Ennis, F.A.; Rothman, A.L. Quantitation of dengue virus specific CD4+ T cells by intracellular cytokine staining. J. Immunol. Methods 2004, 284, 89–97. [Google Scholar] [CrossRef]
- Bollinger, T.; Leutz, A.; Leliavski, A.; Skrum, L.; Kovac, J.; Bonacina, L.; Benedict, C.; Lange, T.; Westermann, J.; Oster, H. Circadian clocks in mouse and human CD4+ T cells. PLoS ONE 2011, 6, e29801. [Google Scholar] [CrossRef] [PubMed]
- Fortier, E.E.; Rooney, J.; Dardente, H.; Hardy, M.-P.; Labrecque, N.; Cermakian, N. Circadian variation of the response of T cells to antigen. J. Immunol. 2011, 187, 6291–6300. [Google Scholar] [CrossRef] [PubMed]
- Crooke, S.N.; Ovsyannikova, I.G.; Poland, G.A.; Kennedy, R.B. Immunosenescence and human vaccine immune responses. Immun. Ageing 2019, 16, 25. [Google Scholar] [CrossRef] [PubMed]
- Pera, A.; Campos, C.; López, N.; Hassouneh, F.; Alonso, C.; Tarazona, R.; Solana, R. Immunosenescence: implications for response to infection and vaccination in older people. Maturitas 2015, 82, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Dirix, V.; Verscheure, V.; Goetghebuer, T.; Hainaut, M.; Debrie, A.-S.; Locht, C.; Mascart, F. Cytokine and antibody profiles in 1-year-old children vaccinated with either acellular or whole-cell pertussis vaccine during infancy. Vaccine 2009, 27, 6042–6047. [Google Scholar] [CrossRef] [PubMed]


| Characteristic | Children 7–10 yrs (n = 19) | Adolescents 11–15 yrs (n = 24) | Young Adults 20–34 yrs (n = 15) | Older Adults 60–70 yrs (n = 15) |
|---|---|---|---|---|
| Sex–no. (%) | ||||
| Male | 10 (53) | 16 (67) | 9 (60) | 10 (67) |
| Female | 9 (47) | 8 (33) | 6 (40) | 5 (33) |
| Age–yrs | ||||
| Median | 8 | 13,5 | 28 | 65 |
| Range | 7–9 | 11–15 | 21–34 | 61–69 |
| Primary Vaccination Background | ||||
| Acellular Pertussis Vaccination | 100% | 46% | 0% | 0% |
| Whole-Cell Pertussis Vaccination | 0% | 54% | 100% | Unknown proportion |
| No Vaccination | 0% | 0% | 0% | Unknown proportion |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lambert, E.E.; Corbière, V.; van Gaans-van den Brink, J.A.M.; Duijst, M.; Venkatasubramanian, P.B.; Simonetti, E.; Huynen, M.; Diavatopoulos, D.D.; Versteegen, P.; Berbers, G.A.M.; et al. Uncovering Distinct Primary Vaccination-Dependent Profiles in Human Bordetella pertussis Specific CD4+ T-Cell Responses Using a Novel Whole Blood Assay. Vaccines 2020, 8, 225. https://doi.org/10.3390/vaccines8020225
Lambert EE, Corbière V, van Gaans-van den Brink JAM, Duijst M, Venkatasubramanian PB, Simonetti E, Huynen M, Diavatopoulos DD, Versteegen P, Berbers GAM, et al. Uncovering Distinct Primary Vaccination-Dependent Profiles in Human Bordetella pertussis Specific CD4+ T-Cell Responses Using a Novel Whole Blood Assay. Vaccines. 2020; 8(2):225. https://doi.org/10.3390/vaccines8020225
Chicago/Turabian StyleLambert, Eleonora E., Véronique Corbière, Jacqueline A. M. van Gaans-van den Brink, Maxime Duijst, Prashanna Balaji Venkatasubramanian, Elles Simonetti, Martijn Huynen, Dimitri D. Diavatopoulos, Pauline Versteegen, Guy A. M. Berbers, and et al. 2020. "Uncovering Distinct Primary Vaccination-Dependent Profiles in Human Bordetella pertussis Specific CD4+ T-Cell Responses Using a Novel Whole Blood Assay" Vaccines 8, no. 2: 225. https://doi.org/10.3390/vaccines8020225
APA StyleLambert, E. E., Corbière, V., van Gaans-van den Brink, J. A. M., Duijst, M., Venkatasubramanian, P. B., Simonetti, E., Huynen, M., Diavatopoulos, D. D., Versteegen, P., Berbers, G. A. M., Mascart, F., & van Els, C. A. C. M. (2020). Uncovering Distinct Primary Vaccination-Dependent Profiles in Human Bordetella pertussis Specific CD4+ T-Cell Responses Using a Novel Whole Blood Assay. Vaccines, 8(2), 225. https://doi.org/10.3390/vaccines8020225






