CD8+ Tumour-Infiltrating Lymphocytes and Tumour Microenvironment Immune Types as Biomarkers for Immunotherapy in Sinonasal Intestinal-Type Adenocarcinoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Samples
2.2. Immunohistochemistry
2.3. Statistical Analysis
3. Results
3.1. Tumour Stage and Histological Subtype Are Related to Longer Survival
3.2. The Prognostic Value of CD8+ TILs Is Independent of Tumour Stage and Histological Subtype
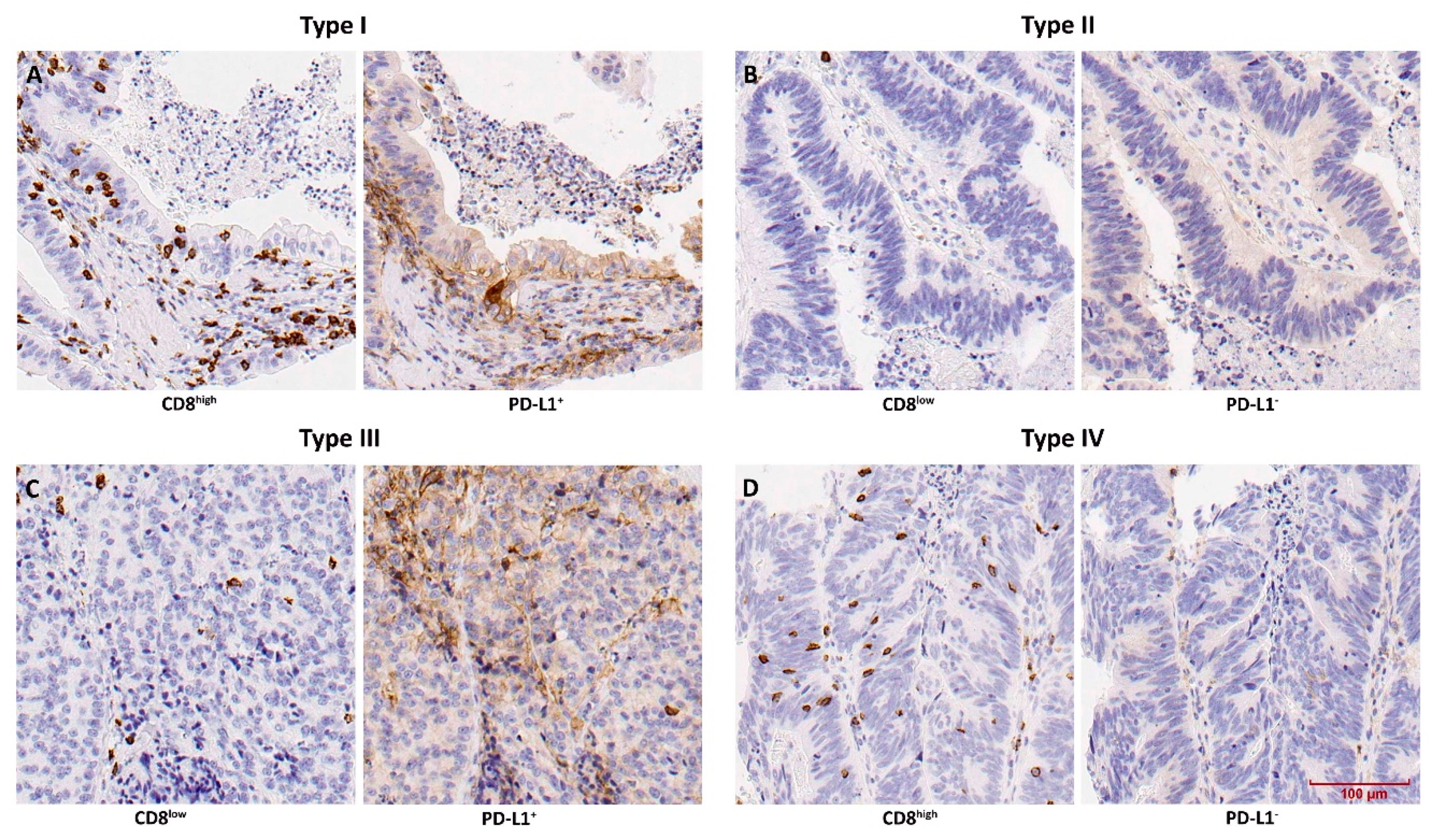
3.3. TMIT Types Display Different Clinical Outcomes
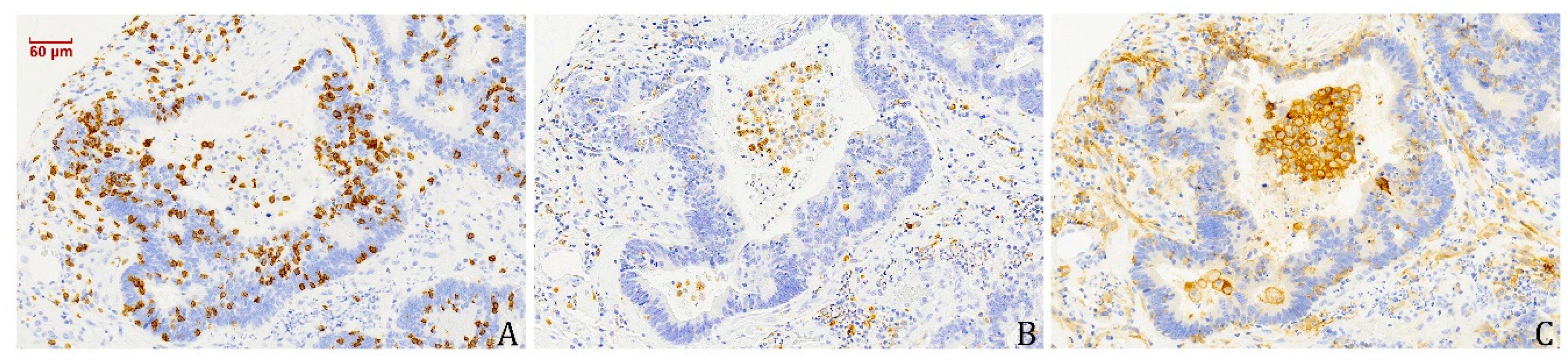
3.4. PD-L1-Expressing Tumour Cells and Macrophages Co-Occur with CD8+ TILs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Binazzi, A.; Corfiati, M.; Di Marzio, D.; Cacciatore, A.M.; Zajacovà, J.; Mensi, C.; Galli, P.; Miligi, L.; Calisti, R.; Romeo, E.; et al. Sinonasal cancer in the Italian national surveillance system: Epidemiology, occupation, and public health implications. Am. J. Ind. Med. 2018, 61, 239–250. [Google Scholar] [CrossRef]
- Youlden, D.R.; Cramb, S.M.; Peters, S.; Porceddu, S.V.; Møller, H.; Fritschi, L.; Baade, P.D. International comparisons of the incidence and mortality of sinonasal cancer. Cancer Epidemiol. 2013, 37, 770–779. [Google Scholar] [CrossRef] [PubMed]
- Choussy, O.; Ferron, C.; Védrine, P.O.; Toussaint, B.; Liétin, B.; Marandas, P.; Babin, E.; De Raucourt, D.; Reyt, E.; Cosmidis, A.; et al. Adenocarcinoma of ethmoid: A GETTEC retrospective multicenter study of 418 cases. Laryngoscope 2008, 118, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Llorente, J.L.; López, F.; Suárez, C.; Hermsen, M.A. Sinonasal carcinoma: Clinical, pathological, genetic and therapeutic advances. Nat. Rev. Clin. Oncol. 2014, 11, 460–472. [Google Scholar] [CrossRef] [PubMed]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Arsenic, metals, fibres, and dusts. IARC Monogr. Eval. Carcinog. Risks Hum. 2012, 100, 11–465. [Google Scholar]
- Bonzini, M.; Battaglia, P.; Parassoni, D.; Casa, M.; Facchinetti, N.; Turri-Zanoni, M.; Borchini, R.; Castelnuovo, P.; Ferrario, M.M. Prevalence of occupational hazards in patients with different types of epithelial sinonasal cancers. Rhinology 2013, 51, 31–36. [Google Scholar] [CrossRef]
- El-Naggar, A.K.; Chan, J.; Grandis, J.; Takata, T.; Slootweg, P. WHO Classification of Tumors Pathology and Genetics of Head and Neck Tumors, 4th ed.; IARC Press: Lyon, France, 2017. [Google Scholar]
- Nicolai, P.; Schreiber, A.; Bolzoni Villaret, A.; Lombardi, D.; Morassi, L.; Raffetti, E.; Donato, F.; Battaglia, P.; Turri-Zanoni, M.; Bignami, M.; et al. Intestinal type adenocarcinoma of the ethmoid: Outcomes of a treatment regimen based on endoscopic surgery with or without radiotherapy. Head Neck 2016, 38 (Suppl. S1), E996–E1003. [Google Scholar] [CrossRef]
- Cantu, G.; Solero, C.L.; Miceli, R.; Mattana, F.; Riccio, S.; Colombo, S.; Pompilio, M.; Lombardo, G.; Formillo, P.; Quattrone, P. Anterior craniofacial resection for malignant paranasal tumors: A monoinstitutional experience of 366 cases. Head Neck 2012, 34, 78–87. [Google Scholar] [CrossRef]
- Bhayani, M.K.; Yilmaz, T.; Sweeney, A.; Calzada, G.; Roberts, D.B.; Levine, N.B.; DeMonte, F.; Hanna, E.Y.; Kupferman, M.E. Sinonasal adenocarcinoma: A 16-year experience at a single institution. Head Neck 2014, 36, 1490–1496. [Google Scholar] [CrossRef]
- Vergez, S.; du Mayne, M.D.; Coste, A.; Gallet, P.; Jankowski, R.; Dufour, X.; Righini, C.; Reyt, E.; Choussy, O.; Serrano, E.; et al. Multicenter study to assess endoscopic resection of 159 sinonasal adenocarcinomas. Ann. Surg. Oncol. 2014, 21, 1384–1390. [Google Scholar] [CrossRef]
- Hermsen, M.A.; Riobello, C.; García-Marín, R.; Cabal, V.N.; Suárez-Fernández, L.; López, F.; Llorente, J.L. Translational genomics of sinonasal cancers. Semin. Cancer Biol. 2020, 61, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Mehra, R.; Seiwert, T.Y.; Gupta, S.; Weiss, J.; Gluck, I.; Eder, J.P.; Burtness, B.; Tahara, M.; Keam, B.; Kang, H.; et al. Efficacy and safety of pembrolizumab in recurrent/metastatic head and neck squamous cell carcinoma: Pooled analyses after long-term follow-up in KEYNOTE-012. Br. J. Cancer 2018, 119, 153–159. [Google Scholar] [CrossRef]
- Ferris, R.L.; Licitra, L.; Fayette, J.; Even, C.; Blumenschein, G., Jr.; Harrington, K.J.; Guigay, J.; Vokes, E.E.; Saba, N.F.; Haddad, R.; et al. Nivolumab in Patients with Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck: Efficacy and Safety in CheckMate 141 by Prior Cetuximab Use. Clin. Cancer Res. 2019, 25, 5221–5230. [Google Scholar] [CrossRef] [PubMed]
- Chow, L.Q.M.; Haddad, R.; Gupta, S.; Mahipal, A.; Mehra, R.; Tahara, M.; Berger, R.; Eder, J.P.; Burtness, B.; Lee, S.H.; et al. Antitumor activity of pembrolizumab in biomarker-unselected patients with recurrent and/or metastatic head and neck squamous cell carcinoma: Results from the phase Ib KEYNOTE-012 Expansion Cohort. J. Clin. Oncol. 2016, 34, 3838–3845. [Google Scholar] [CrossRef]
- Riobello, C.; Vivanco, B.; Reda, S.; López-Hernández, A.; García-Inclán, C.; Potes-Ares, S.; Cabal, V.N.; López, F.; Llorente, J.L.; Hermsen, M.A. Programmed death ligand-1 expression as immunotherapeutic target in sinonasal cancer. Head Neck 2018, 40, 818–827. [Google Scholar] [CrossRef]
- Quan, H.; Yan, L.; Wang, S.; Wang, S. Clinical relevance and significance of programmed death-ligand 1 expression, tumour-infiltrating lymphocytes, and p16 status in sinonasal squamous cell carcinoma. Cancer Manag. Res. 2019, 11, 4335–4345. [Google Scholar] [CrossRef]
- Classe, M.; Burgess, A.; El Zein, S.; Wassef, M.; Herman, P.; Mortuaire, G.; Leroy, X.; Malouf, G.G.; Verillaud, B. Evaluating the prognostic potential of the Ki67 proliferation index and tumour-infiltrating lymphocytes in olfactory neuroblastoma. Histopathology 2019, 75, 853–864. [Google Scholar] [CrossRef]
- Taube, J.M.; Anders, R.A.; Young, G.D.; Xu, H.; Sharma, R.; McMiller, T.L.; Chen, S.; Klein, A.P.; Pardoll, D.M.; Topalian, S.L.; et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci. Transl. Med. 2012, 4, 127ra137. [Google Scholar] [CrossRef]
- Ock, C.Y.; Keam, B.; Kim, S.; Lee, J.S.; Kim, M.; Kim, T.M.; Jeon, Y.K.; Kim, D.W.; Chung, D.H.; Heo, D.S. Pan-Cancer Immunogenomic Perspective on the Tumour Microenvironment Based on PD-L1 and CD8 T-Cell Infiltration. Clin. Cancer Res. 2016, 22, 2261–2270. [Google Scholar] [CrossRef]
- Tumeh, P.C.; Harview, C.L.; Yearley, J.H.; Shintaku, I.P.; Taylor, E.J.; Robert, L.; Chmielowski, B.; Spasic, M.; Henry, G.; Ciobanu, V.; et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014, 515, 568–571. [Google Scholar] [CrossRef]
- Teng, M.W.; Ngiow, S.F.; Ribas, A.; Smyth, M.J. Classifying Cancers Based on T-cell Infiltration 16 and PD-L1. Cancer Res. 2015, 75, 2139–2145. [Google Scholar] [CrossRef] [PubMed]
- Blank, C.U.; Haanen, J.B.; Ribas, A.; Schumacher, T.N. Cancer Immunology. The “cancer immunogram”. Science 2016, 352, 658–660. [Google Scholar] [CrossRef] [PubMed]
- Sobin, L.H.; Gospodarowicz, M.K.; Wittekind, C.H. (Eds.) International Union Against Cancer (UICC) TNM Classification of Malignant Tumors, 7th ed.; Wiley-Blackwell: Chichester, West Sussex, UK, 2009. [Google Scholar]
- Vassilakopoulou, M.; Avgeris, M.; Velcheti, V.; Kotoula, V.; Rampias, T.; Chatzopoulos, K.; Perisanidis, C.; Kontos, C.K.; Giotakis, A.I.; Scorilas, A.; et al. Evaluation of PD-L1 expression and associated tumor-infiltrating lymphocytes in laryngeal squamous cell carcinoma. Clin. Cancer Res. 2016, 22, 704–713. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.J.; Thirdborough, S.M.; Mellows, T.; Riley, C.; Harris, S.; Suchak, K.; Webb, A.; Hampton, C.; Patel, N.N.; Randall, C.J.; et al. Tumor-infiltrating lymphocytes predict for outcome in HPV-positive oropharyngeal cancer. Br. J. Cancer 2014, 110, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Cao, M.F.; Zhang, X.; Dang, W.Q.; Xiao, J.F.; Liu, Q.; Tan, Y.H.; Tan, Y.Y.; Xu, Y.Y.; Xu, S.L.; et al. The landscape of immune microenvironment in lung adenocarcinoma and squamous cell carcinoma based on PD-L1 expression and tumor-infiltrating lymphocytes. Cancer Med. 2019, 8, 7207–7218. [Google Scholar] [CrossRef]
- Wu, S.; Shi, X.; Sun, J.; Liu, Y.; Luo, Y.; Liang, Z.; Wang, J.; Zeng, X. The significance of programmed cell death ligand 1 expression in resected lung adenocarcinoma. Oncotarget 2017, 8, 16421–16429. [Google Scholar] [CrossRef]
- Brody, R.; Zhang, Y.; Ballas, M.; Siddiqui, M.K.; Gupta, P.; Barker, C.; Midha, A.; Walker, J. PD-L1 expression in advanced NSCLC: Insights into risk stratification and treatment selection from a systematic literature review. Lung Cancer 2017, 112, 200–215. [Google Scholar] [CrossRef]
- Cassetta, L.; Pollard, J.W. Targeting macrophages: Therapeutic approaches in cancer. Nat. Rev. Drug Discov. 2018, 17, 887–904. [Google Scholar] [CrossRef]
- Barnes, T.A.; Amir, E. HYPE or HOPE: The prognostic value of infiltrating immune cells in 8 Cancer. Br. J. Cancer 2017, 117, 451–460. [Google Scholar] [CrossRef]
- Becker, M.; Müller, C.B.; De Bastiani, M.A.; Klamt, F. Becker 2014 histol histopathol 2014: The prognostic impact of tumor-associated macrophages and intra-tumoral apoptosis in non-small cell lung cancer. Histol. Histopathol. 2014, 29, 21–31. [Google Scholar] [PubMed]
- Rakaee, M.; Busund, L.R.; Jamaly, S.; Paulsen, E.E.; Richardsen, E.; Andersen, S.; Al-Saad, S.; Bremnes, R.M.; Donnem, T.; Kilvaer, T.K. Prognostic Value of Macrophage Phenotypes in Resectable Non-Small Cell Lung Cancer Assessed by Multiplex Immunohistochemistry. Neoplasia 2019, 21, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Edin, S.; Wikberg, M.L.; Oldenborg, P.A.; Palmqvist, R. Macrophages: Good Guys in Colorectal Cancer. Oncoimmunology 2013, 2, e23038. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.K.; Mantovani, A. Macrophage plasticity and interaction with lymphocyte subsets: Cancer as a paradigm. Nat. Immunol. 2010, 11, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Noh, B.J.; Kwak, J.Y.; Eom, D.W. Immune classification for the PD-L1 expression and tumour-infiltrating lymphocytes in colorectal adenocarcinoma. BMC Cancer 2020, 20, 58. [Google Scholar] [CrossRef] [PubMed]
- Koh, J.; Ock, C.Y.; Kim, J.W.; Nam, S.K.; Kwak, Y.; Yun, S.; Ahn, S.H.; Park, D.J.; Kim, H.H.; Kim, W.H.; et al. Clinicopathologic implications of immune classification by PD-L1 expression and CD8-positive tumor-infiltrating lymphocytes in stage II and III gastric cancer patients. Oncotarget 2017, 8, 26356–26367. [Google Scholar] [CrossRef]
- Fridman, W.H.; Zitvogel, L.; Sautès-Fridman, C.; Kroemer, G. The immune contexture in cancer prognosis and treatment. Nat. Rev. Clin. Oncol. 2017, 14, 717–734. [Google Scholar] [CrossRef]


| Clinical Features | All | CD8+ TI Ls | TMIT Type | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0% | 1–10% | >10% | Significance | I | II | III | IV | Significance | ||
| All | 133 | 47 | 76 | 10 | 8 | 96 | 27 | 2 | ||
| Gender | 0.467 | 0.854 | ||||||||
| Female | 2 (2) | 0 (0) | 2 (3) | 0 (0) | 0 (0) | 2 (2) | 0 (0) | 0 (0) | ||
| Male | 131 (98) | 47 (100) | 74 (97) | 10 (100) | 8 (100) | 94 (98) | 27 (100) | 2 (100) | ||
| Disease stage | 0.696 a | 0.776 a | ||||||||
| I | 30 (22) | 12 (25) | 18 (23) | 0 (0) | 0 (0) | 22 (23) | 8 (30) | 0 (0) | ||
| II | 17 (13) | 5 (11) | 8 (11) | 4 (40) | 4 (50) | 13 (14) | 0 (0) | 0 (0) | ||
| III | 45 (34) | 16 (34) | 25 (33) | 4 (40) | 2 (25) | 31 (32) | 10 (37) | 2 (100) | ||
| IV-a | 16 (12) | 8 (17) | 6 (8) | 2 (20) | 2 (25) | 10 (10) | 4 (15) | 0 (0) | ||
| IV-b | 25 (19) | 6 (13) | 19 (25) | 0 (0) | 0 (0) | 20 (21) | 5 (18) | 0 (0) | ||
| Histological type | 0.007 b | 0.188 b | ||||||||
| Papillary | 13 (10) | 3 (6) | 9 (12) | 1 (10) | 1 (13) | 8 (8) | 4 (15) | 0 (0) | ||
| Colonic | 80 (60) | 23 (49) | 48 (63) | 9 (90) | 7 (87) | 56 (59) | 15 (55) | 2 (100) | ||
| Solid | 10 (7) | 3 (6) | 7 (9) | 0 (0) | 0 (0) | 6 (6) | 4 (15) | 0 (0) | ||
| Mucinous | 30 (23) | 18 (39) | 12 (16) | 0 (0) | 0 (0) | 26 (27) | 4 (15) | 0 (0) | ||
| Recurrence | 0.165 | 0.079 | ||||||||
| No | 70 (53) | 20 (43) | 43 (57) | 7 (70) | 6 (75) | 44 (46) | 19 (70) | 1 (50) | ||
| Yes | 63 (47) | 27 (57) | 33 (43) | 3 (30) | 2 (25) | 52 (54) | 8 (30) | 1 (50) | ||
| Metastasis | 0.249 | 0.389 | ||||||||
| No | 120 (90) | 40 (85) | 70 (92) | 10 (100) | 8 (100) | 84 (88) | 26 (96) | 2 (100) | ||
| Yes | 13 (10) | 7 (15) | 6 (8) | 0 (0) | 0 (0) | 12 (12) | 1 (4) | 0 (0) | ||
| Patient status | 0.021 c | 0.054 c | ||||||||
| Alive | 60 (45) | 20 (43) | 32 (42) | 8 (80) | 6 (75) | 42 (44) | 10 (37) | 2 (100) | ||
| Died of disease | 53 (40) | 19 (40) | 34 (45) | 0 (0) | 0 (0) | 42 (44) | 11 (41) | 0 (0) | ||
| Died other causes | 20 (15) | 8 (17) | 10 (13) | 2 (20) | 2 (25) | 12 (12) | 6 (22) | 0 (0) | ||
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Log Rank | Significance | HR (95% CI) | Significance | |
| Tumour stage (stages I–III versus IVa, IVb) | 50.498 | p = 0.000 | 5.15 (3.06–8.70) | p = 0.000 |
| Histological type (papillary-colonic versus solid-mucinous) | 15.085 | p = 0.002 | 1.21 (0.73–2.03) | p = 0.461 |
| CD8+ TILs (negative/low versus high) | 4.829 | p = 0.028 | 0.16 (0.04–0.67) | p = 0.012 |
| PD-L1 Expression Tumour Cells | PD-L1 Expression Macrophages | ||||||
|---|---|---|---|---|---|---|---|
| Negative | Positive | Significance | Negative | Positive | Significance | ||
| CD8+ TILs | 0% | 41 | 6 | 45 | 2 | ||
| 1–10% | 55 | 21 | p = 0.000 | 60 | 16 | p = 0.001 | |
| >10% | 2 | 8 | 5 | 5 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Marín, R.; Reda, S.; Riobello, C.; Cabal, V.N.; Suárez-Fernández, L.; Vivanco, B.; López, F.; Llorente, J.L.; Hermsen, M.A. CD8+ Tumour-Infiltrating Lymphocytes and Tumour Microenvironment Immune Types as Biomarkers for Immunotherapy in Sinonasal Intestinal-Type Adenocarcinoma. Vaccines 2020, 8, 202. https://doi.org/10.3390/vaccines8020202
García-Marín R, Reda S, Riobello C, Cabal VN, Suárez-Fernández L, Vivanco B, López F, Llorente JL, Hermsen MA. CD8+ Tumour-Infiltrating Lymphocytes and Tumour Microenvironment Immune Types as Biomarkers for Immunotherapy in Sinonasal Intestinal-Type Adenocarcinoma. Vaccines. 2020; 8(2):202. https://doi.org/10.3390/vaccines8020202
Chicago/Turabian StyleGarcía-Marín, Rocío, Sara Reda, Cristina Riobello, Virginia N. Cabal, Laura Suárez-Fernández, Blanca Vivanco, Fernando López, José L. Llorente, and Mario A. Hermsen. 2020. "CD8+ Tumour-Infiltrating Lymphocytes and Tumour Microenvironment Immune Types as Biomarkers for Immunotherapy in Sinonasal Intestinal-Type Adenocarcinoma" Vaccines 8, no. 2: 202. https://doi.org/10.3390/vaccines8020202
APA StyleGarcía-Marín, R., Reda, S., Riobello, C., Cabal, V. N., Suárez-Fernández, L., Vivanco, B., López, F., Llorente, J. L., & Hermsen, M. A. (2020). CD8+ Tumour-Infiltrating Lymphocytes and Tumour Microenvironment Immune Types as Biomarkers for Immunotherapy in Sinonasal Intestinal-Type Adenocarcinoma. Vaccines, 8(2), 202. https://doi.org/10.3390/vaccines8020202







