Identification of Toxoplasma Gondii Tyrosine Hydroxylase (TH) Activity and Molecular Immunoprotection against Toxoplasmosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statements
2.2. Mice and Parasites
2.3. Preparation of Soluble Tachyzoite and Bradyzoite Antigens
2.4. Total RNA Extraction of T. Gondii
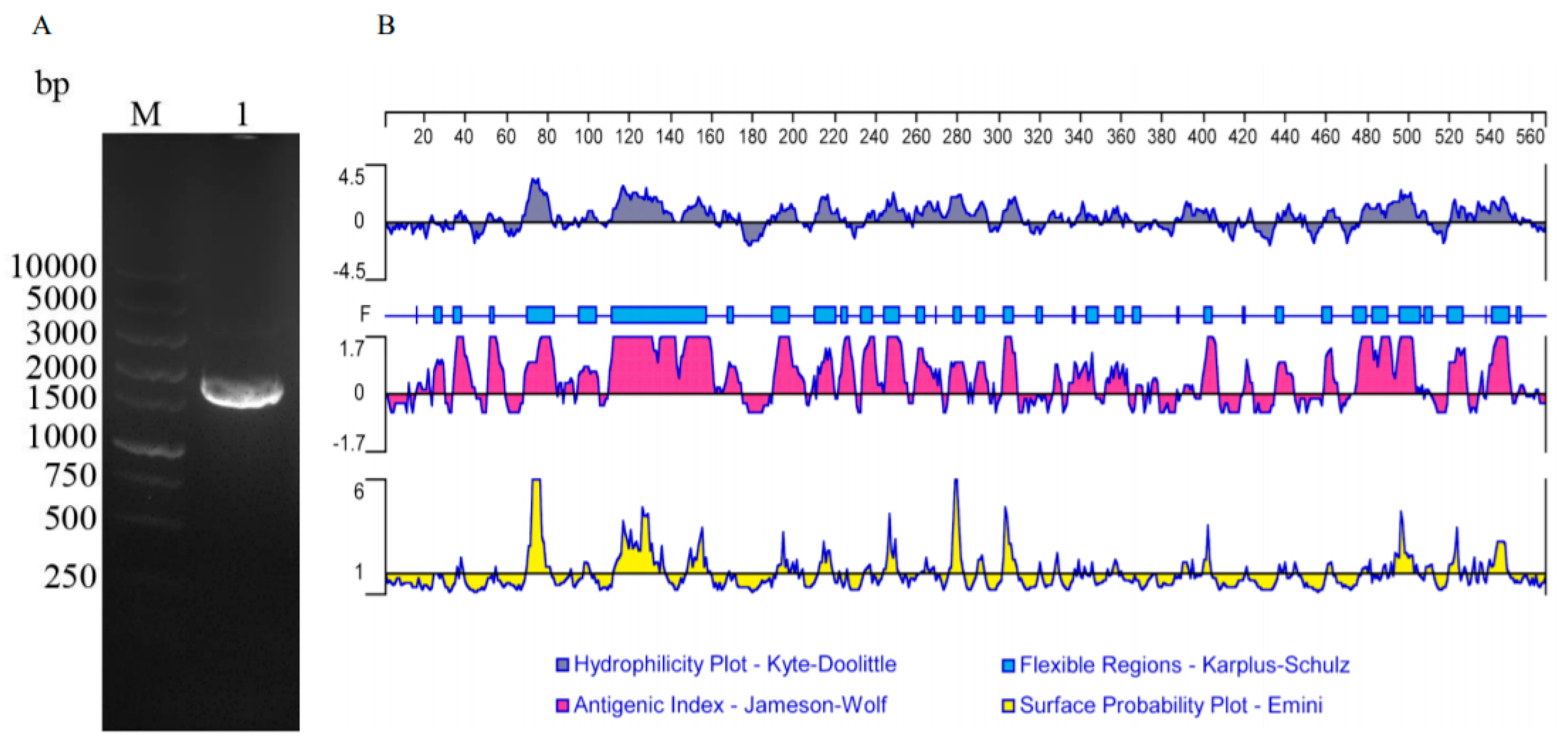
2.5. The Amplification of the ORF of TgTH
2.6. Analyzing Sequences
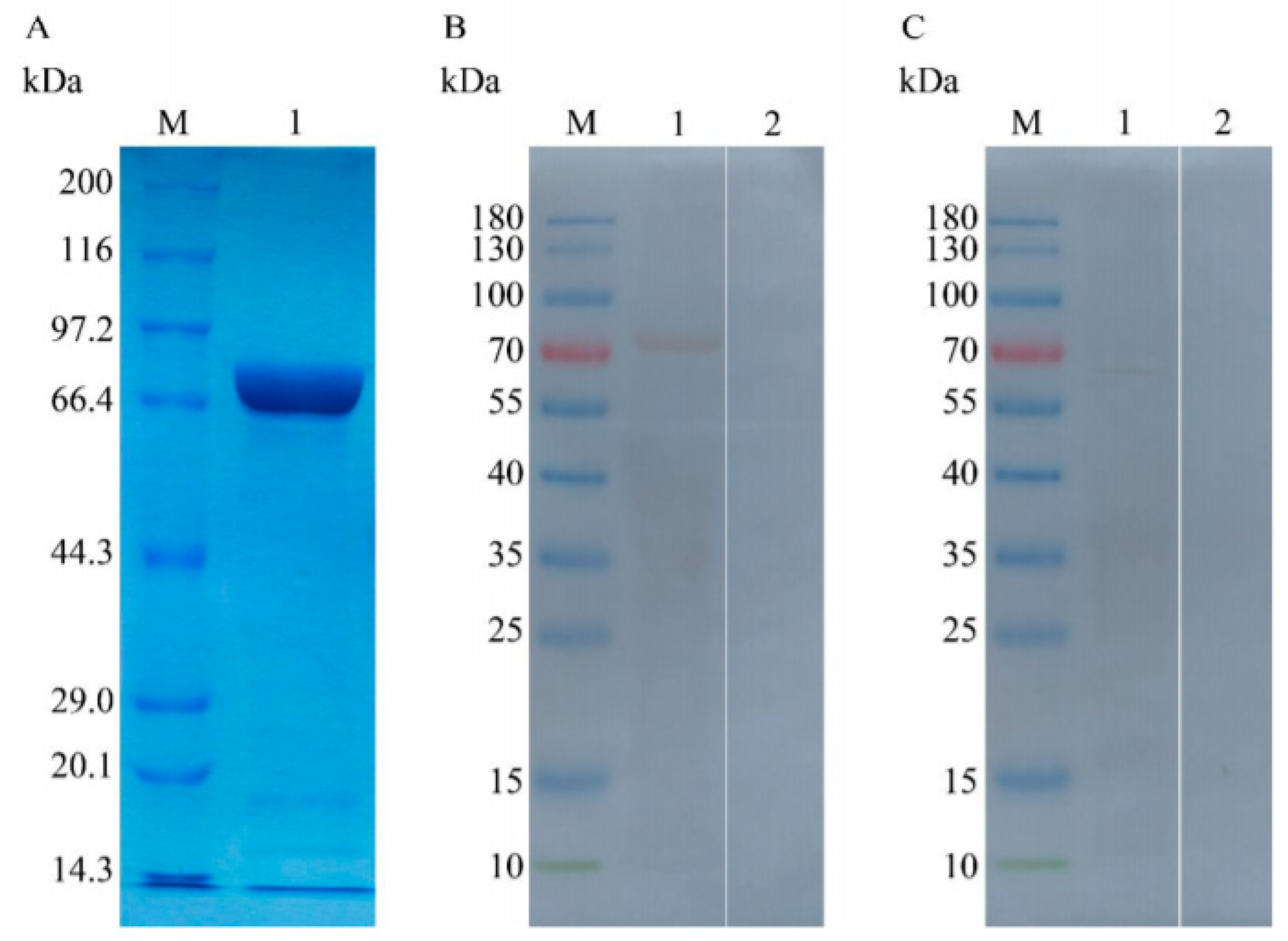
2.7. Expressing and Purifying the Proteins TgTH and pET-32a
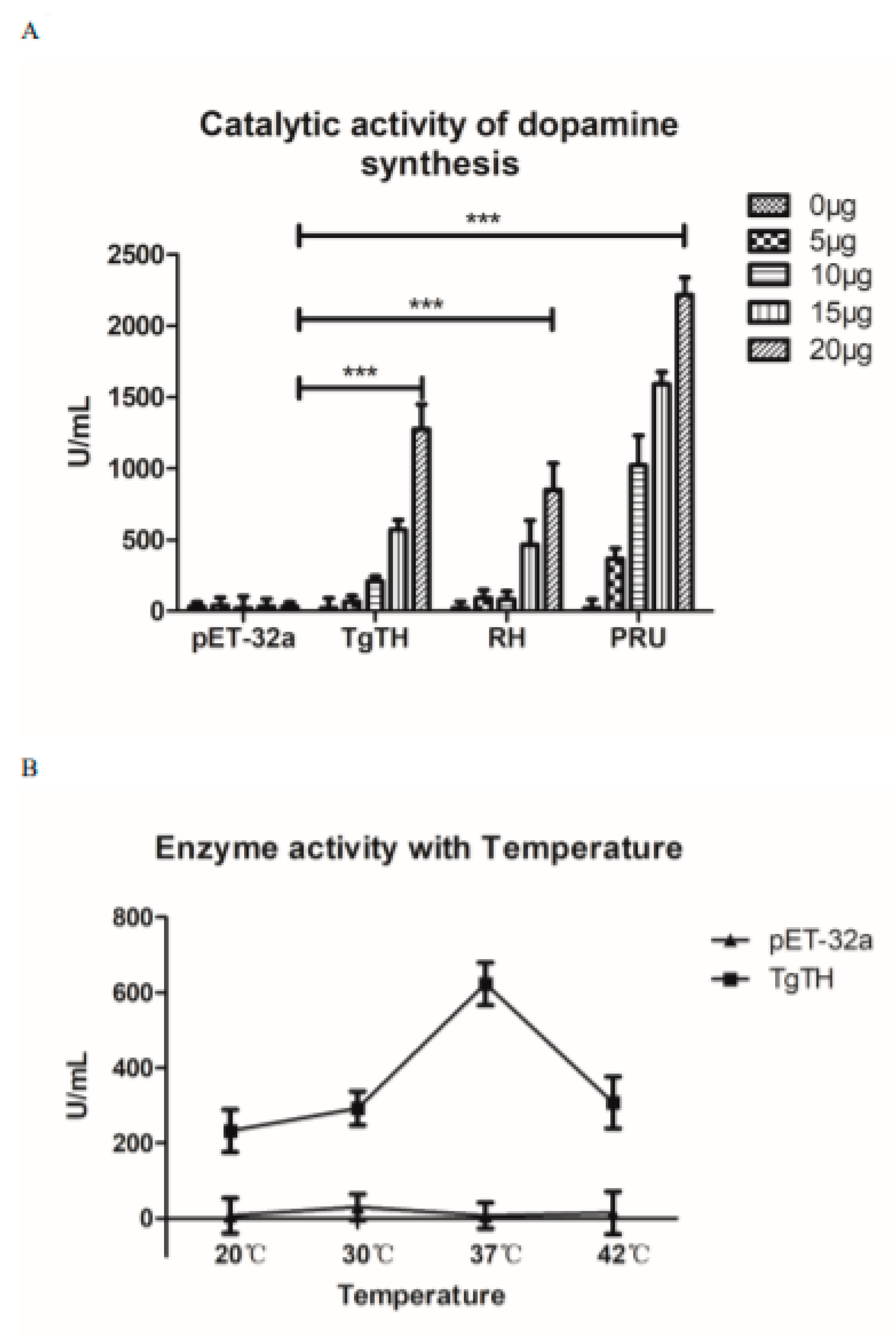
2.8. Enzyme Activity Test
2.9. Antisera against Recombined TgTH and T. gondii
2.10. Analyses of Natural and Recombined TgTH by Immunoblot
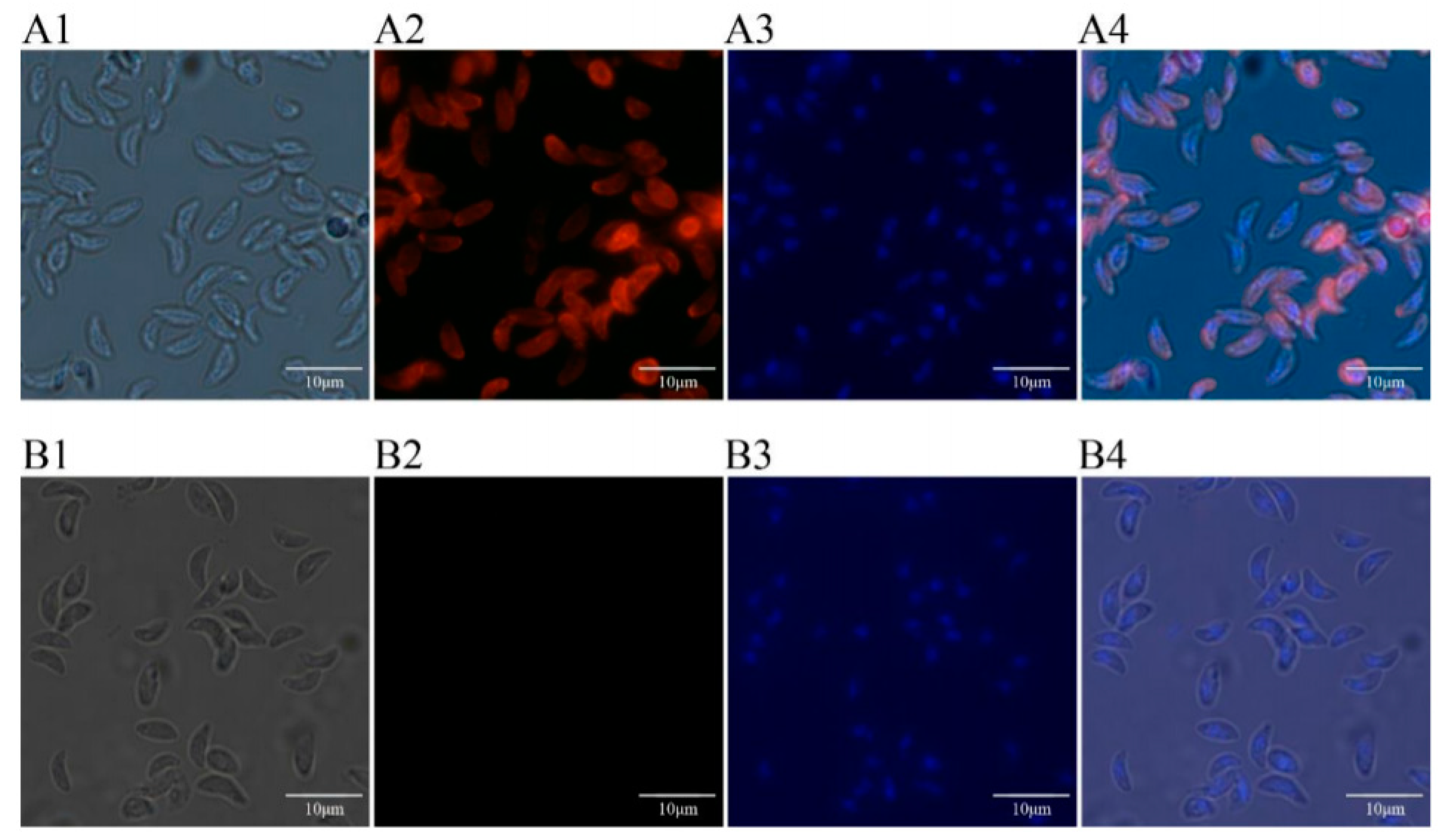
2.11. Expression and Location of TgTH in the Tachyzoite of T. Gondii by Immunofluorescence
2.12. Immunization and Challenge Infection
2.13. The Use of Enzymelinked Immunosorbent Assay (ELISA) for Determining Antibody Levels inSsera
2.14. Cytokine Determination
2.15. Analyses of Statistics
3. Results
3.1. Cloning and Sequence Analysis of TgTH
3.2. Expressing and Purifying Recombined TgTH
3.3. Analysing the Native and Recombined TgTH Using Immunoblot
3.4. Enzyme Activity Analysis of Dopamine Production
3.5. Expressions and Location of TgTH in Tachychites of T. Gondii
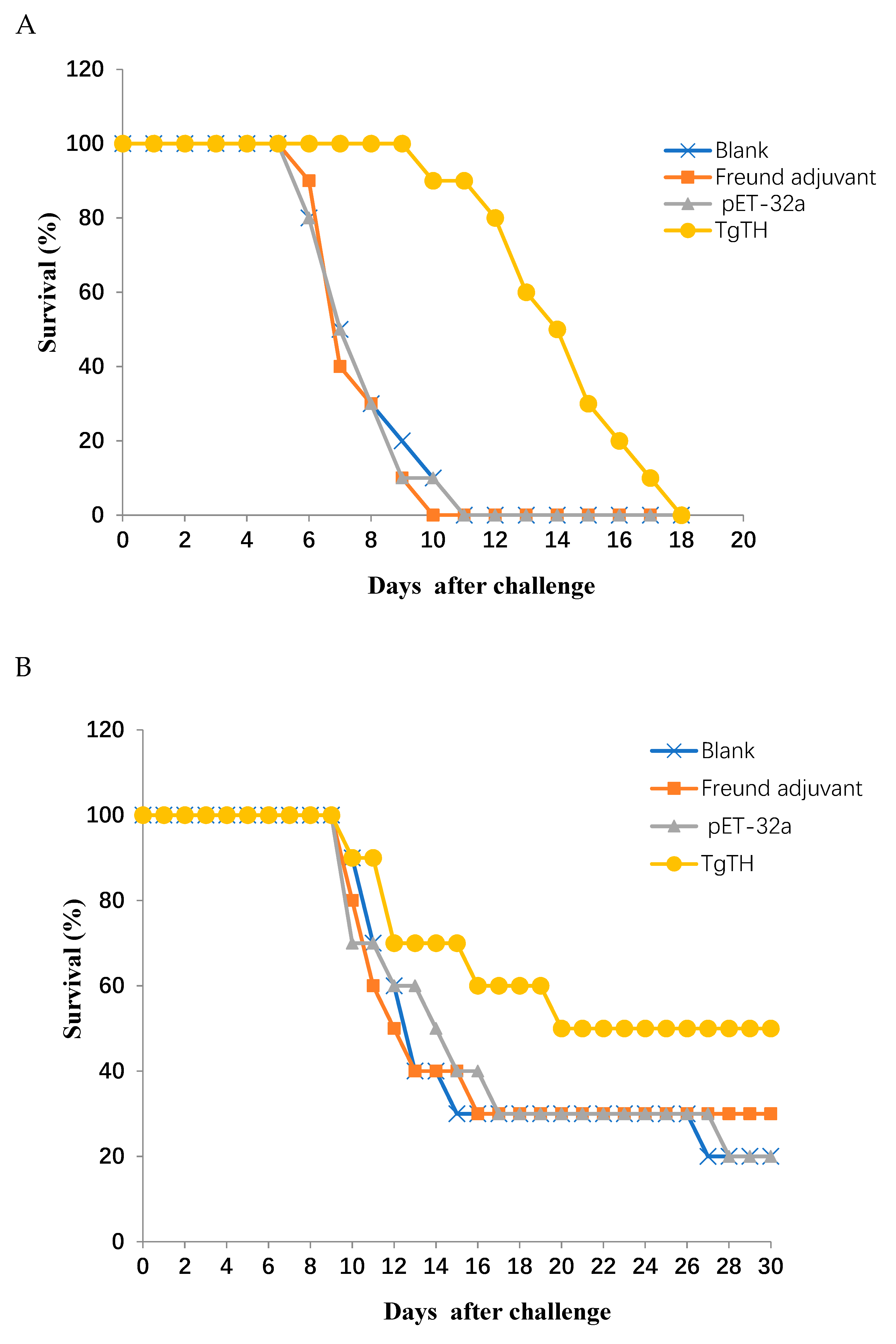
3.6. Evaluating the Protective Effect of Inoculation on theE rodents against Challenge of Pathogen
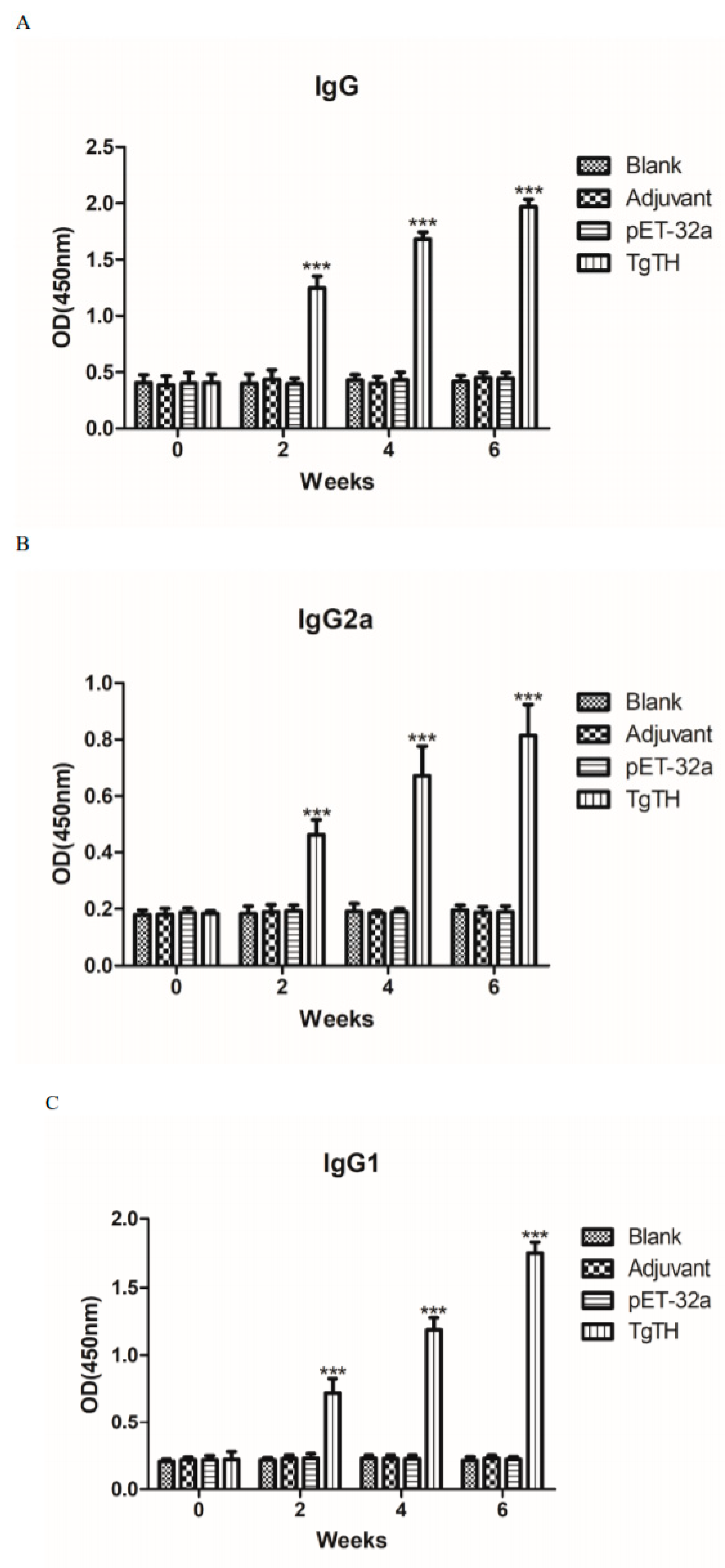
3.7. Humoral Immunoreactions
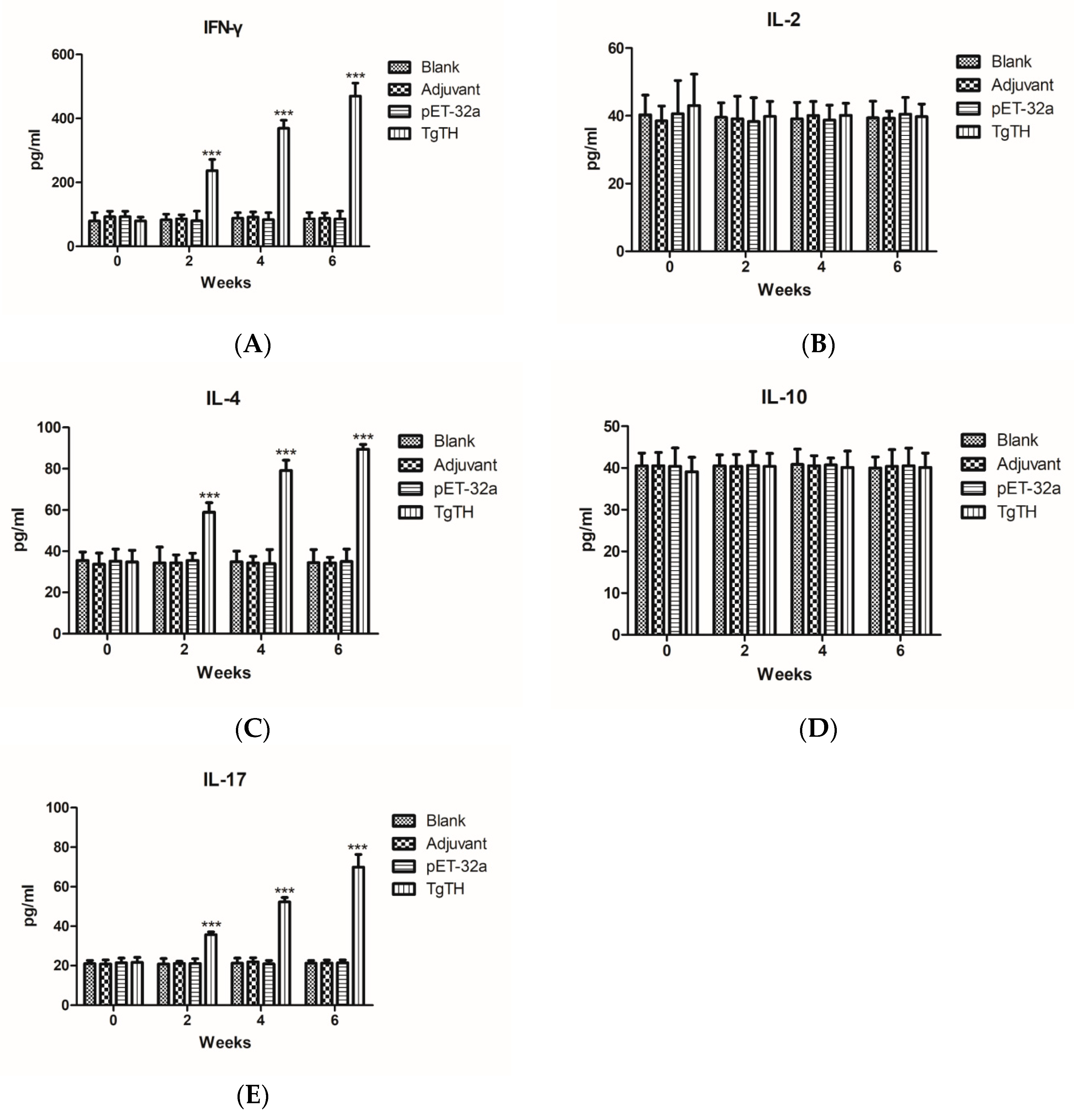
3.8. Cytokine Levels in Sera of Immunized Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Calderaro, A.; Piccolo, G.; Gorrini, C.; Peruzzi, S.; Zerbini, L.; Bommezzadri, S.; Dettori, G.; Chezzi, C. Comparison between two real-time PCR assays and a nested-PCR for the detection of Toxoplasma gondii. Acta Biomed. Ateneo Parm. 2006, 77, 75. [Google Scholar]
- Montoya, J.G.; Liesenfeld, O. Toxoplasmosis. Lancet 2004, 363, 1965–1976. [Google Scholar] [CrossRef]
- Bal, A.; Dhooria, S.; Agarwal, R.; Garg, M.; Das, A. Multiple and atypical opportunistic infections in a HIV patient with Toxoplasma myocarditis. Cardiovasc. Pathol. Off. J. Soc. Cardiovasc. Pathol. 2014, 23, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.F.; Dubey, J.P. Toxoplasma gondii abortion storm in sheep on a Texas farm and isolation of mouse virulent atypical genotype T. gondii from an aborted lamb from a chronically infected ewe. Vet. Parasitol. 2013, 192, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Chessa, G.; Chisu, V.; Porcu, R.; Masala, G. Molecular characterization of Toxoplasma gondii Type II in sheep abortion in Sardinia, Italy. Parasite 2014, 21, 6. [Google Scholar] [CrossRef] [PubMed]
- Gebremedhin, E.Z.; Abebe, A.H.; Tessema, T.S.; Tullu, K.D.; Medhin, G.; Vitale, M.; Di Marco, V.; Cox, E.; Dorny, P. Seroepidemiology of Toxoplasma gondii infection in women of child-bearing age in central Ethiopia. BMC Infect. Dis. 2013, 13, 101. [Google Scholar] [CrossRef]
- Innes, E.A. Vaccination against Toxoplasma gondii: An increasing priority for collaborative research? Expert Rev. Vaccines 2010, 9, 1117–1119. [Google Scholar] [CrossRef]
- Kur, J.; Holec-Gasior, L.; Hiszczynska-Sawicka, E. Current status of toxoplasmosis vaccine development. Expert Rev. Vaccines 2009, 8, 791–808. [Google Scholar] [CrossRef]
- Garrison, E.M.; Arrizabalaga, G. Disruption of a mitochondrial MutS DNA repair enzyme homologue confers drug resistance in the parasite Toxoplasma gondii. Mol. Microbiol. 2009, 72, 425–441. [Google Scholar] [CrossRef]
- Nagamune, K.; Moreno, S.N.; Sibley, L.D. Artemisinin-resistant mutants of Toxoplasma gondii have altered calcium homeostasis. Antimicrob. Agents Chemother. 2007, 51, 3816–3823. [Google Scholar] [CrossRef]
- Flegr, J. Influence of latent Toxoplasma infection on human personality, physiology and morphology: Pros and cons of the Toxoplasma-human model in studying the manipulation hypothesis. J. Exp. Biol. 2013, 216, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Webster, J.P.; Kaushik, M.; Bristow, G.C.; McConkey, G.A. Toxoplasma gondii infection, from predation to schizophrenia: Can animal behaviour help us understand human behaviour? J. Exp. Biol. 2013, 216, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Berdoy, M.; Webster, J.P.; Macdonald, D.W. Fatal attraction in rats infected with Toxoplasma gondii. Proc. Biol. Sci. 2000, 267, 1591–1594. [Google Scholar] [CrossRef] [PubMed]
- Vyas, A.; Kim, S.-K.; Giacomini, N.; Boothroyd, J.C.; Sapolsky, R.M. Behavioral changes induced by Toxoplasma infection of rodents are highly specific to aversion of cat odors. Proc. Natl. Acad. Sci. USA 2007, 104, 6442–6447. [Google Scholar] [CrossRef]
- Webster, J.P.; Mcconkey, G.A. Toxoplasma gondii-altered host behaviour: Clues as to mechanism of action. Folia Parasitol. 2010, 57, 95–104. [Google Scholar] [CrossRef]
- Prandovszky, E.; Gaskell, E.; Martin, H.; Dubey, J.P.; Webster, J.P.; McConkey, G.A. The Neurotropic Parasite Toxoplasma Gondii Increases Dopamine Metabolism. PLoS ONE 2011, 6, e23866. [Google Scholar] [CrossRef]
- Skallová, A.; Kodym, P.; Frynta, D.; Flegr, J. The role of dopamine in Toxoplasma-induced behavioural alterations in mice: An ethological and ethopharmacological study. Parasitology 2006, 133, 525. [Google Scholar] [CrossRef]
- Stibbs, H.H. Changes in brain concentrations of catecholamines and indoleamines in Toxoplasma gondii infected mice. Ann. Trop. Med. Parasitol. 1985, 79, 153–157. [Google Scholar] [CrossRef]
- Martin, H.L.; Alsaady, I.; Howell, G.; Prandovszky, E.; Peers, C.; Robinson, P.; McConkey, G.A. Effect of parasitic infection on dopamine biosynthesis in dopaminergic cells. Neuroscience 2015, 306, 50–62. [Google Scholar] [CrossRef]
- Gaskell, E.A.; Smith, J.E.; Pinney, J.W.; Westhead, D.R.; McConkey, G.A. A unique dual activity amino acid hydroxylase in Toxoplasma gondii. PLoS ONE 2009, 4, e4801. [Google Scholar] [CrossRef]
- Wang, Z.T.; Verma, S.K.; Dubey, J.P.; Sibley, L.D. The aromatic amino acid hydroxylase genes AAH1 and AAH2 in Toxoplasma gondii contribute to transmission in the cat. PLoS Pathog. 2017, 13, e1006272. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.Z.; Xu, Y.; Wang, M.; Chen, J.; Huang, S.Y.; Gao, Q.; Zhu, X.Q. Vaccination with Toxoplasma gondii calcium-dependent protein kinase 6 and rhoptry protein 18 encapsulated in poly(lactide-co-glycolide) microspheres induces long-term protective immunity in mice. BMC Infect. Dis. 2016, 16, 168. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Yanming, S.; Ruofeng, Y.; Muleke, C.I.; Guangwei, Z.; Lixin, X.; Xiangrui, L. Vaccination of goats with recombinant galectin antigen induces partial protection against Haemonchus contortus infection. Parasite Immunol. 2007, 29, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, Y.; Wang, M.; Xie, Q.; Li, P.; Zuo, S.; Kong, L.; Wang, C.; Wang, S. Immune Protection of Rhoptry Protein 21 (ROP21) of Toxoplasma gondii as a DNA Vaccine Against Toxoplasmosis. Front. Microbiol. 2018, 9, 909. [Google Scholar] [CrossRef]
- Lamberton, P.H.L.; Donnelly, C.A.; Webster, J.P. Specificity of the Toxoplasma gondii-altered behaviour to definitive versus non-definitive host predation risk. Parasitology 2008, 135, 1143–1150. [Google Scholar] [CrossRef]
- Webster, J.P.; Lamberton, P.H.L.; Donnelly, C.A.; Torrey, E.F. Parasites as causative agents of human affective disorders? The impact of anti-psychotic, mood-stabilizer and anti-parasite medication on Toxoplasma gondii’s ability to alter host behavior. Proc. R. Soc. B Biol. Sci. 2006, 273, 1023–1030. [Google Scholar] [CrossRef]
- Chen, J.; Huang, S.Y.; Li, Z.Y.; Yuan, Z.G.; Zhou, D.H.; Petersen, E.; Zhang, N.Z.; Zhu, X.Q. Protective immunity induced by a DNA vaccine expressing eIF4A of Toxoplasma gondii against acute toxoplasmosis in mice. Vaccine 2013, 31, 1734–1739. [Google Scholar] [CrossRef]
- Hassan, I.A.; Wang, S.; Xu, L.; Yan, R.; Song, X.; Li, X. Immunoglobulin and cytokine changes induced following immunization with a DNA vaccine encoding Toxoplasma gondii selenium-dependent glutathione reductase protein. Exp. Parasitol. 2014, 146, 1–10. [Google Scholar] [CrossRef]
- Verma, R.; Khanna, P. Development of Toxoplasma gondii vaccine: A global challenge. Hum. Vaccin. Immunother. 2013, 9, 291–293. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Y.; Shuai, W.; Xie, Q.; Nan, X.; Li, P.; Hong, G.; Liu, Q.; Li, X. Molecular Characterization and Protective Immunity of Rhoptry Protein 35 (ROP35) of Toxoplasma gondii as a DNA Vaccine. Vet. Parasitol. 2018, 260, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, Y.; Xie, Q.; Li, P.; Nan, X.; Kong, L.; Zeng, D.; Ding, Z.; Wang, S. The Molecular Characterization and Immunity Identification of Rhoptry Protein 22 of Toxoplasma gondii as a DNA Vaccine Candidate against Toxoplasmosis. J. Eukaryot. Microbiol. 2019, 66, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Tao, Q.; Fang, R.; Zhang, W.; Wang, Y.; Cheng, J.; Li, Y.; Fang, K.; Khan, M.K.; Hu, M.; Zhou, Y.; et al. Protective immunity induced by a DNA vaccine-encoding Toxoplasma gondii microneme protein 11 against acute toxoplasmosis in BALB/c mice. Parasitol. Res. 2013, 112, 2871–2877. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Zhou, A.; Lv, G.; Meng, M.; Sun, M.; Bai, Y.; Han, Y.; Wang, L.; Zhou, H.; Cong, H.; et al. Toxoplasma gondii cathepsin proteases are undeveloped prominent vaccine antigens against toxoplasmosis. BMC Infect. Dis. 2013, 13, 207. [Google Scholar] [CrossRef] [PubMed]
- Hassan, I.A.; Wang, S.; Xu, L.; Yan, R.; Song, X.; Li, X. DNA vaccination with a gene encoding Toxoplasma gondii Deoxyribose Phosphate Aldolase (TgDPA) induces partial protective immunity against lethal challenge in mice. Parasites Vectors 2014, 7, 431. [Google Scholar] [CrossRef]
- Hassan, I.A.; Wang, S.; Xu, L.; Yan, R.; Song, X.; XiangRui, L. Immunological response and protection of mice immunized with plasmid encoding Toxoplasma gondii glycolytic enzyme malate dehydrogenase. Parasite Immunol. 2014, 36, 674–683. [Google Scholar] [CrossRef]
- Paintlia, M.K.; Kaur, S.; Gupta, I.; Ganguly, N.K.; Mahajan, R.C.; Malla, N. Specific IgA response, T-cell subtype and cytokine profile in experimental intravaginal trichomoniasis. Parasitol. Res. 2002, 88, 338–343. [Google Scholar] [CrossRef]
- Xu, X.; Huang, H.; Wang, Q.; Cai, M.; Qian, Y.; Han, Y.; Wang, X.; Gao, Y.; Yuan, M.; Xu, L. IFN-γ-producing Th1-like regulatory T cells may limit acute cellular renal allograft rejection: Paradoxical post-transplantation effects of IFN-γ. Immunobiology 2016, 222, 280. [Google Scholar] [CrossRef]
- Liao, W.; Lin, J.X.; Leonard, W.J. IL-2 family cytokines: New insights into the complex roles of IL-2 as a broad regulator of T helper cell differentiation. Curr. Opin. Immunol. 2011, 23, 598–604. [Google Scholar] [CrossRef]
- Isabelle, D.; Kathy, J.; Hanan, C.; Sandrine, D.; Nicolas, P.; Laure, T.; Oberdan, L.; Véronique, F. Neonatal follicular Th cell responses are impaired and modulated by IL-4. J. Immunol. 2013, 191, 1231–1239. [Google Scholar]
- Beebe, A.M.; Cua, D.J.; Malefyt, R.D.W. The role of interleukin-10 in autoimmune disease: Systemic lupus erythematosus (SLE) and multiple sclerosis (MS). Cytokine Growth Factor Rev. 2015, 13, 403–412. [Google Scholar] [CrossRef]
- Wei, H.; Yin, L.; Feng, S.; Wang, X.; Yang, K.; Zhang, A.; Zhou, H. Dual-parallel inhibition of IL-10 and TGF-β1 controls LPS-induced inflammatory response via NF-κB signaling in grass carp monocytes/macrophages. Fish Shellfish Immunol. 2015, 44, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Wang, Q.; Yang, B.; Wu, L.; Cheng, G.; Kuang, H. Withasteroid B from D. metel L. regulates immune responses by modulating the JAK/STAT pathway and the IL-17+ RORγt+ /IL-10+ FoxP3+ ratio. Clin. Exp. Immunol. 2017, 190, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.T.; Gao, J.M.; Wu, Y.P.; Tang, P.; Hide, G.; Lai, D.H.; Lun, Z.R. Recombinant α-actinin subunit antigens of Trichomonas vaginalis as potential vaccine candidates in protecting against trichomoniasis. Parasites Vectors 2017, 10, 83. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Prasad, R.; Katiyar, S.K.; Yusuf, N.; Elmets, C.A.; Xu, H. Interleukin-17 mediated inflammatory responses are required for ultraviolet radiation-induced immune suppression. Photochem. Photobiol. 2015, 91, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.T.; Yao, X.T.; Peng, Q.; Chen, D.K. The protective and pathogenic roles of IL-17 in viral infections: Friend or foe? Open Biol. 2019, 9, 190109. [Google Scholar] [CrossRef]
- van Dalen, R.; De La Cruz Diaz, J.S.; Rumpret, M.; Fuchsberger, F.F.; van Teijlingen, N.H.; Hanske, J.; Rademacher, C.; Geijtenbeek, T.B.H.; van Strijp, J.A.G.; Weidenmaier, C.; et al. Langerhans Cells Sense Staphylococcus aureus Wall Teichoic Acid through Langerin To Induce Inflammatory Responses. MBio 2019, 10. [Google Scholar] [CrossRef]
- Wright, J.F.; Guo, Y.; Quazi, A.; Luxenberg, D.P.; Bennett, F.; Ross, J.F.; Qiu, Y.; Whitters, M.J.; Tomkinson, K.N.; Dunussijoannopoulos, K. Identification of an IL-17F/IL-17A heterodimer in activated human CD4+ T cells. J. Biol. Chem. 2007, 282, 13447–13455. [Google Scholar] [CrossRef]

| Groups | The Number of Brain Cysts (Mean ± SD) | Cysts Decrease Ratio (%) | The Size of Brain Cysts (px) (Mean ± SD) | Size Decrease Ratio (%) |
|---|---|---|---|---|
| Blank | 2375 ± 884 a | 0.00 a | 518.80 ± 50.50 a | 0.00 a |
| Freund adjuvant | 2333 ± 520 a | 1.77 a | 492.30 ± 57.17 a | 5.11 a |
| pET-32a | 2313 ± 265 a | 2.61 a | 495.20 ± 61.77 a | 4.55 a |
| TgTH | 1275 ± 224 b | 46.32 b | 293.50 ± 46.45 b | 43.43 b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Li, Y.; Li, H.; Song, X.; Ma, Z.; Lu, H.; Liu, S.; Zhao, Y.; Tan, M.; Wang, S.; et al. Identification of Toxoplasma Gondii Tyrosine Hydroxylase (TH) Activity and Molecular Immunoprotection against Toxoplasmosis. Vaccines 2020, 8, 158. https://doi.org/10.3390/vaccines8020158
Zhang Z, Li Y, Li H, Song X, Ma Z, Lu H, Liu S, Zhao Y, Tan M, Wang S, et al. Identification of Toxoplasma Gondii Tyrosine Hydroxylase (TH) Activity and Molecular Immunoprotection against Toxoplasmosis. Vaccines. 2020; 8(2):158. https://doi.org/10.3390/vaccines8020158
Chicago/Turabian StyleZhang, Zhenchao, Yuhua Li, Haoran Li, Xiaoxiao Song, Zhongshan Ma, Haoran Lu, Shuyue Liu, Yi Zhao, Mengyao Tan, Shuai Wang, and et al. 2020. "Identification of Toxoplasma Gondii Tyrosine Hydroxylase (TH) Activity and Molecular Immunoprotection against Toxoplasmosis" Vaccines 8, no. 2: 158. https://doi.org/10.3390/vaccines8020158
APA StyleZhang, Z., Li, Y., Li, H., Song, X., Ma, Z., Lu, H., Liu, S., Zhao, Y., Tan, M., Wang, S., & Li, X. (2020). Identification of Toxoplasma Gondii Tyrosine Hydroxylase (TH) Activity and Molecular Immunoprotection against Toxoplasmosis. Vaccines, 8(2), 158. https://doi.org/10.3390/vaccines8020158





