Recombinant Measles AIK-C Vaccine Strain Expressing Influenza HA Protein
Abstract
1. Introduction
2. Materials and Methods
2.1. Virus Strains and Cells
2.2. Construction of the Recombinant AIK-C Vaccine Strain
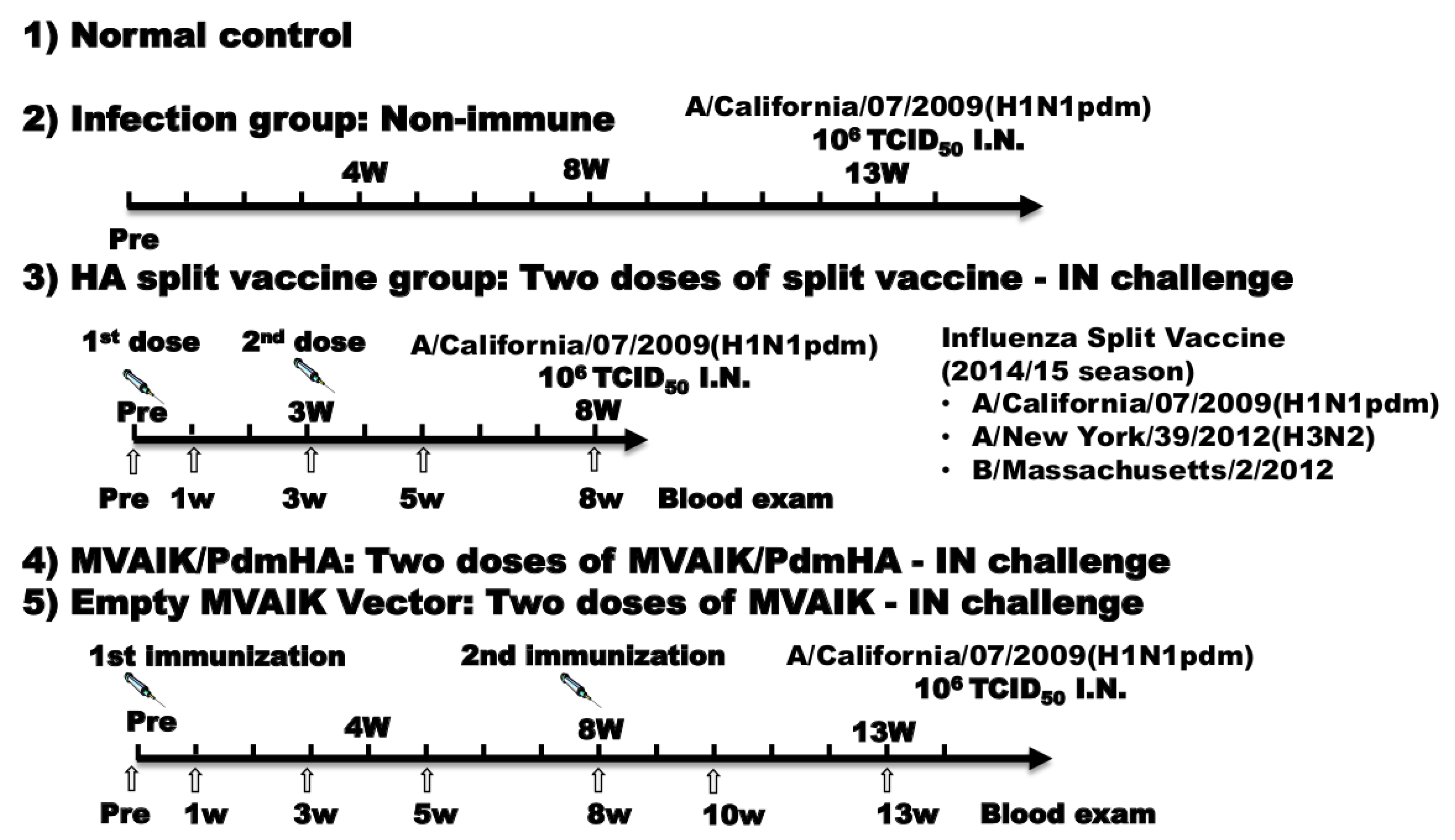
2.3. Experimental Protocol
2.4. Serology
2.5. Recovery of Influenza Virus and Titration of Infectivity
2.6. Immunostaining
2.7. Electron Microscopy
2.8. Histopathology
2.9. Quantification of Cytokine mRNA
3. Results
3.1. Virus Growth and Expression of Influenza HA Antigen
3.2. Measles PA and Influenza HI Antibodies
3.3. Protection against Influenza H1N1 Challenge
3.4. Recovery of Infectious Virus and Detection of the Influenza Antigen
3.5. Quantification of IFN-α, IL-1β, TNF-α, RANTES, and IFN-γ mRNA
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CPE | cytopathic effects |
| F | fusion |
| GMT | geometric mean titer |
| HA | hemagglutinin |
| HI | influenza hemagglutinin inhibition |
| IFN | interferon |
| IL | interleukin |
| PA | particle agglutination |
| Pdm | pandemic |
| RANTES | regulated on activation, normal T cell expressed and secreted |
| RBC | red blood cells |
| RSV | respiratory syncytial virus |
| RT-PCR | reverse transcription-polymerase chain reaction |
| SEM | scanning electron-microscope |
| TCID50 | 50% tissue culture infective dose |
| TNF-α | tumor necrotic factor-α |
References
- Molinari, N.A.; Ortega-Sanchez, I.R.; Messonnier, M.L.; Thompson, W.W.; Wortley, P.M.; Weintraub, E.; Bridges, C.B. The annual impact of seasonal influenza in the US: Measuring disease burden and costs. Vaccine 2007, 25, 5086–5096. [Google Scholar] [CrossRef] [PubMed]
- Jayasundara, K.; Soobiah, C.; Thommes, E.; Tricco, A.C.; Chit, A. Natural attack rate of influenza in unvaccinated children and adults: A meta-regression analysis. BMC Infect. Dis. 2014, 14, 670. [Google Scholar] [CrossRef] [PubMed]
- Guerche-Séblain, C.E.I.; Moureau, A.; Schiffler, C.; Dupuy, M.; Pepin, S.; Samson, S.I.; Vanhems, P.; Schellevis, F. Epidemiology and burden of influenza in healthy children aged 6 to 35 months: Analysis of data from the placebo arm of a phase III efficacy trial. BMC Infect. Dis. 2019, 19, 308. [Google Scholar] [CrossRef] [PubMed]
- Dugan, V.G.; Blanton, L.; Elal, A.I.A.; Alabi, N.; Barnes, J.; Brammer, L.; Burns, E.; Cummings, C.N.; Davis, T.; Flannery, B.; et al. Update: Influenza activity -United States, 1 October–25 November 2017. Morb. Mortal. Wkly. Rep. 2017, 66, 1318–1326. [Google Scholar] [CrossRef][Green Version]
- Ziegler, T.; Mamahit, A.; Cox, N.J. 65 years of influenza surveillance by a World Health Organization-coordinated global network. Influenza Other Respir. Viruses 2018, 12, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, C.; Rezza, G.; Ricciardi, W. Strategies in recommending influenza vaccination in Europe and US. Hum. Vaccin. Immunother. 2018, 14, 693–698. [Google Scholar] [CrossRef]
- Grohskopf, L.A.; Sokolow, L.Z.; Broder, K.R.; Walter, E.B.; Bresee, J.S.; Fry, A.M.; Jernigan, D.B. Prevention and control of seasonal influenza with vaccines: Recommendations of the Advisory Committee on Immunization Practices - United States, 2017–2018 Influenza Season. Morb. Mortal. Wkly. Rep. 2017, 66, 1–20. [Google Scholar] [CrossRef]
- Jorgensen, P.; Mereckiene, J.; Cotter, S.; Johansen, K.; Tsolova, S.; Brown, C. How close are countries of the WHO European Region to achieving the goal of vaccinating 75% of key risk groups against influenza? Results from national surveys on seasonal influenza vaccination programmes, 2008/2009 to 2014/2015. Vaccine 2018, 36, 442–452. [Google Scholar] [CrossRef]
- Greenberg, D.P.; Robertson, C.A.; Noss, M.J.; Blatter, M.M.; Biedenbender, R.; Decker, M.D. Safety and immunogenicity of a quadrivalent inactivated influenza vaccine compared to licensed trivalent inactivated influenza vaccines in adults. Vaccine 2013, 31, 770–776. [Google Scholar] [CrossRef]
- Osterholm, M.T.; Kelley, N.S.; Sommer, A.; Belongia, E.A. Efficacy and effectiveness of influenza vaccines: A systematic review and meta-analysis. Lancet Infect. Dis. 2013, 12, 36–44. [Google Scholar] [CrossRef]
- DiazGranados, C.A.; Denis, M.; Plotkin, S. Seasonal influenza vaccine efficacy and its determinants in children and non-elderly adults: A systematic review with meta-analyses of controlled trials. Vaccine 2012, 31, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Belongia, E.A.; Simpson, M.D.; King, J.P.; Sundaram, M.E.; Kelley, N.S.; Osterholm, M.T.; MaLean, H.Q. Variable influenza vaccine effectiveness by subtype: A systematic review and meta-analysis of test-negative design studies. Lancet Infect. Dis. 2016, 16, 942–951. [Google Scholar] [CrossRef]
- Tricco, A.C.; Chit, A.; Soobiah, C.; Hallett, D.; Meier, G.; Chen, M.; Tashkandi, M.; Bauch, C.T.; Loeb, M. Comparing influenza vaccine efficacy against mismatched and matched strains: A systematic review and meta-analysis. BMC Med. 2013, 11, 153. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, T.; Nagai, T.; Okui, T.; Tsutsumi, H.; Nagata, N.; Yano, S.; Nakayama, T.; Okuno, Y.; Kamiya, H. Poor immune responses to influenza vaccination in infants. Vaccine 2004, 22, 3404–3410. [Google Scholar] [CrossRef]
- Siegrist, C.A. Vaccine Immunology, 6th ed.; Plotkin, S.A., Orenstein, W.A., Offit, P.A., Eds.; Elsevier Saunders: Philadelphia, PA, USA, 2013; pp. 14–32. [Google Scholar]
- Nakayama, T. An inflammatory response is essential for the development of adaptive immunity-immunogenicity and immunotoxicity. Vaccine 2016, 34, 5815–5818. [Google Scholar] [CrossRef]
- Poland, G.A.; Ovsyannikova, I.G.; Jacobson, R.M. Immunogenetics of seasonal influenza vaccine response. Vaccine 2008, 26, D35–D40. [Google Scholar] [CrossRef]
- Wong, S.S.; Webby, R.J. Traditional and new influenza vaccines. Clin. Microbiol. Rev. 2013, 26, 476–492. [Google Scholar] [CrossRef]
- Zangwill, K.M.; Droge, J.; Mendelman, P.; Marcy, S.M.; Partridge, S.; Chiu, C.Y.; Jing, J.; Chang, S.J.; Cho, I.; Ward, J.I. Prospective, randomized, placebo-controlled evaluation of the safety and immunogenicity of three lots of intranasal trivalent influenza vaccine among young children. Pediatr. Infect. Dis. J. 2001, 20, 740–746. [Google Scholar] [CrossRef]
- Mohn, K.G.I.; Smith, I.; Sjursen, H.; Coxa, R.J. Immune responses after live attenuated influenza vaccination. Hum. Vaccin. Immunother. 2018, 14, 571–578. [Google Scholar] [CrossRef]
- Valkenburg, S.A.; Li, O.T.W.; Li, A.; Bull, M.; Waldmann, T.A.; Perera, L.P.; Peiris, M.; Poon, L.L. Protection by universal influenza vaccine is mediated by memory CD4 T cells. Vaccine 2018, 36, 4198–4206. [Google Scholar] [CrossRef]
- Sant, A.J.; DiPiazza, A.T.; Nayak, J.L.; Rattan, A.; Richards, K.A. CD4 T cells in protection from influenza virus: Viral antigen specificity and functional potential. Immunol. Rev. 2018, 284, 91–105. [Google Scholar] [CrossRef] [PubMed]
- Hoelscher, M.A.; Garg, S.; Bangari, D.S.; Belser, J.A.; Lu, X.; Stephenson, I.; Bright, R.A.; Katz, J.M.; Mittal, S.K.; Sambhara, S. Development of adenoviral-vector-based pandemic influenza vaccine against antigenically distinct human H5N1 strains in mice. Lancet 2006, 367, 475–481. [Google Scholar] [CrossRef]
- Mooney, A.J.; Li, Z.; Gabbard, J.D.; He, B.; Tompkins, S.M. Recombinant parainfluenza virus 5 vaccine encoding the influenza hemagglutinin protects against H5N1 highly pathogenic avian influenza virus infection following intranasal or intramuscular vaccination of BALB/c mice. J. Virol. 2013, 87, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Kreijtz, J.H.C.M.; Suezer, Y.; de Mutsert, G.; van Amerongen, G.; Schwantes, A.; van den Brand, J.M.A.; Fouchier, R.A.M.; Löwer, J.; Osterhaus, A.D.M.A.; Sutter, G.; et al. MVA-based H5N1 vaccine affords cross-clade protection in mice against influenza A/H5N1 viruses at low doses and after single immunization. PLoS ONE 2009, 4, e7790. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Liu, X.; Jiao, X.; Liu, X. Newcastle disease virus (NDV) recombinant expressing the hemagglutinin of H7N9 avian influenza virus protects chickens against NDV and highly pathogenic avian influenza A (H7N9) virus challenges. Vaccine 2017, 35, 6585–6590. [Google Scholar] [CrossRef]
- Nakayama, T.; Sawada, A.; Yamaji, Y.; Ito, T. Recombinant measles AIK-C vaccine strain expressing heterologous virus antigens. Vaccine 2016, 34, 292–295. [Google Scholar] [CrossRef]
- Higuchi, A.; Toriniwa, H.; Komiya, T.; Nakayama, T. Recombinant measles AIK-C vaccine strain expressing the prM-E antigen of Japanese encephalitis virus. PLoS ONE 2016, 11, e0150213. [Google Scholar] [CrossRef]
- Sawada, A.; Komase, K.; Nakayama, T. AIK-C measles vaccine expressing fusion protein respiratory syncytial virus induces protective antibodies in cotton rats. Vaccine 2011, 29, 1481–1490. [Google Scholar] [CrossRef] [PubMed]
- Green, M.G.; Huey, D.; Niewiesk, S. The cotton rat (Sigmodon hispidus) as an animal model for respiratory tract infections with human pathogens. Lab. Anim. (NY) 2013, 42, 170–176. [Google Scholar] [CrossRef]
- Truelove, S.; Zhu, H.; Lessler, J.; Riley, S.; Read, J.M.; Wang, S.; Kwok, K.O.; Guan, Y.; Jiang, C.Q.; Cummings, D.A.T. A comparison of hemagglutination inhibition and neutralization assays for characterizing immunity to seasonal influenza A. Influenza Other Respir. Viruses 2016, 10, 518–524. [Google Scholar] [CrossRef]
- Yamaji, Y.; Sawada, A.; Yasui, Y.; Ito, T.; Nakayama, T. Simultaneous administration of recombinant measles viruses expressing respiratory syncytial virus fusion (F) and nucleo (N) proteins induced humoral and cellular immune responses in cotton rats. Vaccines 2019, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Sawada, A.; Nakayama, T. Experimental animal model for analyzing immunobiological responses following vaccination with formalin-inactivated respiratory syncytial virus. Microbiol. Immunol. 2016, 60, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Black, S.; Nicolay, U.; Vesikari, T.; Knuf, M.; Del Giudice, G.; Cioppa, G.D.; Tsai, T.; Clemens, R.; Rappuoli, R. Hemagglutination inhibition antibody titers as a correlate of protection for inactivated influenza vaccines in children. Pediatr. Infect. Dis. J. 2011, 30, 1081–1085. [Google Scholar] [CrossRef] [PubMed]
- Caspard, H.; Heikkinen, T.; Belshe, R.B.; Ambrose, C.S. A systematic review of the efficacy of live attenuated influenza vaccine upon revaccination of children. Hum. Vaccin. Immunother. 2016, 12, 1721–1727. [Google Scholar] [CrossRef][Green Version]
- Mohn, K.G.I.; Bredholt, G.; Brokstad, K.A.; Pathirana, R.D.; Aarstad, H.J.; Tøndel, C.; Cox, R.J. Longevity of B-Cell and T-Cell responses after live attenuated influenza vaccination in children. J. Infect. Dis. 2015, 211, 1541–1549. [Google Scholar] [CrossRef] [PubMed]
- Makino, S.; Sasaki, K.; Nakamura, N.; Nakagawa, M.; Nakajima, S. Studies on the modification of the live AIK measles vaccine. II. Development and evaluation of the live AIK-C measles vaccine. Kitasato Arch. Exp. Med. 1974, 47, 13–21. [Google Scholar]
- Izadi, S.; Zahraei, S.M.; Salehi, M.; Mohammadi, M.; Tabatabaei, S.M.; Mokhtari-Azad, T. Head-to-head immunogenicity comparison of Edmonston-Zagreb vs. AIK-C measles vaccine strains in infants aged 8-12 months: A randomized clinical trial. Vaccine 2018, 36, 631–636. [Google Scholar] [CrossRef]
- Hien, N.G.; Luan, L.T.; Duong, D.H.; Lee, T.; Ito, T.; Nakayama, T. Clinical trial of measles and rubella combined vaccine produced by PolyVac in Vietnam. Open J. Pediatr. 2018, 8, 178–188. [Google Scholar] [CrossRef][Green Version]
- Ihara, T. The strategy for prevention of measles and rubella prevalence with measles-rubella (MR) vaccine in Japan. Vaccine 2009, 27, 3234–3236. [Google Scholar] [CrossRef]
- Komase, K.; Nakayama, T.; Iijima, M.; Miki, K.; Kawanishi, R.; Uejima, H. The phosphoprotein of attenuated measles AIK-C vaccine strain contributes to its temperature-sensitive phenotype. Vaccine 2006, 24, 826–834. [Google Scholar] [CrossRef]
- Nakayama, T.; Komase, K.; Uzauka, R.; Hoshi, A.; Okafuji, T. Leucine at position 278 of the AIK-C measles virus vaccine strain fusion protein is responsible for reduced syncytium formation. J. Gen. Virol. 2001, 82, 2143–2150. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tidjani, O.; Guérin, N.; Lecam, N.; Grunitsky, B.; Lévy-Bruhl, D.; Xuereff, C.; Tatagan, K. Serological effects of Edmonston-Zagreb, Schwarz, and AIK-C measles vaccine strains given at ages 4–5 or 8–10 months. Lancet 1989, 334, 1357–1360. [Google Scholar] [CrossRef]
- Bolotovski, V.M.; Grabowsky, M.; Clements, C.J.; Albrecht, P.; Brenner, E.R.; Zargaryantzs, A.I.; Litvinov, S.K.; Mikheyeva, I.V. Immunization of 6 and 9 month old infants with AIK-C, Edmonston-Zagreb, Leningrad-16 and Schwarz strains of measles vaccine. Int. J. Epidemiol. 1994, 23, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Nkrumah, F.K.; Osei-Kwasi, M.; Dunyo, S.K.; Koram, K.A.; Afari, E.A. Comparison of AIK-C measles vaccine in infants at 6 months with Schwarz vaccine at 9 months: A randomized controlled trial in Ghana. Bull. WHO 1998, 76, 353–359. [Google Scholar]
- Sato, T.A.; Miyamura, K.; Sakae, K.; Kobune, F.; Inouye, S.; Fujino, R.; Yamazaki, S. Development of a gelatin particle agglutination reagent for measles antibody assay. Arch. Virol. 1997, 142, 1971–1977. [Google Scholar] [CrossRef]
- Fujiyuki, T.; Horie, R.; Yoneda, M.; Kuraishi, T.; Yasui, F.; Kwon, H.J.; Munekata, K.; Ikeda, F.; Hoshi, M.; Kiso, M.; et al. Efficacy of recombinant measles virus expressing highly pathogenic avian influenza virus (HPAIV) antigen against HPAIV infection in monkeys. Sci. Rep. 2017, 7, 12017. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ito, T.; Kumagai, T.; Yamaji, Y.; Sawada, A.; Nakayama, T. Recombinant Measles AIK-C Vaccine Strain Expressing Influenza HA Protein. Vaccines 2020, 8, 149. https://doi.org/10.3390/vaccines8020149
Ito T, Kumagai T, Yamaji Y, Sawada A, Nakayama T. Recombinant Measles AIK-C Vaccine Strain Expressing Influenza HA Protein. Vaccines. 2020; 8(2):149. https://doi.org/10.3390/vaccines8020149
Chicago/Turabian StyleIto, Takashi, Takuji Kumagai, Yoshiaki Yamaji, Akihito Sawada, and Tetsuo Nakayama. 2020. "Recombinant Measles AIK-C Vaccine Strain Expressing Influenza HA Protein" Vaccines 8, no. 2: 149. https://doi.org/10.3390/vaccines8020149
APA StyleIto, T., Kumagai, T., Yamaji, Y., Sawada, A., & Nakayama, T. (2020). Recombinant Measles AIK-C Vaccine Strain Expressing Influenza HA Protein. Vaccines, 8(2), 149. https://doi.org/10.3390/vaccines8020149



