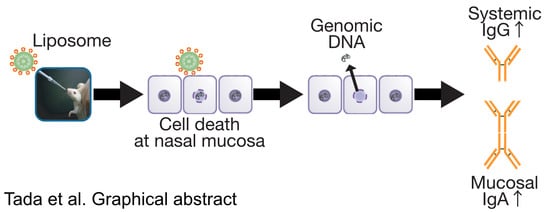
Essential Role of Host Double-Stranded DNA Released from Dying Cells by Cationic Liposomes for Mucosal Adjuvanticity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Materials
2.3. Liposome Preparation
2.4. Immunization and Sampling Schedule
2.5. ELISA Assay for the Detection of OVA-Specific Antibody Titers
2.6. DsDNA Quantification in Nasal Fluids
2.7. Flow Cytometric Assessment of Dying Cells
2.8. Preparation of Murine Genomic DNA
2.9. Mucosal Adjuvant Effects of Nasally Administered Genomic DNA
2.10. In Vivo DNase I Treatment
2.11. Statistical Analysis
3. Results
3.1. Cationic Liposomes Enhance Antigen-Specific Mucosal and Systemic Antibody Responses
3.2. Cationic Liposome Provokes Cell Death Followed by the Release of Host DsDNA at the Site of Injection
3.3. Host DsDNA Exerts Antigen-Specific Systemic and Mucosal Immune Responses
3.4. Mucosal Adjuvant Effect of Cationic Liposomes Relies upon Released Host DsDNA
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fauci, A.S.; Touchette, N.A.; Folkers, G.K. Emerging infectious diseases: A 10-year perspective from the National Institute of Allergy and Infectious Diseases. Emerg. Infect Dis. 2005, 11, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Fauci, A. Infectious diseases: Considerations for the 21st century. Clin. Infect Dis. 2001, 32, 675–685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, H.; Kondoh, M.; Yagi, K.; Kiyono, H.; Kunisawa, J. The Development of Mucosal Vaccine Using Bacterial Function for Targeting Mucosal Tissues. Yakuga Zasshi 2014, 134, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Kunisawa, J.; Gohda, M.; Kiyono, H. Uniqueness of the Mucosal Immune System for the Development of Prospective Mucosal Vaccine. Yakuga Zasshi 2007, 127, 319–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borges, O.; Lebre, F.; Bento, D.; Borchard, G.; Junginger, H.E. Mucosal vaccines: Recent progress in understanding the natural barriers. Pharm. Res. 2010, 27, 211–223. [Google Scholar] [CrossRef]
- Kunisawa, J.; Fukuyama, S.; Kiyono, H. Mucosa-associated lymphoid tissues in the aerodigestive tract: Their shared and divergent traits and their importance to the orchestration of the mucosal immune system. Curr. Mol. Med. 2005, 5, 557–572. [Google Scholar] [CrossRef]
- Ada, G. Vaccines and vaccination. N. Engl. J. Med. 2001, 345, 1042–1053. [Google Scholar] [CrossRef]
- McKee, A.S.; MacLeod, M.K.; Kappler, J.W.; Marrack, P. Immune mechanisms of protection: Can adjuvants rise to the challenge? BMC Biol. 2010, 8, 37. [Google Scholar] [CrossRef] [Green Version]
- McKee, A.S.; Munks, M.W.; Marrack, P. How Do Adjuvants Work? Important Considerations for New Generation Adjuvants. Immunity 2007, 27, 687–690. [Google Scholar] [CrossRef] [Green Version]
- Lamichhane, A.; Azegami, T.; Kiyono, H. The mucosal immune system for vaccine development. Vaccine 2014, 32, 6711–6723. [Google Scholar] [CrossRef] [Green Version]
- Boyaka, P.N. Inducing Mucosal IgA: A Challenge for Vaccine Adjuvants and Delivery Systems. J. Immunol. 2017, 199, 9–16. [Google Scholar] [CrossRef]
- Tada, R.; Hidaka, A.; Iwase, N.; Takahashi, S.; Yamakita, Y.; Iwata, T.; Muto, S.; Sato, E.; Takayama, N.; Honjo, E.; et al. Intranasal Immunization with DOTAP Cationic Liposomes Combined with DC-Cholesterol Induces Potent Antigen-Specific Mucosal and Systemic Immune Responses in Mice. PLoS ONE 2015, 10, e0139785. [Google Scholar] [CrossRef]
- Tada, R.; Muto, S.; Iwata, T.; Hidaka, A.; Kiyono, H.; Kunisawa, J.; Aramaki, Y. Attachment of class B CpG ODN onto DOTAP/DC-chol liposome in nasal vaccine formulations augments antigen-specific immune responses in mice. BMC Res. Notes 2017, 10, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Tada, R.; Hidaka, A.; Kiyono, H.; Kunisawa, J.; Aramaki, Y. Intranasal administration of cationic liposomes enhanced granulocyte–macrophage colony-stimulating factor expression and this expression is dispensable for mucosal adjuvant activity. BMC Res. Notes 2018, 11, 1–8. [Google Scholar] [CrossRef]
- Tada, R.; Suzuki, H.; Takahashi, S.; Negishi, Y.; Kiyono, H.; Kunisawa, J.; Aramaki, Y. Nasal vaccination with pneumococcal surface protein A in combination with cationic liposomes consisting of DOTAP and DC-chol confers antigen-mediated protective immunity against Streptococcus pneumoniae infections in mice. Int. Immunopharmacol. 2018, 61, 385–393. [Google Scholar] [CrossRef]
- Takahashi, S.; Tada, R.; Negishi, Y.; Aramaki, Y. Mechanisms of Enhanced Antigen Delivery to Murine Dendritic Cells by the Cationic Liposomes. Open. J. Immunol. 2017, 07, 85–101. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.Y.; Nuñez, G. Sterile inflammation: Sensing and reacting to damage. Nat. Rev. Immunol. 2010, 10, 826–837. [Google Scholar] [CrossRef] [Green Version]
- Jounai, N.; Kobiyama, K.; Takeshita, F.; Ishii, K.J. Recognition of damage-associated molecular patterns related to nucleic acids during inflammation and vaccination. Front. Cell. Infect. Microbiol. 2012, 2, 168. [Google Scholar] [CrossRef] [Green Version]
- Marichal, T.; Ohata, K.; Bedoret, D.; Mesnil, C.; Sabatel, C.; Kobiyama, K.; Lekeux, P.; Coban, C.; Akira, S.; Ishii, K.J.; et al. DNA released from dying host cells mediates aluminum adjuvant activity. Nat. Med. 2011, 17, 996–1002. [Google Scholar] [CrossRef]
- Iwaoka, S.; Nakamura, T.; Takano, S.; Tsuchiya, S.; Aramaki, Y. Cationic liposomes induce apoptosis through p38 MAP kinase-caspase-8-Bid pathway in macrophage-like RAW264.7 cells. J. Leukoc. Biol. 2006, 79, 184–191. [Google Scholar] [CrossRef]
- Arisaka, M.; Nakamura, T.; Yamada, A.; Negishi, Y.; Aramaki, Y. Involvement of protein kinase Cdelta in induction of apoptosis by cationic liposomes in macrophage-like RAW264.7 cells. Febs. Lett. 2010, 584, 1016–1020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arisaka, M.; Takano, K.; Negishi, Y.; Arima, H.; Aramaki, Y. Involvement of lipid rafts in macrophage apoptosis induced by cationic liposomes. Arch. Biochem. Biophys. 2011, 508, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Takano, K.; Sato, K.; Negishi, Y.; Aramaki, Y. Involvement of actin cytoskeleton in macrophage apoptosis induced by cationic liposomes. Arch. Biochem. Biophys. 2012, 518, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Watari, A.; Hashimoto, E.; Yonemitsu, M.; Kiyono, H.; Yagi, K.; Kondoh, M.; Kunisawa, J. C-Terminal Clostridium perfringens Enterotoxin-Mediated Antigen Delivery for Nasal Pneumococcal Vaccine. PLoS ONE 2015, 10, e0126352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, I.; Sato, A.; Yuki, Y.; Nochi, T.; Takahashi, H.; Sawada, S.; Mejima, M.; Kurokawa, S.; Okada, K.; Sato, S.; et al. Nanogel-based PspA intranasal vaccine prevents invasive disease and nasal colonization by Streptococcus pneumoniae. Infect. Immun. 2013, 81, 1625–1634. [Google Scholar] [CrossRef] [Green Version]
- Lu, G.; Gonzalez, R.; Guo, L.; Wu, C.; Wu, J.; Vernet, G.; Paranhos-Baccalà, G.; Wang, J.; Hung, T. Large-scale seroprevalence analysis of human metapneumovirus and human respiratory syncytial virus infections in Beijing, China. Virol. J. 2011, 8, 62. [Google Scholar] [CrossRef] [Green Version]
- Gillgrass, A.; Ashkar, A.; Rosenthal, K.; Kaushic, C. Prolonged Exposure to Progesterone Prevents Induction of Protective Mucosal Responses following Intravaginal Immunization with Attenuated Herpes Simplex Virus Type 2. J. Virol. 2003, 77, 9845–9851. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, M.; Aoshi, T.; Ozasa, K.; Kusakabe, T.; Momota, M.; Haseda, Y.; Kobari, S.; Kuroda, E.; Kobiyama, K.; Coban, C.; et al. RNA is an Adjuvanticity Mediator for the Lipid-Based Mucosal Adjuvant, Endocine. Sci. Rep. UK 2016, 6, 29165. [Google Scholar] [CrossRef] [Green Version]
- Nace, G.; Evankovich, J.; Eid, R.; Tsung, A. Dendritic Cells and Damage-Associated Molecular Patterns: Endogenous Danger Signals Linking Innate and Adaptive Immunity. J. Innate Immun. 2012, 4, 6–15. [Google Scholar] [CrossRef]
- Vono, M.; Taccone, M.; Caccin, P. The adjuvant MF59 induces ATP release from muscle that potentiates response to vaccination. Proc. Natl. Acad. Sci. USA 2013, 110, 21095–21100. [Google Scholar] [CrossRef] [Green Version]
- Aramaki, Y.; Takano, S.; Arima, H.; Tsuchiya, S. Induction of apoptosis in WEHI 231 cells by cationic liposomes. Pharm. Res. 2000, 17, 515–520. [Google Scholar] [CrossRef]
- Aramaki, Y.; Takano, S.; Tsuchiya, S. Induction of apoptosis in macrophages by cationic liposomes. FEBS Lett. 1999, 460, 472–476. [Google Scholar] [CrossRef] [Green Version]
- Aramaki, Y.; Takano, S.; Tsuchiya, S. Cationic liposomes induce macrophage apoptosis through mitochondrial pathway. Arch. Biochem. Biophys. 2001, 392, 245–250. [Google Scholar] [CrossRef]
- Kono, H.; Rock, K.L. How dying cells alert the immune system to danger. Nat. Rev. Immunol. 2008, 8, 279–289. [Google Scholar] [CrossRef]
- Ishii, K.; Suzuki, K.; Coban, C.; Takeshita, F.; Itoh, Y.; Matoba, H.; Kohn, L.; Klinman, D. Genomic DNA Released by Dying Cells Induces the Maturation of APCs. J. Immunol. 2001, 167, 2602–2607. [Google Scholar] [CrossRef] [Green Version]
- Miquel-Clopés, A.; Bentley, E.; Stewart, J.; Carding, S. Mucosal vaccines and technology. Clin. Exp. Immunol. 2019, 196, 205–214. [Google Scholar] [CrossRef] [Green Version]
- Zhu, D.; Hu, C.; Fan, F.; Qin, Y.; Huang, C.; Zhang, Z.; Lu, L.; Wang, H.; Sun, H.; Leng, X.; et al. Co-delivery of antigen and dual agonists by programmed mannose-targeted cationic lipid-hybrid polymersomes for enhanced vaccination. Biomaterials 2019, 206, 25–40. [Google Scholar] [CrossRef]
- Xia, Y.; Xie, Y.; Yu, Z.; Xiao, H.; Jiang, G.; Zhou, X.; Yang, Y.; Li, X.; Zhao, M.; Li, L.; et al. The Mevalonate Pathway Is a Druggable Target for Vaccine Adjuvant Discovery. Cell 2018, 175, 1059–1073. [Google Scholar] [CrossRef] [Green Version]
- WEIGLE, W.; McCONAHEY, P. The serological cross-reaction between bovine serum albumin and anti-ovalbumin. J. Immunol. Baltim. Md 1950 1962, 88, 121–127. [Google Scholar]
- Wegmann, F.; Gartlan, K.H.; Harandi, A.M.; Brinckmann, S.A.; Coccia, M.; Hillson, W.R.; Kok, W.; Cole, S.; Ho, L.P.; Lambe, T.; et al. Polyethyleneimine is a potent mucosal adjuvant for viral glycoprotein antigens. Nat. Biotechnol. 2012, 30, 883–888. [Google Scholar] [CrossRef] [Green Version]
- Noges, L.E.; White, J.; Cambier, J.C.; Kappler, J.W.; Marrack, P. Contamination of DNase Preparations Confounds Analysis of the Role of DNA in Alum-Adjuvanted Vaccines. J. Immunol. 2016, 197, 1221–1230. [Google Scholar] [CrossRef] [Green Version]
- Desmet, C.J.; Ishii, K.J. Nucleic acid sensing at the interface between innate and adaptive immunity in vaccination. Nat. Rev. Immunol. 2012, 12, 479–491. [Google Scholar] [CrossRef]
- Paludan, S.R.; Bowie, A.G. Immune Sensing of DNA. Immunity 2013, 38, 870–880. [Google Scholar] [CrossRef] [Green Version]
- Carroll, E.C.; Jin, L.; Mori, A.; Muñoz-Wolf, N.; Oleszycka, E.; Moran, H.B.; Mansouri, S.; McEntee, C.P.; Lambe, E.; Agger, E.; et al. The Vaccine Adjuvant Chitosan Promotes Cellular Immunity via DNA Sensor cGAS-STING-Dependent Induction of Type I Interferons. Immunity 2016, 44, 597–608. [Google Scholar] [CrossRef] [Green Version]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tada, R.; Ohshima, A.; Tanazawa, Y.; Ohmi, A.; Takahashi, S.; Kiyono, H.; Kunisawa, J.; Aramaki, Y.; Negishi, Y. Essential Role of Host Double-Stranded DNA Released from Dying Cells by Cationic Liposomes for Mucosal Adjuvanticity. Vaccines 2020, 8, 8. https://doi.org/10.3390/vaccines8010008
Tada R, Ohshima A, Tanazawa Y, Ohmi A, Takahashi S, Kiyono H, Kunisawa J, Aramaki Y, Negishi Y. Essential Role of Host Double-Stranded DNA Released from Dying Cells by Cationic Liposomes for Mucosal Adjuvanticity. Vaccines. 2020; 8(1):8. https://doi.org/10.3390/vaccines8010008
Chicago/Turabian StyleTada, Rui, Akihiro Ohshima, Yuya Tanazawa, Akari Ohmi, Saeko Takahashi, Hiroshi Kiyono, Jun Kunisawa, Yukihiko Aramaki, and Yoichi Negishi. 2020. "Essential Role of Host Double-Stranded DNA Released from Dying Cells by Cationic Liposomes for Mucosal Adjuvanticity" Vaccines 8, no. 1: 8. https://doi.org/10.3390/vaccines8010008
APA StyleTada, R., Ohshima, A., Tanazawa, Y., Ohmi, A., Takahashi, S., Kiyono, H., Kunisawa, J., Aramaki, Y., & Negishi, Y. (2020). Essential Role of Host Double-Stranded DNA Released from Dying Cells by Cationic Liposomes for Mucosal Adjuvanticity. Vaccines, 8(1), 8. https://doi.org/10.3390/vaccines8010008