Efficacy of HPV Vaccination in Women Receiving LEEP for Cervical Dysplasia: A Single Institution’s Experience
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. LEEP Procedure and Histological Findings
2.3. Statistical Analysis
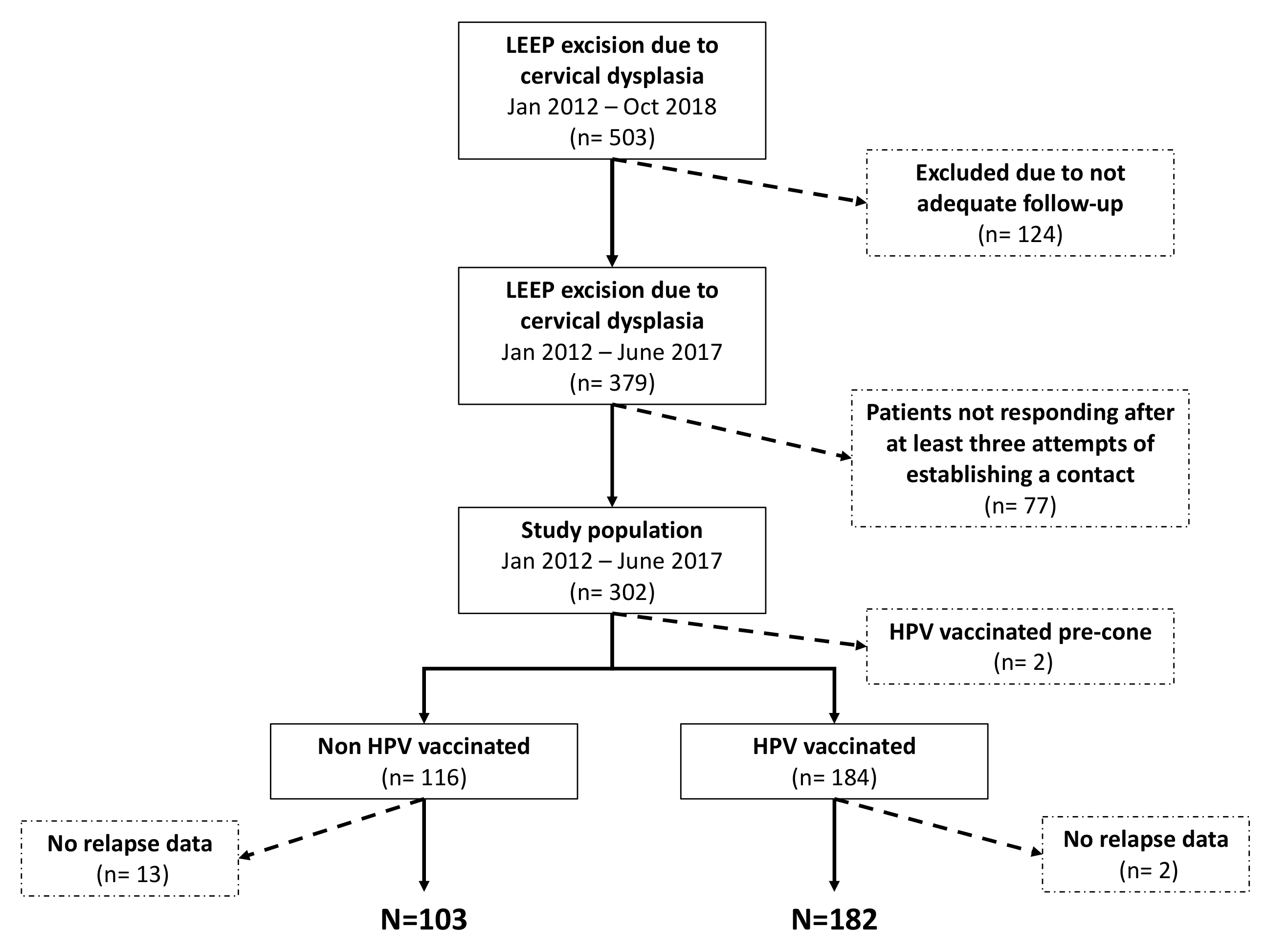
3. Results
3.1. Study Sample
3.2. Effectiveness of Post-LEEP HPV Vaccination
3.3. Predictors of LEEP Failure
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Harper, D.M.; DeMars, L.R. HPV vaccines—A review of the first decade. Gynecol. Oncol. 2017, 14, 196–204. [Google Scholar] [CrossRef]
- Bogani, G.; Maggiore, U.R.L.; Signorelli, M.; Martinelli, F.; Ditto, A.; Sabatucci, I.; Mosca, L.; Lorusso, D.; Raspagliesi, F. The role of human papillomavirus vaccines in cervical cancer: Prevention and treatment. Crit. Rev. Oncol. Hematol. 2018, 122, 92–97. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef]
- Schiller, J.T.; Day, P.M.; Kines, R.C. Current understanding of the mechanism of HPV infection. Gynecol. Oncol. 2010, 118, S12–S17. [Google Scholar] [CrossRef]
- Yoshinouchi, M.; Yamada, T.; Kizaki, M.; Fen, J.; Koseki, T.; Ikeda, Y.; Nishihara, T.; Yamato, K. In vitro and in vivo growth suppression of human papillomavirus 16-positive cervical cancer cells by E6 siRNA. Mol. Ther. 2003, 8, 762–768. [Google Scholar] [CrossRef]
- Duensing, S.; Münger, K. The human papillomavirus type 16 E6 and E7 oncoproteins independently induce numerical and structural chromosome instability. Cancer Res. 2002, 62, 7075–7082. [Google Scholar]
- Burk, R.D.; Terai, M.; Gravitt, P.E.; Brinton, L.A.; Kurman, R.J.; Barnes, W.A.; Greenberg, M.D.; Hadjimichael, O.C.; Fu, L.; McGowan, L.; et al. Distribution of human papillomavirus types 16 and 18 variants in squamous cell carcinomas and adenocarcinomas of the cervix. Cancer Res. 2003, 63, 7215–7220. [Google Scholar]
- Kaufmann, A.M.; Stern, P.L.; Rankin, E.M.; Sommer, H.; Nuessler, V.; Schneider, A.; Adams, M.; Onon, T.S.; Bauknecht, T.; Wagner, U.; et al. Safety and immunogenicity of TA-HPV, a recombinant vaccinia virus expressing modified human papillomavirus (HPV)-16 and HPV-18 E6 and E7 genes, in women with progressive cervical cancer. Clin. Cancer Res. 2002, 8, 3676–3685. [Google Scholar]
- Harro, C.D.; Pang, Y.Y.; Roden, R.B.; Hildesheim, A.; Wang, Z.; Reynolds, M.J.; Mast, T.C.; Robinson, R.; Murphy, B.R.; Karron, R.A.; et al. Safety and immunogenicity trial in adult volunteers of a human papillomavirus 16 L1 virus-like particle vaccine. J. Natl. Cancer Inst. 2001, 93, 284–292. [Google Scholar] [CrossRef]
- European Medicines Agency. Science Medicines Health. Human Papillomavirus Vaccines. 2017. Available online: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/referrals/Human_papillomavirus_vaccines/human_referral_prac_000053.jsp&mid=WC0b01ac05805c516f.Last (accessed on 20 June 2017).
- FDA. Vaccines, Blood & Biologics. 2017. Available online: https://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts (accessed on 30 June 2017).
- Bruni, L.; Diaz, M.; Barrionuevo-Rosas, L.; Herrero, R.; Bray, F.; Bosch, F.X.; de Sanjosé, S.; Castellsagué, X. Global estimates of human papillomavirus vaccination coverage by region and income level: A pooled analysis. Lancet Glob. Health 2016, 4, e453–e463. [Google Scholar] [CrossRef]
- Kang, W.D.; Choi, H.S.; Kim, S.M. Is vaccination with quadrivalent HPV vaccine after loop electrosurgical excision procedure effective in preventing recurrence in patients with high-grade cervical intraepithelial neoplasia (CIN2-3)? Gynecol. Oncol. 2013, 130, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Hildesheim, A.; Gonzalez, P.; Kreimer, A.R.; Wacholder, S.; Schussler, J.; Rodriguez, A.C.; Porras, C.; Schiffman, M.; Sidawy, M.; Schiller, J.T.; et al. Impact of human papillomavirus (HPV) 16 and 18 vaccination on prevalent infections and rates of cervical lesions after excisional treatment. Am. J. Obstet. Gynecol. 2016, 215, 212.e1–212.e15. [Google Scholar] [CrossRef] [PubMed]
- Prato, B.; Ghelardi, A.; Gadducci, A.; Marchetti, I.; Di Cristofano, C.; Di Coscio, G.; Bevilacqua, G.; Genazzani, A.R. Correlation of recurrence rates and times with posttreatment human papillomavirus status in patients treated with loop electrosurgical excision procedure conization for cervical squamous intraepithelial lesions. Int. J. Gynecol. Cancer. 2008, 18, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Alonso, I.; Torné, A.; Puig-Tintoré, L.M.; Esteve, R.; Quinto, L.; Campo, E.; Pahisa, J.; Ordi, J. Pre- and post-conization high-risk HPV testing predicts residual/recurrent disease in patients treated for CIN 2–3. Gynecol. Oncol. 2006, 103, 631–636. [Google Scholar] [CrossRef]
- Manchanda, R.; Baldwin, P.; Crawford, R.; Vowler, S.L.; Moseley, R.; Latimer, J.; Welton, K.; Shafi, M. Effect of margin status on cervical intraepithelial neoplasia recurrence following LLETZ in women over 50 years. BJOG 2008, 115, 1238–1242. [Google Scholar] [CrossRef]
- Zhu, M.; He, Y.; Baak, J.P.; Zhou, X.; Qu, Y.; Sui, L.; Feng, W.; Wang, Q. Factors that influence persistence or recurrence of high-grade squamous intraepithelial lesion with positive margins after the loop electrosurgical excision procedure: A retrospective study. BMC Cancer. 2015, 15, 744. [Google Scholar] [CrossRef]
- Swedish, K.A.; Factor, S.H.; Goldstone, S.E. Prevention of recurrent high-grade anal neoplasia with quadrivalent human papillomavirus vaccination of men who have sex with men: A nonconcurrent cohort study. Clin. Infect. Dis. 2012, 54, 891–898. [Google Scholar] [CrossRef]
- Joura, E.A.; Garland, S.M.; Paavonen, J.; Ferris, D.G.; Perez, G.; Ault, K.A.; Huh, W.K.; Sings, H.L.; James, M.K.; Haupt, R.M. Effect of the human papillomavirus (HPV) quadrivalent vaccine in a subgroup of women with cervical and vulvar disease: Retrospective pooled analysis of trial data. BMJ 2012, 344, e1401. [Google Scholar] [CrossRef]
- Joura, E.A.; Giuliano, A.R.; Iversen, O.E.; Bouchard, C.; Mao, C.; Mehlsen, J.; Moreira, E.D.; Ngan, Y.; Petersen, L.K.; Lazcano-Ponce, E.; et al. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N. Engl. J. Med. 2015, 372, 711–723. [Google Scholar] [CrossRef]
- Ghelardi, A.; Parazzini, F.; Martella, F.; Pieralli, A.; Bay, P.; Tonetti, A.; Svelato, A.; Bertacca, G.; Lombardi, S.; Joura, E.A. SPERANZA project: HPV vaccination after treatment for CIN2. Gynecol. Oncol. 2018, 151, 229–234. [Google Scholar] [CrossRef]
- Pieralli, A.; Bianchi, C.; Auzzi, N.; Fallani, M.G.; Bussani, C.; Fambrini, M.; Cariti, G.; Scarselli, G.; Petraglia, F.; Ghelardi, A. Indication of prophylactic vaccines as a tool for secondary prevention in HPV-linked disease. Arch. Gynecol. Obstet. 2018, 298, 1205–1210. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.C.; Ouh, Y.T.; Sung, M.H.; Park, H.G.; Kim, T.J.; Cho, C.H.; Park, J.S.; Lee, J.K. A phase 1/2a, dose-escalation, safety and preliminary efficacy study of oral therapeutic vaccine in subjects with cervical intraepithelial neoplasia 3. J. Gynecol. Oncol. 2019, 30, e88. [Google Scholar] [CrossRef] [PubMed]
- Tewari, K.S. Therapeutic vaccination using HPV 16 E7 to eradicate CIN3. J. Gynecol. Oncol. 2019, 30, e119. [Google Scholar] [CrossRef] [PubMed]
| Clinico-Pathological Variables | Total | Nonvaccinated | Vaccinated | p-Value | |
|---|---|---|---|---|---|
| All cases | 285 (100.0) | 103 (36.1) | 182 (63.9) | - | |
| Median (IQR) age, years | 39 (32–47) | 41 (36–49) | 37.5 (30–44) | 0.0004 | |
| Oral contraceptive use, n (%) | 46 (16.1) | 16 (13.8) | 32 (17.4) | 0.41 | |
| Infections, n (%) | 6 (2.1) | 3 (2.6) | 3 (1.6) | 0.68 | |
| Vegetarian habits, n (%) | 16 (5.6) | 7 (6.0) | 11 (6.0) | 1.0 | |
| Smoking exposure, n (%) | 69 (24.2) | 28 (24.1) | 46 (25.0) | 0.87 | |
| Total number of sexual partners >3, n (%) | 195 (68.4) | 77 (66.4) | 129 (70.1) | 0.50 | |
| Cervical biopsy, n (%) | Negative | 2 (0.7) | 1 (0.9) | 1 (0.6) | 0.98 |
| CIN1 | 5 (1.8) | 2 (1.8) | 3 (1.7) | ||
| CIN2 | 144 (51.8) | 57 (50.9) | 96 (53.0) | ||
| CIN3 | 113 (40.7) | 45 (40.2) | 72 (39.8) | ||
| CIS | 14 (5.0) | 7 (6.3) | 9 (5.0) | ||
| HPV virotypes, n (%) | HPV 16 | 17 (5.9) | 4 (3.9) | 13 (7.1) | 0.484 |
| HPV 16 + other | 8 (2.9) | 1 (0.2) | 7 (3.8) | ||
| HPV 18 | 2 (0.3) | 0 (0.0) | 2 (1.1) | ||
| HPV 31 | 9 (3.3) | 3 (2.9) | 6 (3.3) | ||
| HPV 51 | 4 (1.6) | 2 (1.9) | 2 (0.1) | ||
| Other | 8 (2.6) | 4 (3.9) | 4 (12.8) | ||
| High-risk * | 237(83.4) | 89 (87.2) | 148 (71.8) | ||
| Immunodepression, n (%) | 1 (0.4) | - | 1 (0.5) | 1.0 | |
| Median (IQR) cone length, mm | 25 (15–35) | 25 (15–35) | 25 (15–30) | 0.62 | |
| Histology cone, n (%) | Negative | 9 (3.2) | 2 (1.7) | 8 (4.4) | 0.44 |
| CIN1 | 43 (15.1) | 14 (12.1) | 31 (16.9) | ||
| CIN2 | 104 (36.5) | 45 (38.8) | 65 (35.3) | ||
| CIN3 | 103 (36.1) | 41 (35.4) | 65 (35.3) | ||
| CIS | 26 (9.1) | 14 (12.1) | 15 (8.2) | ||
| P16 positive, n (%) | 91 (31.9) | 37 (31.9) | 58 (31.5) | 0.95 | |
| Involvement of glandular outlets, n (%) | 129 (45.3) | 58 (50.0) | 76 (41.3) | 0.14 | |
| Positive margin of resection, n (%) | 25 (8.8) | 15 (12.9) | 13 (7.1) | 0.09 | |
| Positive apex, n (%) | 43 (15.1) | 23 (19.8) | 26 (14.1) | 0.19 | |
| Median follow-up time, months (range) | 6 (4–12) | 6 (5–12) | 6 (3–8) | 0.002 | |
| Clinico-Pathological Variables | Nonvaccinated n = 103 | Vaccinated n = 182 | p-Value | |
|---|---|---|---|---|
| Relapse | 17 (16.5) | 13 (7.1) | 0.01 | |
| Histology at recurrence, n (%) | CIN1 | 3/17 (17.7) | 7/13 (53.9) | 0.04 |
| CIN2 | 6/17 (35.3) | 4/13 (30.8) | 0.80 | |
| CIN3 | 8/17 (47.1) | 1/13 (7.7) | 0.02 | |
| CIS | 0/17 (0.0) | 1/13 (7.7) | 0.25 | |
| Clinico-Pathological Variables | Relapse | |
|---|---|---|
| OR (95%CI) | p-Value | |
| Age ≥40 | 1.2 (0.6–2.6) | 0.60 |
| Age | 1.0 (1.0–1.1) | 0.33 |
| Positive margin of resection | 2.3 (0.8–6.5) | 0.13 |
| Positive apex | 1.4 (0.6–3.8) | 0.46 |
| Oral contraceptive use | 1.1 (0.4–2.9) | 0.92 |
| Smoking exposure | 1.1 (0.5–2.7) | 0.76 |
| Parity | 1.1 (0.8–1.6) | 0.59 |
| Vaccinated | 0.4 (0.2–0.8) | 0.02 |
| Clinico-Pathological Variables | Relapse | |
|---|---|---|
| OR (95%CI) | p-Value | |
| Age ≥40 | 1.2 (0.6–2.6) | 0.60 |
| Age | 1.0 (1.0–1.1) | 0.33 |
| Positivity margin of resection | 2.3 (0.8–6.5) | 0.13 |
| Apex positivity | 1.4 (0.6–3.8) | 0.46 |
| Oral contraceptive use | 1.1 (0.4–2.9) | 0.92 |
| Smoking exposure | 1.1 (0.5–2.7) | 0.76 |
| Parity | 1.1 (0.8–1.6) | 0.59 |
| Vaccinated | 0.4 (0.2–0.8) | 0.02 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrillo, M.; Dessole, M.; Tinacci, E.; Saderi, L.; Muresu, N.; Capobianco, G.; Cossu, A.; Dessole, S.; Sotgiu, G.; Piana, A. Efficacy of HPV Vaccination in Women Receiving LEEP for Cervical Dysplasia: A Single Institution’s Experience. Vaccines 2020, 8, 45. https://doi.org/10.3390/vaccines8010045
Petrillo M, Dessole M, Tinacci E, Saderi L, Muresu N, Capobianco G, Cossu A, Dessole S, Sotgiu G, Piana A. Efficacy of HPV Vaccination in Women Receiving LEEP for Cervical Dysplasia: A Single Institution’s Experience. Vaccines. 2020; 8(1):45. https://doi.org/10.3390/vaccines8010045
Chicago/Turabian StylePetrillo, Marco, Margherita Dessole, Elettra Tinacci, Laura Saderi, Narcisa Muresu, Giampiero Capobianco, Antonio Cossu, Salvatore Dessole, Giovanni Sotgiu, and Andrea Piana. 2020. "Efficacy of HPV Vaccination in Women Receiving LEEP for Cervical Dysplasia: A Single Institution’s Experience" Vaccines 8, no. 1: 45. https://doi.org/10.3390/vaccines8010045
APA StylePetrillo, M., Dessole, M., Tinacci, E., Saderi, L., Muresu, N., Capobianco, G., Cossu, A., Dessole, S., Sotgiu, G., & Piana, A. (2020). Efficacy of HPV Vaccination in Women Receiving LEEP for Cervical Dysplasia: A Single Institution’s Experience. Vaccines, 8(1), 45. https://doi.org/10.3390/vaccines8010045









