Evaluation of the Safety and Protection Efficacy of spiC and nmpC or rfaL Deletion Mutants of Salmonella Enteritidis as Live Vaccine Candidates for Poultry Non-Typhoidal Salmonellosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Development of S. Enteritidis Mutant Strains
2.2. Chickens
2.3. Virulence Assessment
2.4. Biochemical Test and Growth Ability In Vitro
2.5. Bacterial Vaccination and Changes in Body Weight in Chickens
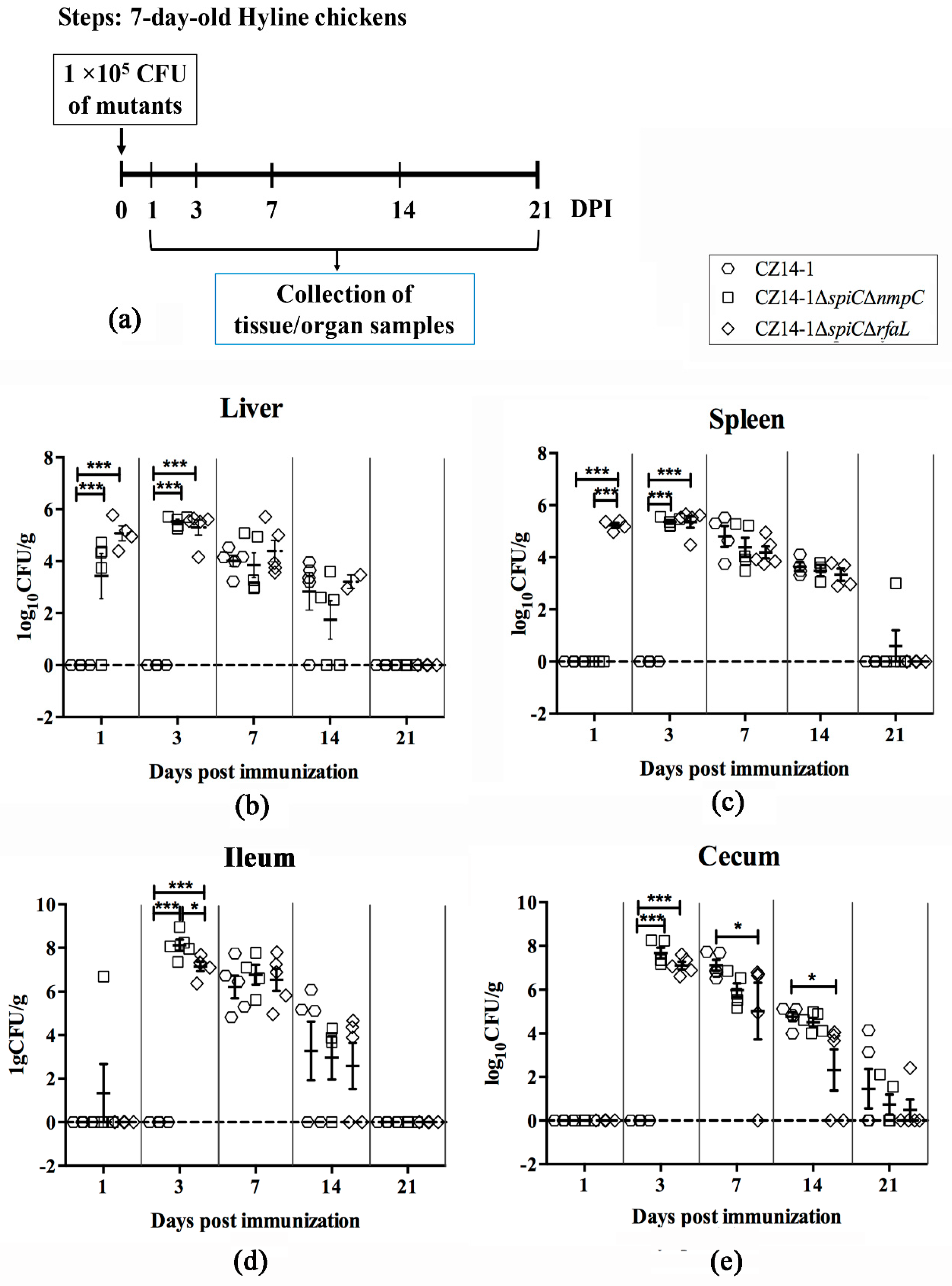
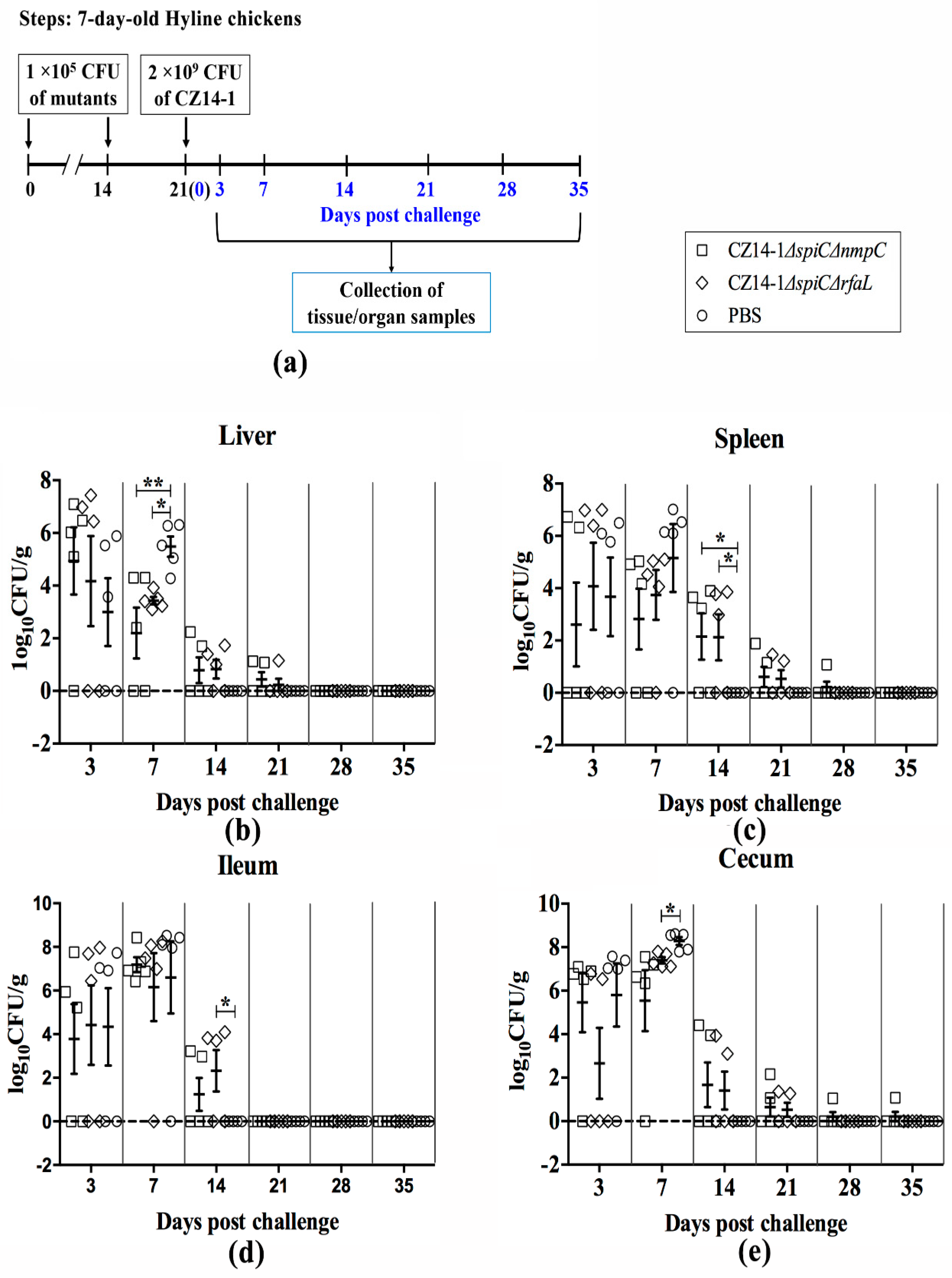
2.6. Persistence and Clearance of Bacteria in Chicken Tissues and Organs
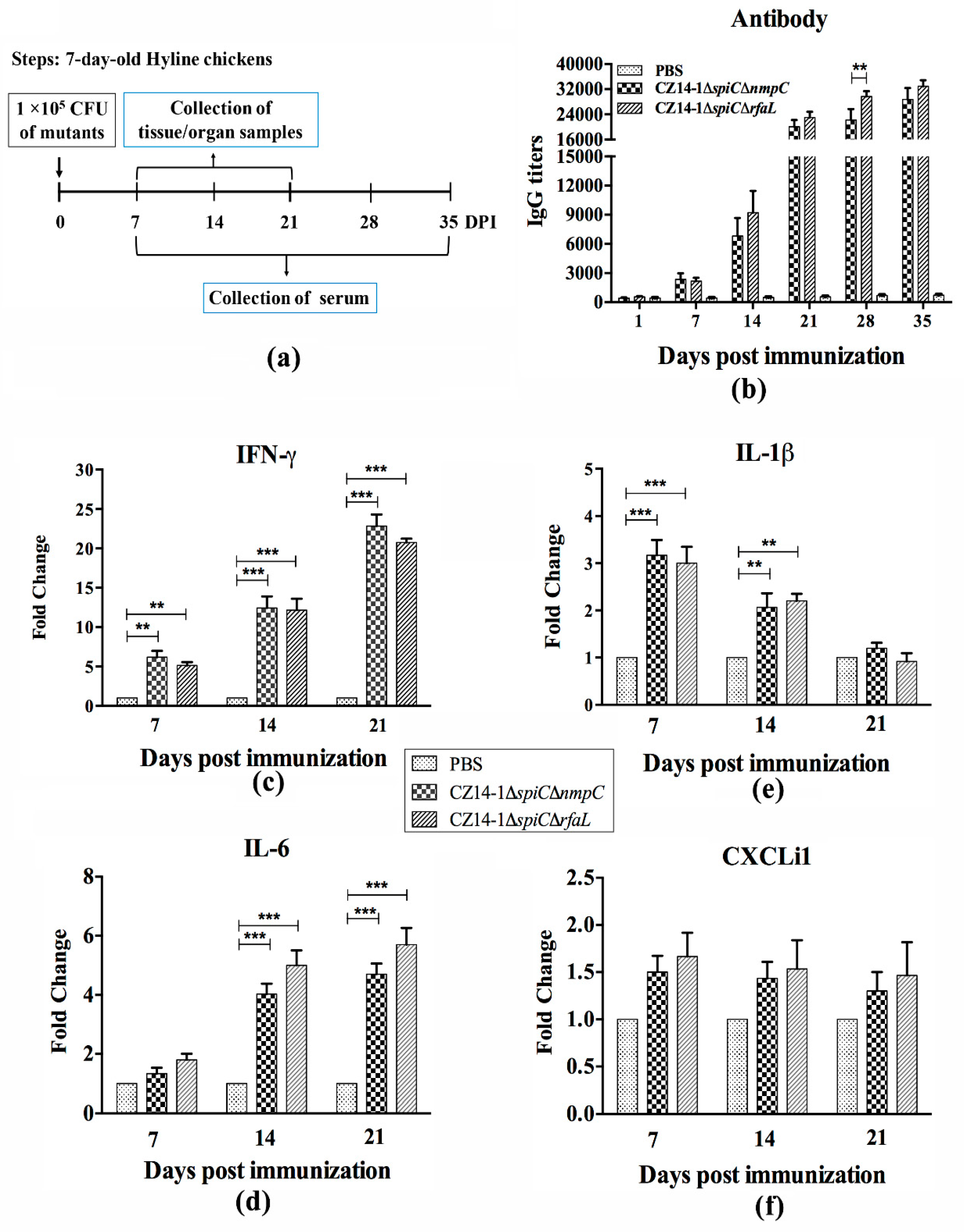
2.7. Immune Response
2.8. Immune Protection Assessment
2.9. Statistical Analysis
3. Results
3.1. Virulence of the Two Vaccine Candidates
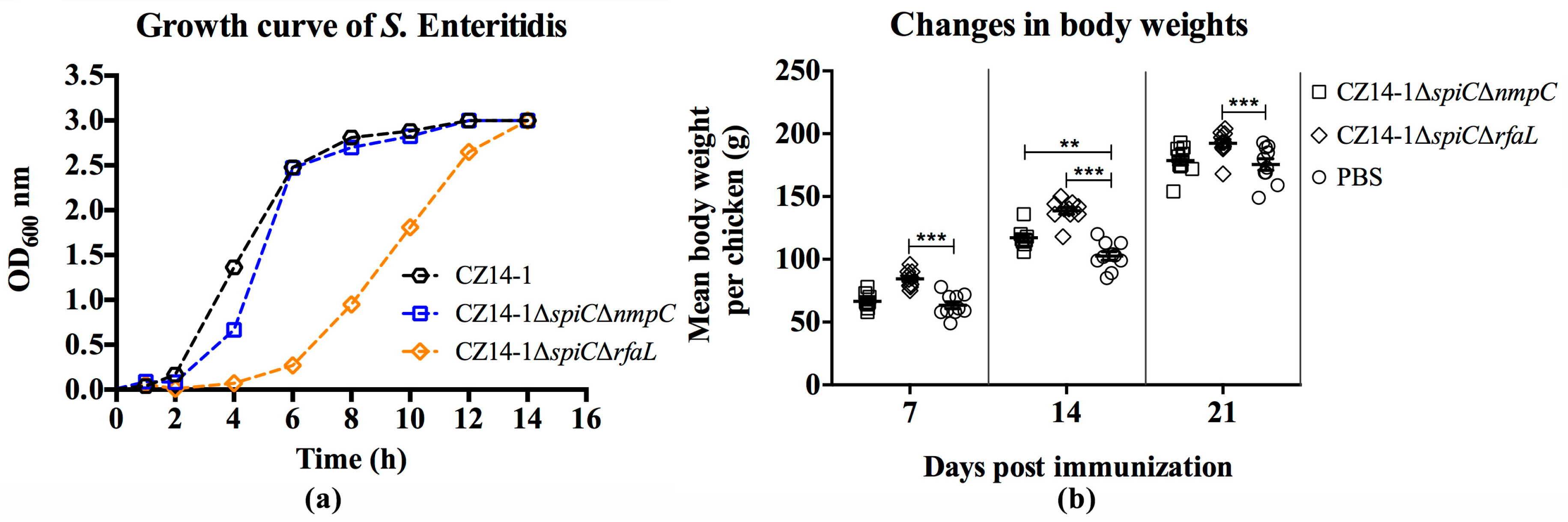
3.2. Biochemical Property and Growth Ability of Mutant Strains
3.3. Changes in Body Weight Following Immunization
3.4. Bacterial Colonization and Persistence in Chicken Tissues and Organs
3.5. Immune Response
3.6. Immune Protection
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Centers for Disease Control and Prevention. National Enteric Disease Surveillance: Salmonella Annual Report, 2016; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2016.
- EFSA. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2017. EFSA J. 2018, 16, 5500. [Google Scholar]
- M’ikanatha, N.M.; Sandt, C.H.; Localio, A.R.; Tewari, D.; Rankin, S.C.; Whichard, J.M.; Altekruse, S.F.; Lautenbach, E.; Folster, J.P.; Russo, A. Multidrug-resistant Salmonella isolates from retail chicken meat compared with human clinical isolates. Foodborne Pathog. Dis. 2017, 7, 929–934. [Google Scholar] [CrossRef]
- Davies, R.H.; Breslin, M. Persistence of Salmonella Enteritidis phage type 4 in the environment and arthropod vectors on an empty free-range chicken farm. Environ. Microbiol. 2003, 5, 79–84. [Google Scholar] [CrossRef]
- Gast, R. Microbiology of shell egg production in the United State. In Producing Sage Eggs—Micorbial Ecology of Salmonella; Ricke, C., Richard, K., Eds.; Academic Press: London, UK, 2016; pp. 25–44. [Google Scholar]
- Tollefson, L.; Miller, M.A. Antibiotic use in food animals: Controlling the human health impact. J. AOAC Int. 2000, 83, 245–254. [Google Scholar] [PubMed]
- Djordjevic, S.P.; Cain, A.K.; Evershed, N.J.; Falconer, L.; Levings, R.S.; Lightfoot, D.; Hall, R.M. Emergence and evolution of multiply antibiotic-resistant Salmonella enterica serovar Paratyphi B D-tartrate-utilizing strains containing SGI1. Antimicrob. Agents Chemother. 2009, 53, 2319–2326. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, G.A.; Donato, T.C.; Baptista, A.A.; Corrêa, I.M.; Garcia, K.C.; Andreatti Filho, R.L. Bacteriophage-induced reduction in Salmonella Enteritidis counts in the crop of broiler chickens undergoing preslaughter feed withdrawal. Poult. Sci. 2014, 93, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Schmidt, K.; Marks, D.; Hatter, S.; Marshall, A.; Albino, L.; Ebner, P. Treatment of Salmonella-contaminated eggs and pork with a broad-spectrum, single bacteriophage: Assessment of efficacy and resistance development. Foodborne Pathog. Dis. 2016, 13, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Wigley, P.; Barrow, P.; Villareal-Ramos, B. Immunity to Salmonella in domestic (food animal) species. Vaccines against S. enterica infections. Vaccines for chickens. In Salmonella Infections. Clinical, Immunological and Molecular Aspects; Mastroeni, P., Maskell, D., Eds.; Cambridge University Press: Cambridge, UK, 2006; pp. 308–309. [Google Scholar]
- Fei, X.; Yin, K.; Yin, C.; Hu, Y.; Li, J.; Zhou, Z.; Tian, Y.; Geng, S.; Chen, X.; Pan, Z.; et al. Analyses of prevalence and molecular typing reveal the spread of antimicrobial-resistant Salmonella infection across two breeder chicken farms. Poult. Sci. 2018, 97, 4374–4383. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cai, Y.; Zhang, J.; Liu, D.; Gong, X.; Pan, Z.; Geng, S.; Jiao, X. Controversy surrounding the function of spiC protein in Salmonella: An overview. Front. Microbiol. 2019, 10, 1784. [Google Scholar] [CrossRef]
- Geng, S.; Jiao, X.; Barrow, P.; Pan, Z.; Chen, X. Virulence determinants of Salmonella Gallinarum biovar Pullorum identified by PCR signature-tagged mutagenesis and the spiC mutant as a candidate live attenuated vaccine. Vet. Microbiol. 2014, 168, 388–394. [Google Scholar] [CrossRef]
- Cheng, Z.; Yin, J.; Kang, X.; Geng, S.; Hu, M.; Pan, Z.; Jiao, X. Safety and protective efficacy of a spiC and crp deletion mutant of Salmonella Gallinarum as a live attenuated vaccine for fowl typhoid. Res. Vet. Sci. 2016, 107, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Jiao, Y.; Li, Z.; Zhu, S.; Fei, X.; Geng, S.; Pan, Z.; Chen, X.; Li, Q.; Jiao, X. Safety, protective immunity, and diva capability of a rough mutant Salmonella Pullorum vaccine candidate in broilers. Front. Microbiol. 2017, 8, 547. [Google Scholar] [CrossRef] [PubMed]
- Van Oirschot, J.T.; Bruschke, C.J.; van Rijn, P.A. Vaccination of cattle against bovine viral diarrhorea. Vet. Microbiol. 1999, 64, 169–183. [Google Scholar] [CrossRef]
- Kong, Q.; Yang, J.; Liu, Q.; Alamuri, P.; Roland, K.L.; Curtiss, R., 3rd. Effect of deletion of genes involved in lipopolysaccharide core and o-antigen synthesis on virulence and immunogenicity of Salmonella enterica serovar Typhimurium. Infect. Immun. 2011, 79, 4227–4239. [Google Scholar] [CrossRef] [PubMed]
- Selke, M.; Meens, J.; Springer, S.; Frank, R.; Gerlach, G.F. Immunization of pigs to prevent disease in humans: Construction and protective efficacy of a Salmonella enterica serovar Typhimurium live negative-marker vaccine. Infect. Immun. 2007, 75, 2476–2483. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pérez, J.M.; McGarry, M.A.; Marolda, C.L.; Valvano, M.A. Functional analysis of the large periplasmic loop of the Escherichia coli K-12 Waal O-antigen ligase. Mol. Microbiol. 2008, 70, 1424–1440. [Google Scholar] [CrossRef] [PubMed]
- Nagy, G.; Palkovics, T.; Otto, A.; Kusch, H.; Kocsis, B.; Dobrindt, U.; Engelmann, S.; Hecker, M.; Emody, L.; Pal, T.; et al. “Gently rough”: The vaccine potential of a Salmonella enterica regulatory lipopolysaccharide mutant. J. Infect. Dis. 2008, 198, 1699–1706. [Google Scholar] [CrossRef]
- Gil-Cruz, C.; Bobat, S.; Marshall, J.L.; Kingsley, R.A.; Ross, E.A.; Henderson, I.R.; Leyton, D.L.; Coughlan, R.E.; Khan, M.; Jensen, K.T.; et al. The porin OmpD from nontyphoidal Salmonella is a key target for a protective B1b cell antibody response. Proc. Natl. Acad. Sci. USA 2009, 106, 9803–9808. [Google Scholar] [CrossRef]
- Zhang, Y.; Dominguez-Medina, C.; Cumley, N.J.; Heath, J.N.; Essex, S.J.; Bobat, S.; Schager, A.; Goodall, M.; Kracker, S.; Buckley, C.D.; et al. IgG1 is required for optimal protection after immunization with the purified porin OmpD from Salmonella Typhimurium. J. Immunol. 2017, 199, 4103–4109. [Google Scholar] [CrossRef]
- Datsenko, K.A.; Wanner, B.L. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 2000, 97, 6640–6645. [Google Scholar] [CrossRef]
- Li, Q.; Hu, Y.; Xu, Y.; Chen, J.; Fang, L.; Liu, Z.; Jiao, X. A gene knock-in method used to purify plasmid pSPI12 from Salmonella enterica serovar Pullorum and characterization of IpaJ. J. Microbiol. Methods 2014, 98, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Reed, L.J.; Muench, H. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 1938, 27, 493–497. [Google Scholar]
- Li, Q.; Yuan, Y.; Wang, X.; Chen, J.; Wu, Y.; Wang, X.; Xu, L.; Yin, K.; Liu, Z.; Yin, C.; et al. Comparative study of Salmonella enterica serovar Enteritidis genes expressed within avian and murine macrophages via selective capture of transcribed sequences (SCOTS). Appl. Microbiol. Biotechnol. 2018, 102, 6567–6579. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Xu, L.; Li, Y.; Liu, Z.; Gu, D.; Li, Q.; Jiao, X. Construction of pSPI12-cured Salmonella enterica serovar Pullorum and identification of IpaJ as an immune response modulator. Avian Pathol. 2018, 47, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Woodward, M.J.; Gettinby, G.; Breslin, M.F.; Corkish, J.D.; Houghton, S. The efficacy of salenvac, a Salmonella enterica subsp. enterica serotype Enteritidis iron-restricted bacterin vaccine, in laying chickens. Avian Pathol. 2002, 31, 383–392. [Google Scholar] [CrossRef]
- Babu, U.; Dalloul, R.A.; Okamura, M.; Lillehoj, H.S.; Xie, H.; Raybourne, R.B.; Gaines, D.; Heckert, R.A. Salmonella Enteritidis clearance and immune responses in chickens following Salmonella vaccination and challenge. Vet. Immunol. Immunopathol. 2004, 101, 251–257. [Google Scholar] [CrossRef]
- Singh, B.R. Salmonella vaccines for animals and birds and their future perspective. Open Vaccine J. 2009, 2, 100–112. [Google Scholar] [CrossRef]
- Tran, T.Q.; Quessy, S.; Letellier, A.; Desrosiers, A.; Boulianne, M. Immune response following vaccination against Salmonella Enteritidis using 2 commercial bacterins in laying hens. Can. J. Vet. Res. 2010, 74, 185–192. [Google Scholar]
- Barrow, P.A. Salmonella infections: Immune and non-immune protection with vaccines. Avian Pathol. 2007, 36, 1–13. [Google Scholar] [CrossRef]
- Zhang-Barber, L.; Turner, A.K.; Barrow, P.A. Vaccination for control of Salmonella in poultry. Vaccine 1999, 17, 2538–2545. [Google Scholar] [CrossRef]
- Adriaensen, C.; De Greve, H.; Tian, J.Q.; De Craeye, S.; Gubbels, E.; Eeckhaut, V.; Van Immerseel, F.; Ducatelle, R.; Kumar, M.; Hernalsteens, J.P. A live Salmonella enterica serovar Enteritidis vaccine allows serological differentiation between vaccinated and infected animals. Infect. Immun. 2007, 75, 2461–2468. [Google Scholar] [CrossRef] [PubMed]
- Methner, U.; Barrow, P.A.; Berndt, A.; Rychlik, I. Salmonella Enteritidis with double deletion in phoP fliC--a potential live Salmonella vaccine candidate with novel characteristics for use in chickens. Vaccine 2011, 29, 3248–3253. [Google Scholar] [CrossRef] [PubMed]
- Ochman, H.; Soncini, F.C.; Solomon, F.; Groisman, E.A. Identification of a pathogenicity island required for Salmonella survival in host cells. Proc. Natl. Acad. Sci. USA 1996, 93, 7800–7804. [Google Scholar] [CrossRef] [PubMed]
- Uchiya, K.; Barbieri, M.A.; Funato, K.; Shah, A.H.; Stahl, P.D.; Groisman, E.A. A Salmonella virulence protein that inhibits cellular trafficking. EMBO J. 1999, 18, 3924–3933. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Li, B.; Yan, S.; Pan, Z.; Geng, S.; Jiao, X. Construction of genetically engineered attenuated vaccines Salmonella Entertidis C50041ΔspiCΔcrp. Chin. J. Zoonoses 2015, 31, 97–101. [Google Scholar]
- Mastroeni, P.; Chabalgoity, J.A.; Dunstan, S.J.; Maskell, D.J.; Dougan, G. Salmonella: Immune responses and vaccines. Vet. J. 2001, 161, 132–164. [Google Scholar] [CrossRef]
- Chausse, A.M.; Grepinet, O.; Bottreau, E.; Robert, V.; Hennequet-Antier, C.; Lalmanach, A.C.; Lecardonnel, J.; Beaumont, C.; Velge, P. Susceptibility to Salmonella carrier-state: A possible Th2 response in susceptible chicks. Vet. Immunol. Immunopathol. 2014, 159, 16–28. [Google Scholar] [CrossRef]
- Gal-Mora, O. Persistent infection and long-term carriage of typhoidal and nontyphoidal Salmonellae. Clin. Microbiol. Rev. 2019, 32, e00088–e000118. [Google Scholar] [CrossRef]
- Lalsiamthara, J.; Kamble, N.M.; Lee, J.H. A live attenuated Salmonella Enteritidis secreting detoxified heat labile toxin enhances mucosal immunity and confers protection against wild-type challenge in chickens. Vet. Res. 2016, 47, 60. [Google Scholar] [CrossRef]
| Strains | Challenge Dose (CFU) | No. of Deaths/Total No. of Chickens | LD50 (CFU) |
|---|---|---|---|
| CZ14-1 | 1 × 108 | 10/10 | 2.24 × 104 |
| 1 × 107 | 9/10 | ||
| 1 × 106 | 7/10 | ||
| 1 × 105 | 6/10 | ||
| 1 × 104 | 4/10 | ||
| CZ14-1∆spiC∆nmpC | 1 × 1010 | 10/10 | 1.58 × 107 |
| 1 × 109 | 10/10 | ||
| 1 × 108 | 10/10 | ||
| 1 × 107 | 5/10 | ||
| 1 × 106 | 2/10 | ||
| CZ14-1∆spiC∆rfaL | 1 × 1010 | 10/10 | 7.76 × 106 |
| 1 × 109 | 10/10 | ||
| 1 × 108 | 10/10 | ||
| 1 × 107 | 7/10 | ||
| 1 × 106 | 3/10 |
| Strains | Challenge Dose (CFU) | No. of Deaths/Total No. of Chickens | LD50 (CFU) |
|---|---|---|---|
| CZ14-1 | 1 × 108 | 10/10 | 1.26 × 104 |
| 1 × 107 | 10/10 | ||
| 1 × 106 | 9/10 | ||
| 1 × 105 | 6/10 | ||
| 1 × 104 | 5/10 | ||
| CZ14-1∆spiC∆nmpC | 1 × 109 | 10/10 | 2.57 × 107 |
| 1 × 108 | 10/10 | ||
| 1 × 107 | 5/10 | ||
| 1 × 106 | 2/10 | ||
| 1 × 105 | 0/10 | ||
| CZ14-1∆spiC∆rfaL | 1 × 109 | 10/10 | 4.07 × 106 |
| 1 × 108 | 10/10 | ||
| 1 × 107 | 6/10 | ||
| 1 × 106 | 3/10 | ||
| 1 × 105 | 0/10 |
| Group | Vaccination | No. | Challenge | Survivors/Total (Survival Rate) | |||
|---|---|---|---|---|---|---|---|
| Strain | Dose (CFU) | Strain | Route | Dose | |||
| A | CZ14-1∆spiC∆nmpC | 1 × 105 | 20 | CZ14-1 | i.m.a | 2 × 109 | 16/20 (80%) |
| B | CZ14-1∆spiC∆rfaL | 1 × 105 | 20 | CZ14-1 | i.m. | 2 × 109 | 15/20 (75%) |
| C | PBS | - | 20 | CZ14-1 | i.m. | 2 × 109 | 1/20 (5%) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Zhu, Y.; Ren, J.; Qiao, Z.; Yin, C.; Xian, H.; Yuan, Y.; Geng, S.; Jiao, X. Evaluation of the Safety and Protection Efficacy of spiC and nmpC or rfaL Deletion Mutants of Salmonella Enteritidis as Live Vaccine Candidates for Poultry Non-Typhoidal Salmonellosis. Vaccines 2019, 7, 202. https://doi.org/10.3390/vaccines7040202
Li Q, Zhu Y, Ren J, Qiao Z, Yin C, Xian H, Yuan Y, Geng S, Jiao X. Evaluation of the Safety and Protection Efficacy of spiC and nmpC or rfaL Deletion Mutants of Salmonella Enteritidis as Live Vaccine Candidates for Poultry Non-Typhoidal Salmonellosis. Vaccines. 2019; 7(4):202. https://doi.org/10.3390/vaccines7040202
Chicago/Turabian StyleLi, Qiuchun, Yue Zhu, Jingwei Ren, Zhuang Qiao, Chao Yin, Honghong Xian, Yu Yuan, Shizhong Geng, and Xinan Jiao. 2019. "Evaluation of the Safety and Protection Efficacy of spiC and nmpC or rfaL Deletion Mutants of Salmonella Enteritidis as Live Vaccine Candidates for Poultry Non-Typhoidal Salmonellosis" Vaccines 7, no. 4: 202. https://doi.org/10.3390/vaccines7040202
APA StyleLi, Q., Zhu, Y., Ren, J., Qiao, Z., Yin, C., Xian, H., Yuan, Y., Geng, S., & Jiao, X. (2019). Evaluation of the Safety and Protection Efficacy of spiC and nmpC or rfaL Deletion Mutants of Salmonella Enteritidis as Live Vaccine Candidates for Poultry Non-Typhoidal Salmonellosis. Vaccines, 7(4), 202. https://doi.org/10.3390/vaccines7040202





