Feasibility of Using a Luminescence-Based Method to Determine Serum Bactericidal Activity against Neisseria gonorrhoeae
Abstract
1. Introduction
2. Materials and Methods
2.1. Culture of Bacteria
2.2. Human and Murine Sera
2.3. Comparison of CFU and ATP Readouts
2.4. Measurement of Serum Sensitivity
2.5. Quantification of SBA
2.6. Statistical Analysis
3. Results
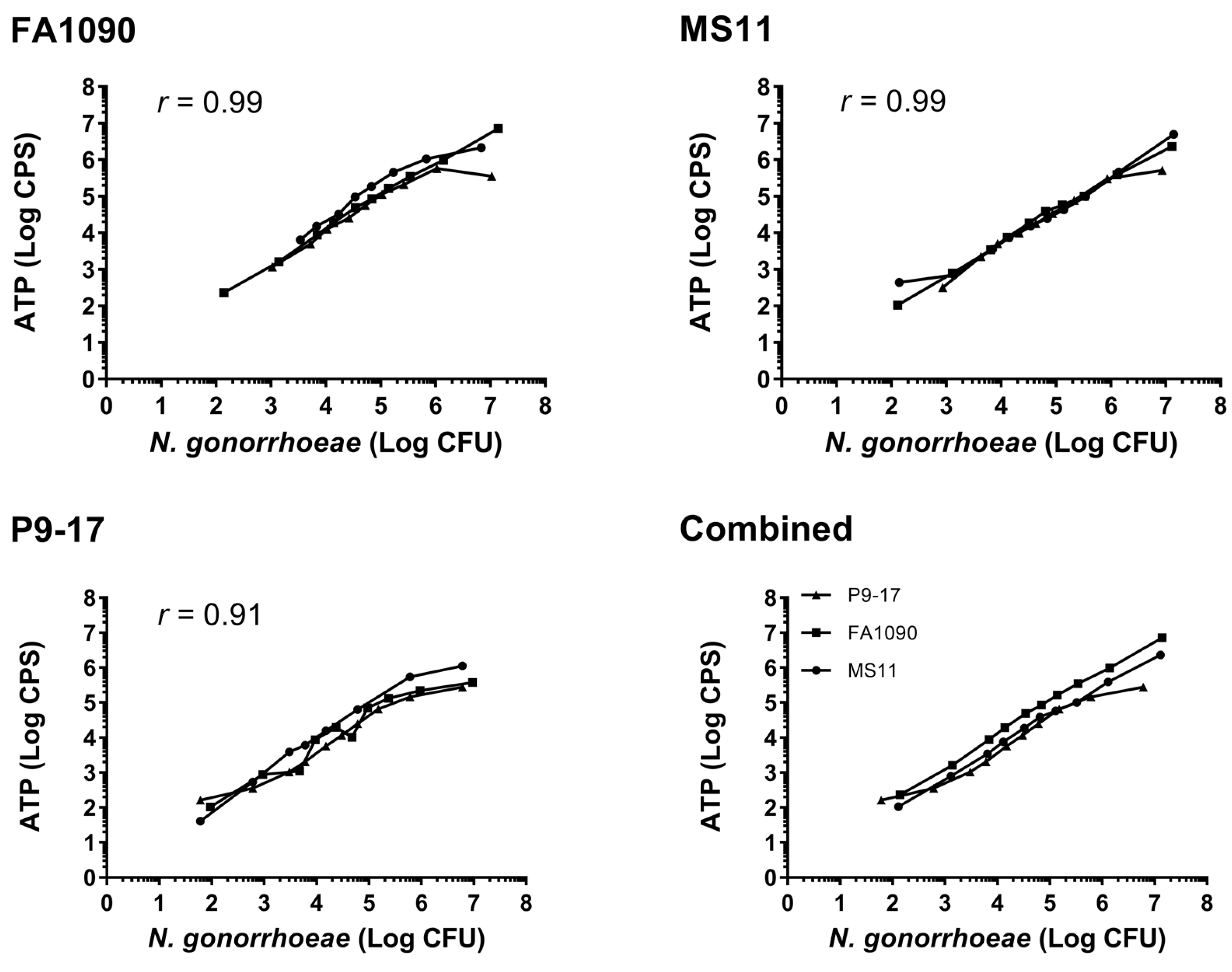
3.1. Comparison of Luminescent and CFU Readouts
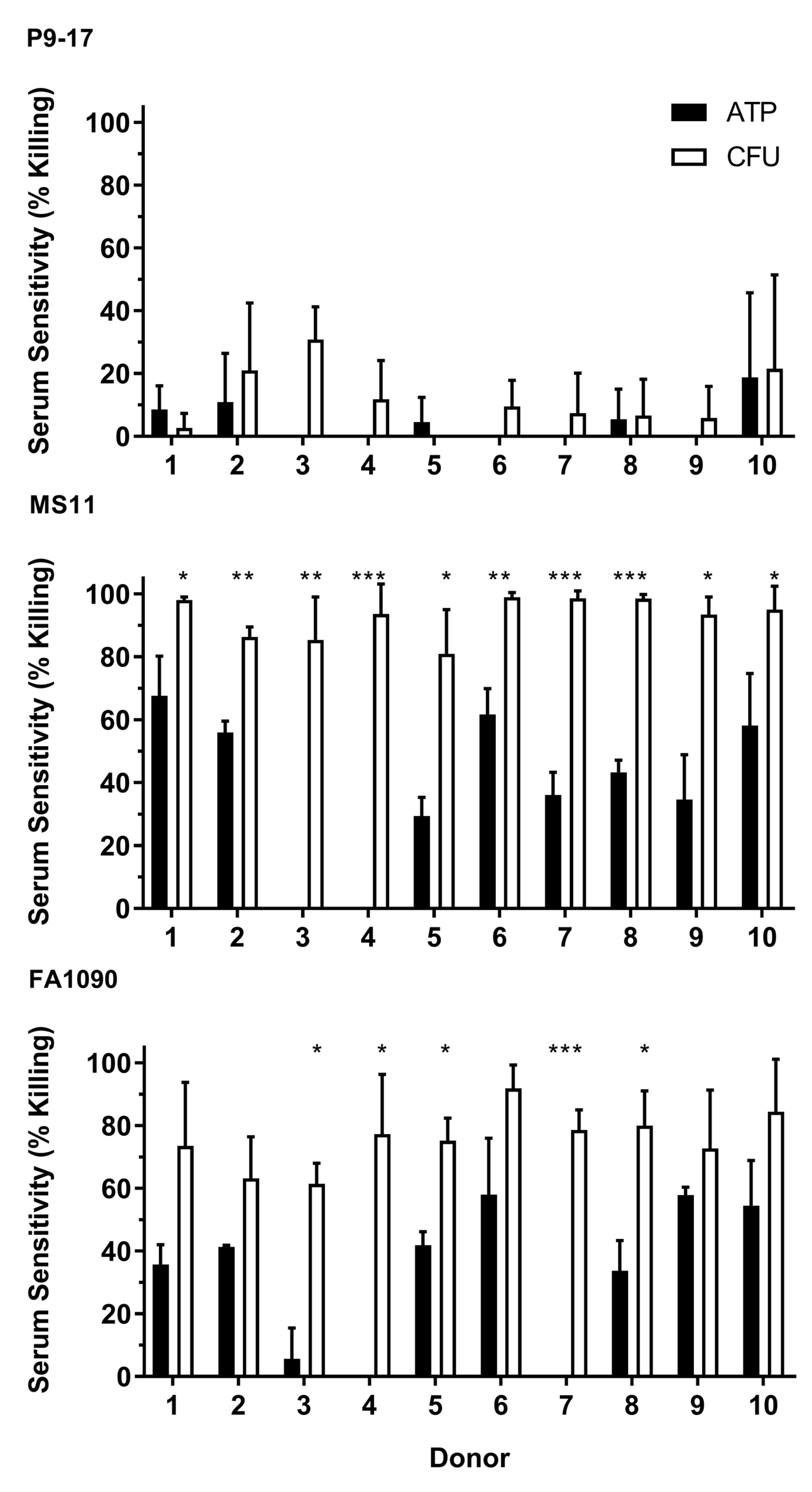
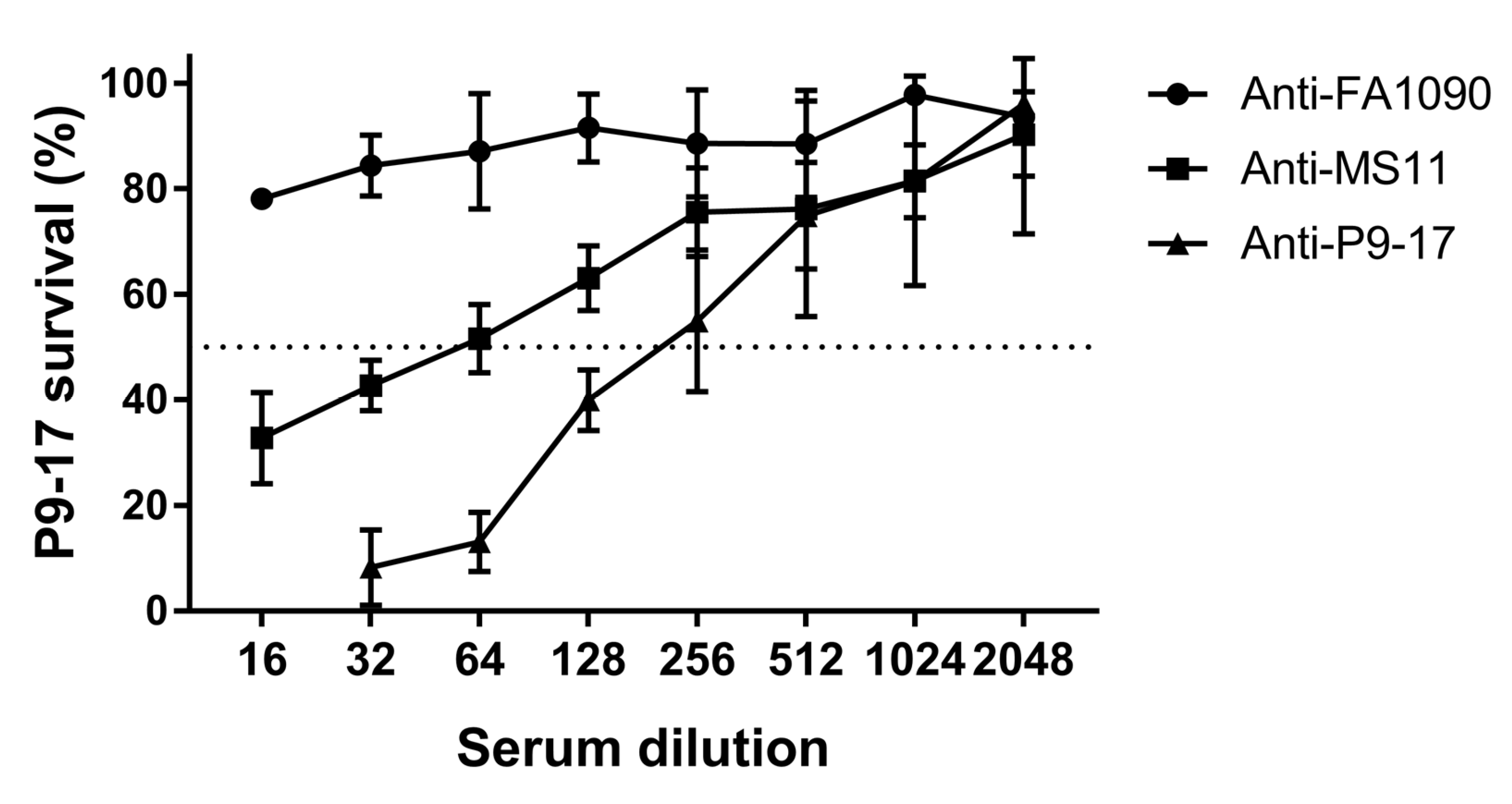
3.2. Use of Bioluminescent Assay to Screen for NHS Sensitivity
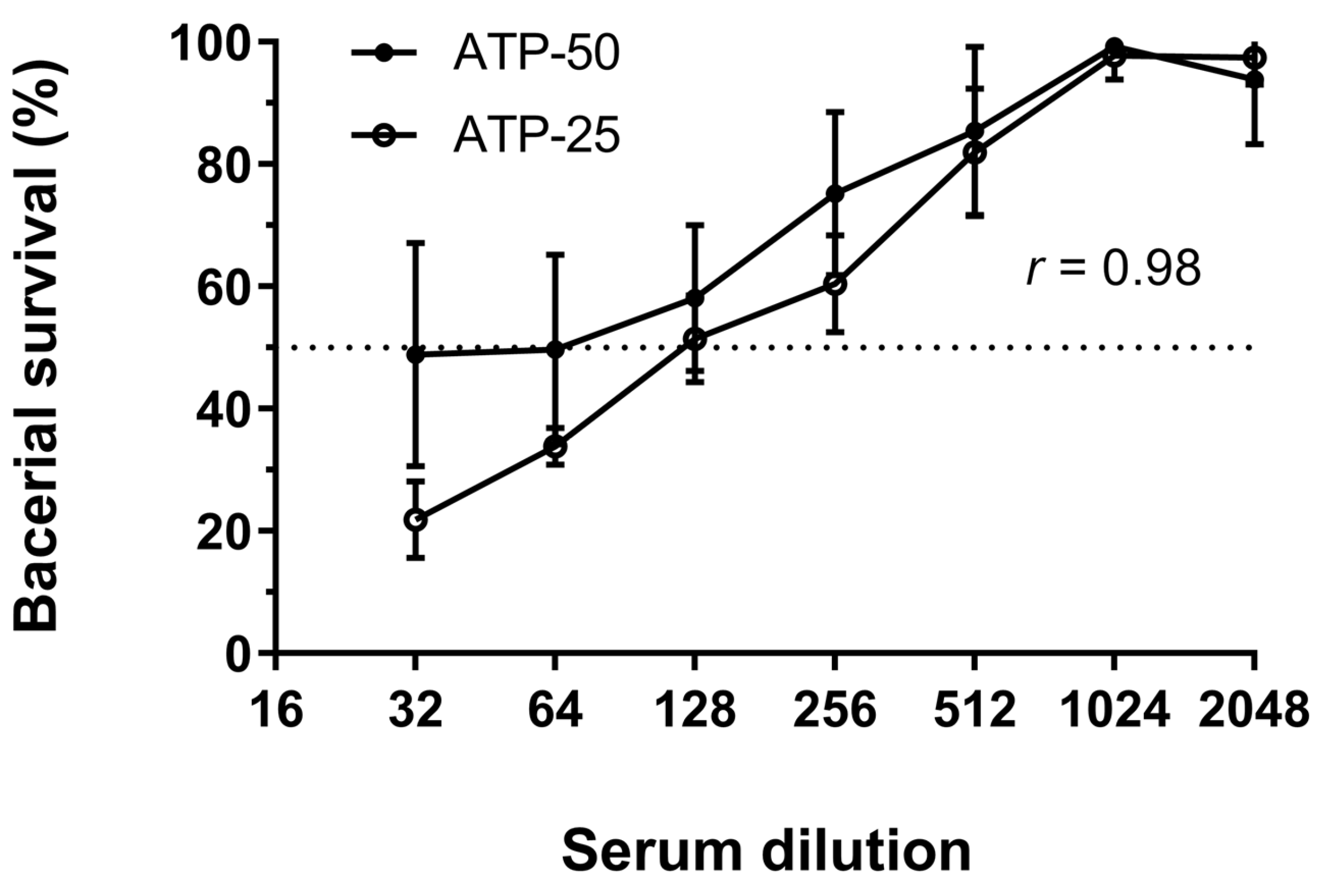
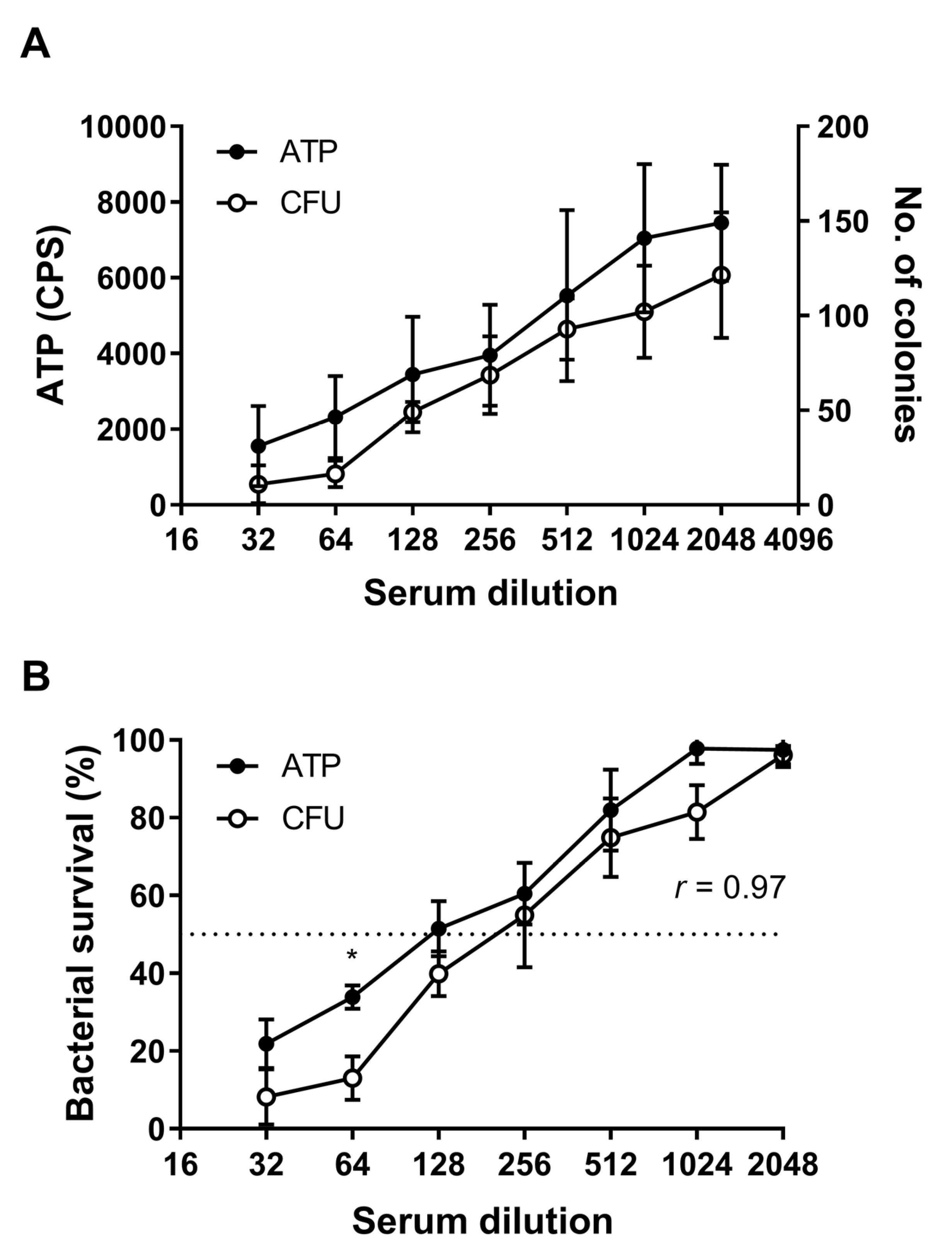
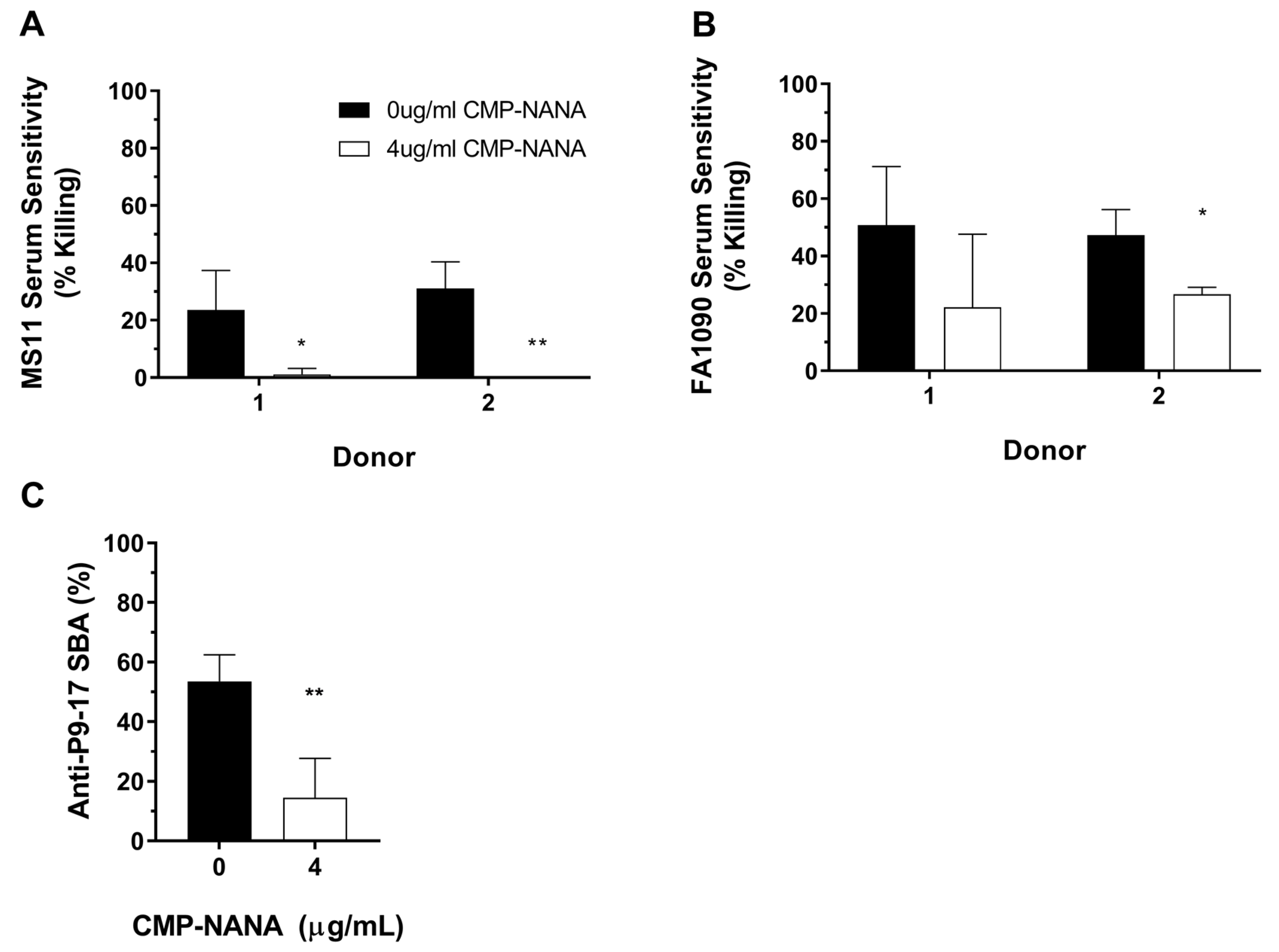
3.3. Quantification of SBA by Detection of ATP
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Newman, L.; Rowley, J.; Vander Hoorn, S.; Wijesooriya, N.S.; Unemo, M.; Low, N.; Temmerman, M. Global Estimates of the Prevalence and Incidence of Four Curable Sexually Transmitted Infections in 2012 Based on Systematic Review and Global Reporting. PLoS ONE 2015, 10, e0143304. [Google Scholar] [CrossRef]
- World Health Organisation. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. Available online: https://www.who.int/medicines/publications/WHO-PPL-Short_Summary_25Feb-ET_NM_WHO.pdf. 2017 (accessed on 11 January 2019).
- Wi, T.; Lahra, M.M.; Ndowa, F.; Bala, M.; Dillon, J.A.R.; Ramon-Pardo, P.; Unemo, M. Antimicrobial resistance in Neisseria gonorrhoeae: Global surveillance and a call for international collaborative action. PLoS Med. 2017, 14, e1002344. [Google Scholar] [CrossRef]
- Fifer, H.; Natarajan, U.; Jones, L.; Alexander, S.; Hughes, G.; Golparian, D.; Unemo, M. Failure of Dual Antimicrobial Therapy in Treatment of Gonorrhea. N. Engl. J. Med. 2016, 374, 2504–2506. [Google Scholar] [CrossRef] [PubMed]
- Public Health England. UK Case of Neisseria Gonorrhoeae with High-Level Resistance to Azithromycin and Resistance to Ceftriaxone Acquired Abroad. Health Protect. Rep. Adv. Access Rep. 2018, 12. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/694655/hpr1118_MDRGC.pdf (accessed on 10 January 2019).
- Australian Government Department of Health. Gonorrhoea Health Alert. Available online: http://www.health.gov.au/internet/main/publishing.nsf/Content/ohp-gonorrhoea.htm (accessed on 10 January 2019).
- Hill, S.A.; Masters, T.L.; Wachter, J. Gonorrhea-an evolving disease of the new millennium. Microb. Cell. 2016, 3, 371–389. [Google Scholar] [CrossRef] [PubMed]
- Galvin, S.R.; Cohen, M.S. The role of sexually transmitted diseases in HIV transmission. Nat. Rev. Micro. 2004, 2, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, K.H.; Schneider, H.; Cross, A.S.; Boslego, J.W.; Hoover, D.L.; Staley, T.L.; Deal, C.D. Inflammatory cytokines produced in response to experimental human gonorrhea. J. Infect. Dis. 1995, 172, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, M.M.; Sparling, P.F.; Cohen, M.S.; Shafer, W.M.; Deal, C.D.; Jerse, A.E. Experimental Gonococcal Infection in Male Volunteers: Cumulative Experience with Neisseria gonorrhoeae Strains FA1090 and MS11mkC. Front. Microbiol. 2011, 2, 123. [Google Scholar] [CrossRef]
- Hedges, S.R.; Mayo, M.S.; Mestecky, J.; Hook, E.W., 3rd; Russell, M.W. Limited local and systemic antibody responses to Neisseria gonorrhoeae during uncomplicated genital infections. Infect. Immun. 1999, 67, 3937–3946. [Google Scholar]
- Schmidt, K.A.; Schneider, H.; Lindstrom, J.A.; Boslego, J.W.; Warren, R.A.; Van de Verg, L.; Griffiss, J.M. Experimental gonococcal urethritis and reinfection with homologous gonococci in male volunteers. Sex. Transm. Dis. 2001, 28, 555–564. [Google Scholar] [CrossRef]
- Fox, K.K.; Thomas, J.C.; Weiner, D.H.; Davis, R.H.; Sparling, P.F.; Cohen, M.S. Longitudinal evaluation of serovar-specific immunity to Neisseria gonorrhoeae. Am. J. Epidemiol. 1999, 149, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Plummer, F.A.; Simonsen, J.N.; Chubb, H.; Slaney, L.; Kimata, J.; Bosire, M.; Ngugi, E.N. Epidemiologic evidence for the development of serovar-specific immunity after gonococcal infection. J. Clinic. Investig. 1989, 83, 1472–1476. [Google Scholar] [CrossRef] [PubMed]
- Lovett, A.; Duncan, J.A. Human Immune Responses and the Natural History of Neisseria gonorrhoeae Infection. Front Immunol. 2018, 9, 3187. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hammer, L.A.; Liu, W.; Hobbs, M.M.; Zielke, R.A.; Sikora, A.E.; Russell, M.W. Experimental vaccine induces Th1-driven immune responses and resistance to Neisseria gonorrhoeae infection in a murine model. Mucosal Immunol. 2017. [Google Scholar] [CrossRef]
- Liu, Y.; Egilmez, N.K.; Russell, M.W. Enhancement of adaptive immunity to Neisseria gonorrhoeae by local intravaginal administration of microencapsulated interleukin 12. J. Infect. Dis. 2013, 208, 1821–1829. [Google Scholar] [CrossRef]
- Edwards, J.L.; Jennings, M.P.; Apicella, M.A.; Seib, K.L. Is gonococcal disease preventable? The importance of understanding immunity and pathogenesis in vaccine development. Crit. Rev. Microbiol. 2016, 42, 928–941. [Google Scholar] [CrossRef]
- Gulati, S.; Shaughnessy, J.; Ram, S.; Rice, P.A. Targeting Lipooligosaccharide (LOS) for a Gonococcal Vaccine. Front Immunol. 2019, 10, 321. [Google Scholar] [CrossRef]
- Jerse, A.E.; Deal, C.D. Vaccine research for gonococcal infections: Where are we? Sex. Transm. Infect. 2013, 89 (Suppl. 4), iv63-8. [Google Scholar] [CrossRef]
- Rice, P.A.; Shafer, W.M.; Ram, S.; Jerse, A.E. Neisseria gonorrhoeae: Drug Resistance, Mouse Models, and Vaccine Development. Annu Rev Microbiol. 2017, 71, 665–686. [Google Scholar] [CrossRef]
- Frasch, C.E.; Borrow, R.; Donnelly, J. Bactericidal antibody is the immunologic surrogate of protection against meningococcal disease. Vaccine 2009, 27 (Suppl. 2), B112–B116. [Google Scholar] [CrossRef]
- Marri, P.R.; Paniscus, M.; Weyand, N.J.; Rendon, M.A.; Calton, C.M.; Hernandez, D.R.; So, M. Genome sequencing reveals widespread virulence gene exchange among human Neisseria species. PLoS ONE 2010, 5, e11835. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, E.D.; Broker, M.; Wassil, J.; Welsch, J.A.; Borrow, R. Serum bactericidal antibody assays-The role of complement in infection and immunity. Vaccine 2015, 33, 4414–4421. [Google Scholar] [CrossRef] [PubMed]
- Rice, P.A.; McCormack, W.M.; Kasper, D.L. Natural serum bactericidal activity against Neisseria gonorrhoeae isolates from disseminated, locally invasive, and uncomplicated disease. J Immunol. 1980, 124, 2105–2109. [Google Scholar] [PubMed]
- Schoolnik, G.K.; Buchanan, T.M.; Holmes, K.K. Gonococci causing disseminated gonococcal infection are resistant to the bactericidal action of normal human sera. J. Clinic. Investig. 1976, 58, 1163–1173. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.E.; Watt, P.J.; Glynn, A.A. Gonococci in urethral exudates possess a virulence factor lost on subculture. Nature 1970, 227, 382–384. [Google Scholar] [CrossRef] [PubMed]
- McQuillen, D.P.; Gulati, S.; Rice, P.A. Complement-mediated bacterial killing assays. Methods Enzymol. 1994, 236, 137–147. [Google Scholar] [CrossRef]
- Necchi, F.; Saul, A.; Rondini, S. Development of a high-throughput method to evaluate serum bactericidal activity using bacterial ATP measurement as survival readout. PLoS ONE 2017, 12, e0172163. [Google Scholar] [CrossRef]
- Necchi, F.; Saul, A.; Rondini, S. Setup of luminescence-based serum bactericidal assay against Salmonella Paratyphi, A. J. Immunol. Methods 2018, 461, 117–121. [Google Scholar] [CrossRef]
- Wetzler, L.M.; Barry, K.; Blake, M.S.; Gotschlich, E.C. Gonococcal lipooligosaccharide sialylation prevents complement-dependent killing by immune sera. Infect. Immun. 1992, 60, 39–43. [Google Scholar]
- Gulati, S.; Rice, P.A.; Ram, S. Complement-Dependent Serum Bactericidal Assays for Neisseria gonorrhoeae. Methods Mol. Biol. 2019, 1997, 267–280. [Google Scholar] [CrossRef]
- Fredriksen, J.H.; Rosenqvist, E.; Wedege, E.; Bryn, K.; Bjune, G.; Froholm, L.O.; Rye, U. Production, characterization and control of MenB-vaccine “Folkehelsa”: An outer membrane vesicle vaccine against group B meningococcal disease. NIPH Ann. 1991, 14, 67–80. [Google Scholar] [PubMed]
- Lee, H.S.; Boulton, I.C.; Reddin, K.; Wong, H.; Halliwell, D.; Mandelboim, O.; Gray-Owen, S. DNeisserial outer membrane vesicles bind the coinhibitory receptor carcinoembryonic antigen-related cellular adhesion molecule 1 and suppress CD4+ T lymphocyte function. Infect. Immun. 2007, 75, 4449–4455. [Google Scholar] [CrossRef] [PubMed]
- Almonacid-Mendoza, H.L.; Humbert, M.V.; Dijokaite, A.; Cleary, D.W.; Soo, Y.; Hung, M.C.; Christodoulides, M. Structure of the Recombinant Neisseria gonorrhoeae Adhesin Complex Protein (rNg-ACP) and Generation of Murine Antibodies with Bactericidal Activity against Gonococci. mSphere 2018, 3. [Google Scholar] [CrossRef] [PubMed]
- Burton, R.L.; Nahm, M.H. Development and validation of a fourfold multiplexed opsonization assay (MOPA4) for pneumococcal antibodies. Clinic. Vaccine Immunol. CVI 2006, 13, 1004–14009. [Google Scholar] [CrossRef] [PubMed]
- Burton, R.L.; Nahm, M.H. Development of a fourfold multiplexed opsonophagocytosis assay for pneumococcal antibodies against additional serotypes and discovery of serological subtypes in Streptococcus pneumoniae serotype 20. Clinic. Vaccine Immunol. CVI 2012, 19, 835–841. [Google Scholar] [CrossRef]
- Bacterial Respiratory Pathogen Reference Laboratory. Available online: http://www.vaccine.uab.edu. 2018. (accessed on 18 December 2018).
- Ram, S.; Sharma, A.K.; Simpson, S.D.; Gulati, S.; McQuillen, D.P.; Pangburn, M.K.; Rice, P.A. A Novel Sialic Acid Binding Site on Factor H Mediates Serum Resistance of Sialylated Neisseria gonorrhoeae. J. Exp. Med. 1998, 187, 743–752. [Google Scholar] [CrossRef]
- Heesterbeek, D.A.C.; Angelier, M.L.; Harrison, R.A.; Rooijakkers, S.H.M. Complement and Bacterial Infections: From Molecular Mechanisms to Therapeutic Applications. J. Innate. Immun. 2018, 10, 455–464. [Google Scholar] [CrossRef]
- Piekarowicz, A.; Klyz, A.; Majchrzak, M.; Stein, D.C. Oral Immunization of Rabbits with S. enterica Typhimurium Expressing Neisseria gonorrhoeae Filamentous Phage Phi6 Induces Bactericidal Antibodies Against, N. gonorrhoeae. Sci. Rep. 2016, 6, 22549. [Google Scholar] [CrossRef]
- Zielke, R.A.; Wierzbicki, I.H.; Weber, J.V.; Gafken, P.R.; Sikora, A.E. Quantitative proteomics of the Neisseria gonorrhoeae cell envelope and membrane vesicles for the discovery of potential therapeutic targets. Mol. Cell. Proteomics 2014, 13, 1299–12317. [Google Scholar] [CrossRef]
- Chen, A.; Seifert, H.S. Structure-function studies of the Neisseria gonorrhoeae major outer membrane porin. Infect. Immun. 2013, 81, 4383–4391. [Google Scholar] [CrossRef]
- Gulati, S.; Zheng, B.; Reed, G.W.; Su, X.; Cox, A.D.; St Michael, F.; Rice, P.A. Immunization against a saccharide epitope accelerates clearance of experimental gonococcal infection. PLoS Pathog. 2013, 9, e1003559. [Google Scholar] [CrossRef] [PubMed]
- Feinen, B.; Jerse, A.E.; Gaffen, S.L.; Russell, M.W. Critical role of Th17 responses in a murine model of Neisseria gonorrhoeae genital infection. Mucosal Immunol. 2010, 3, 312–321. [Google Scholar] [CrossRef]
- Jerse, A.E. Experimental gonococcal genital tract infection and opacity protein expression in estradiol-treated mice. Infect. Immun. 1999, 67, 5699–5708. [Google Scholar] [PubMed]
- Rice, P.A. Molecular basis for serum resistance in Neisseria gonorrhoeae. Clinic. Microbiol. Rev. 1989, 2, S112–S117. [Google Scholar] [CrossRef] [PubMed]
- Arnold, R.; Galloway, Y.; McNicholas, A.; O’Hallahan, J. Effectiveness of a vaccination programme for an epidemic of meningococcal B in New Zealand. Vaccine 2011, 29, 7100–7106. [Google Scholar] [CrossRef]
- Brooks, G.F.; Ingwer, I. Studies on the relationships between serum bactericidal activity and uncomplicated genital infections due to Neisseria gonorrhoeae. J. Infect. Dis. 1978, 138, 333–339. [Google Scholar] [CrossRef]
- Petousis-Harris, H.; Paynter, J.; Morgan, J.; Saxton, P.; McArdle, B.; Goodyear-Smith, F.; Black, S. Effectiveness of a group B outer membrane vesicle meningococcal vaccine against gonorrhoea in New Zealand: A retrospective case-control study. Lancet 2017, 390, 1603–1610. [Google Scholar] [CrossRef]
- Whelan, J.; Klovstad, H.; Haugen, I.L.; Holle, M.R.; Storsaeter, J. Ecologic Study of Meningococcal B Vaccine and Neisseria gonorrhoeae Infection, Norway. Emerg. Infect. Dis. 2016, 22, 1137–1139. [Google Scholar] [CrossRef]
- Perez, O.; del Campo, J.; Cuello, M.; Gonzalez, E.; Nunez, N.; Cabrera, O.; Romeu, B. Mucosal approaches in Neisseria vaccinology. VacciMonitor 2009, 18, 53–55. [Google Scholar]
- Longtin, J.; Dion, R.; Simard, M.; Betala Belinga, J.-F.; Longtin, Y.; Lefebvre, B.; De Wals, P. Possible Impact of Wide-scale Vaccination Against Serogroup B Neisseria Meningitidis on Gonorrhea Incidence Rates in One Region of Quebec, Canada. Open Forum Infect. Dis. 2017, 4, S734–S735. [Google Scholar] [CrossRef]
- O’Hallahan, J.; Lennon, D.; Oster, P.; Lane, R.; Reid, S.; Mulholland, K.; Martin, D. From secondary prevention to primary prevention: A unique strategy that gives hope to a country ravaged by meningococcal disease. Vaccine 2005, 23, 2197–21201. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clow, F.; O’Hanlon, C.J.; Christodoulides, M.; Radcliff, F.J. Feasibility of Using a Luminescence-Based Method to Determine Serum Bactericidal Activity against Neisseria gonorrhoeae. Vaccines 2019, 7, 191. https://doi.org/10.3390/vaccines7040191
Clow F, O’Hanlon CJ, Christodoulides M, Radcliff FJ. Feasibility of Using a Luminescence-Based Method to Determine Serum Bactericidal Activity against Neisseria gonorrhoeae. Vaccines. 2019; 7(4):191. https://doi.org/10.3390/vaccines7040191
Chicago/Turabian StyleClow, Fiona, Conor J O’Hanlon, Myron Christodoulides, and Fiona J Radcliff. 2019. "Feasibility of Using a Luminescence-Based Method to Determine Serum Bactericidal Activity against Neisseria gonorrhoeae" Vaccines 7, no. 4: 191. https://doi.org/10.3390/vaccines7040191
APA StyleClow, F., O’Hanlon, C. J., Christodoulides, M., & Radcliff, F. J. (2019). Feasibility of Using a Luminescence-Based Method to Determine Serum Bactericidal Activity against Neisseria gonorrhoeae. Vaccines, 7(4), 191. https://doi.org/10.3390/vaccines7040191




