Maternally-Derived Antibodies Protect against Challenge with Highly Pathogenic Avian Influenza Virus of the H7N3 Subtype
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Cells, Eggs, and Chickens
2.3. Viruses
2.4. Vaccine Preparation
2.5. Vaccination of Mothers and Generation of Hatchlings with and without MDA
2.6. Challenge of 21-Day-Old Chickens with or without MDAs
2.7. Virus Shedding after Challenge
2.8. Hemagglutination Inhibition (HI) Assay
2.9. Statistical Analysis
3. Results
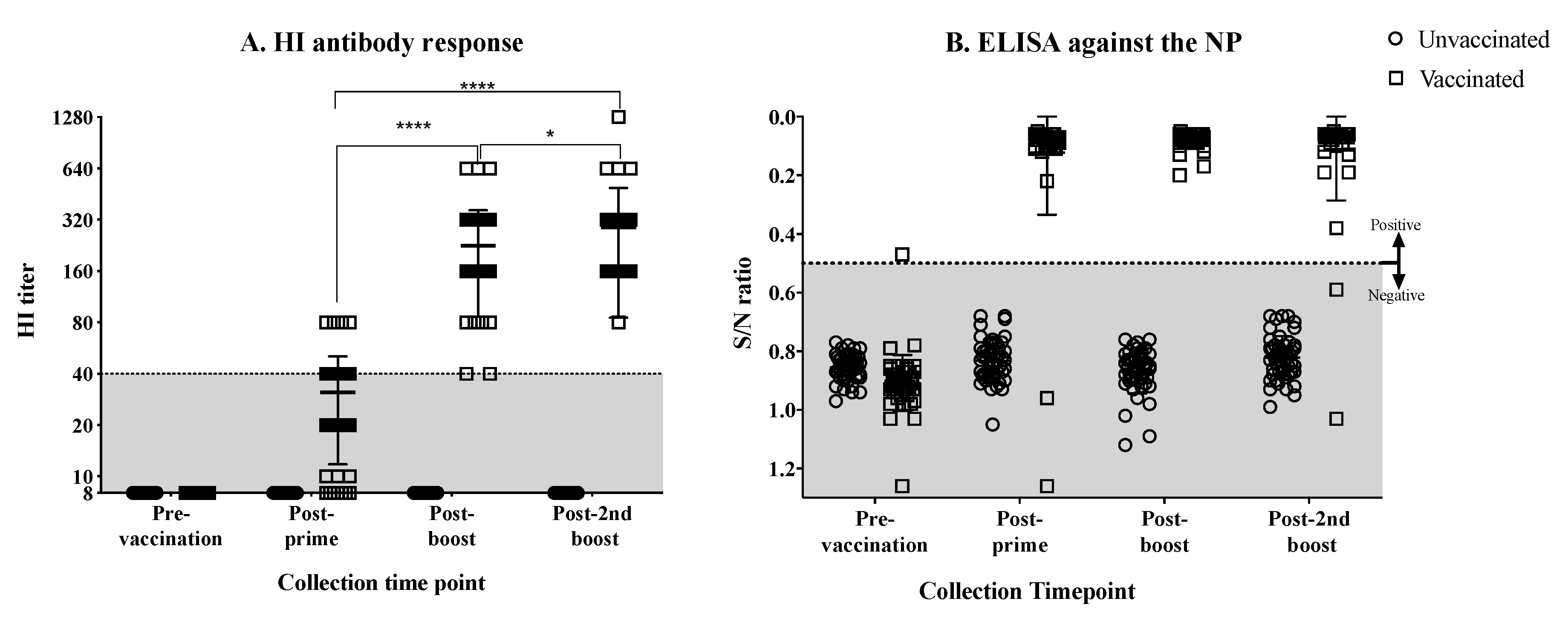
3.1. Antibody Response against the H7N3 Influenza Virus in Vaccinated Hens
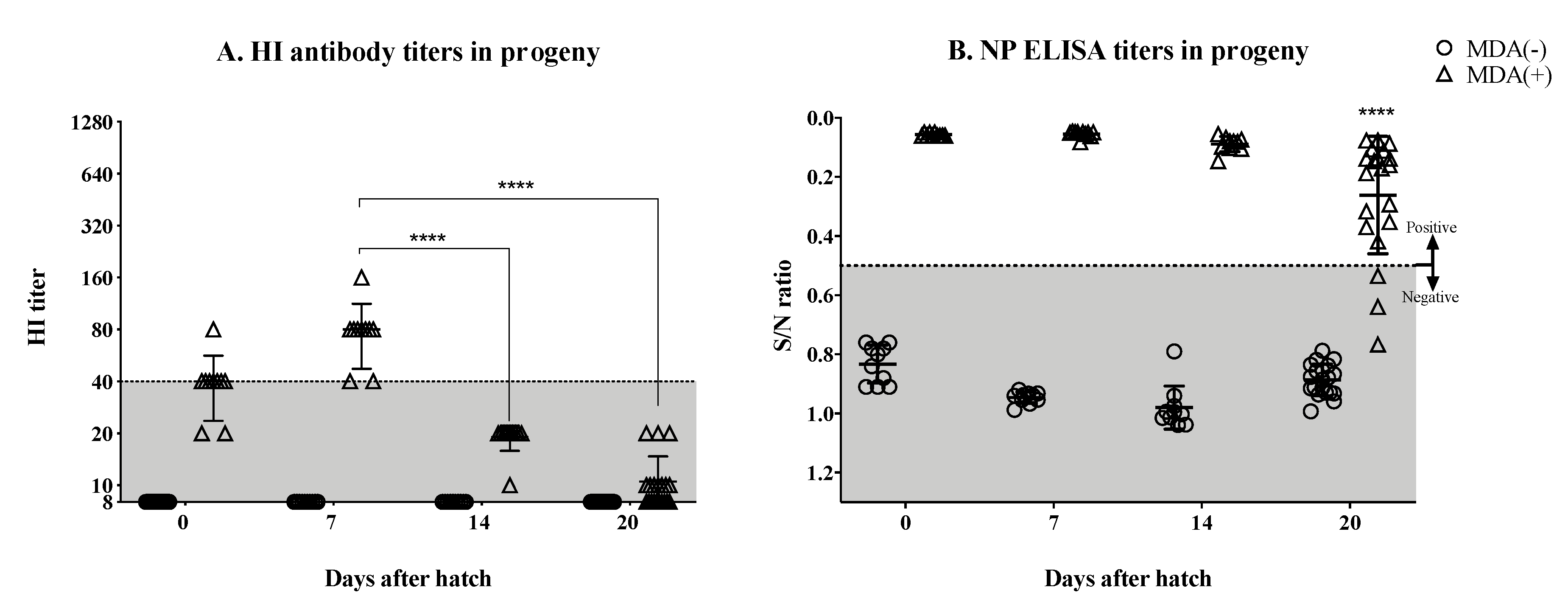
3.2. Significant Drop in the Hatchlings’ HI Titers by the Second Week Post-Hatch
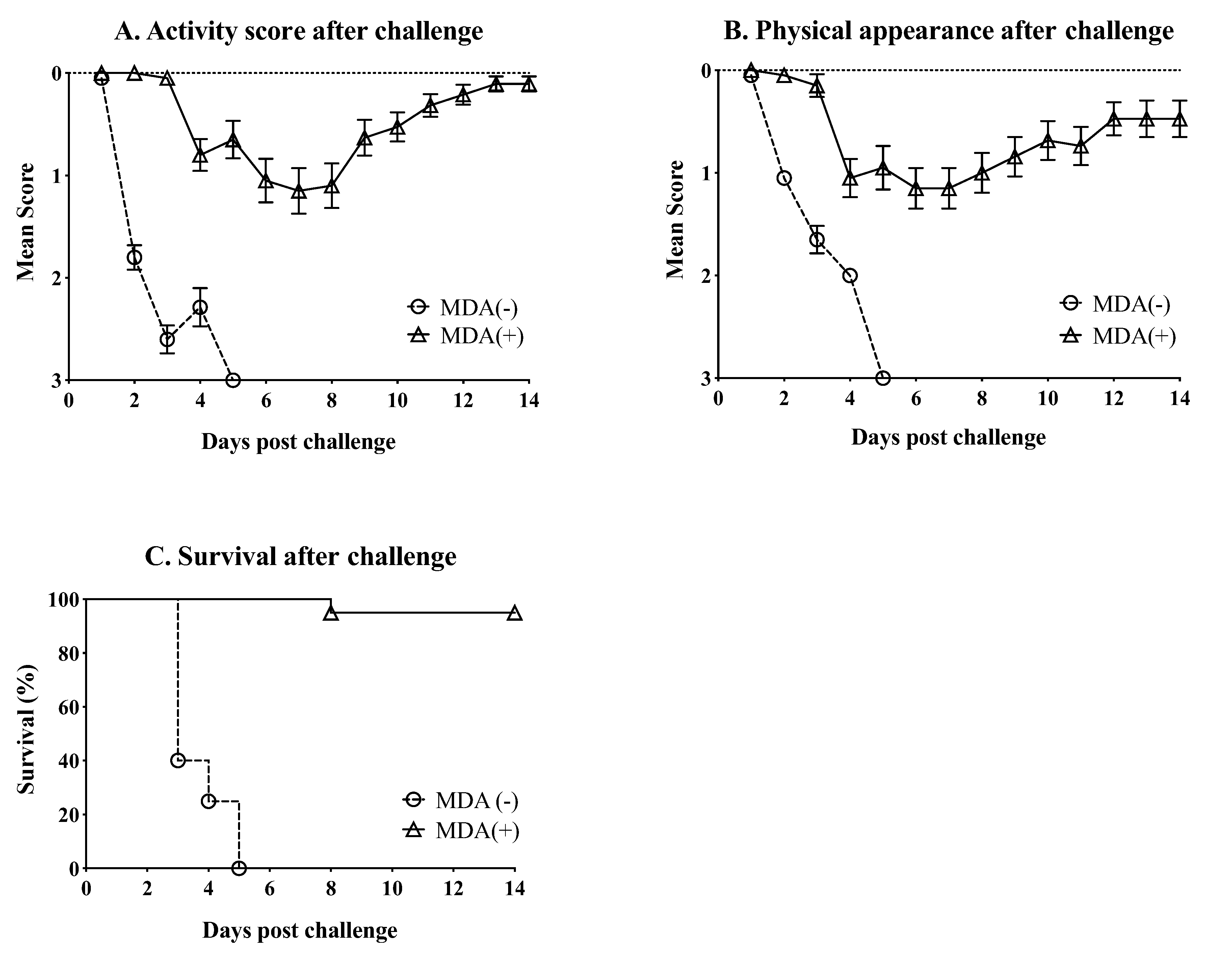
3.3. MDA Ameliorate the Clinical Outcome and Partially Protect against Mortality after Challenge with HPAIV
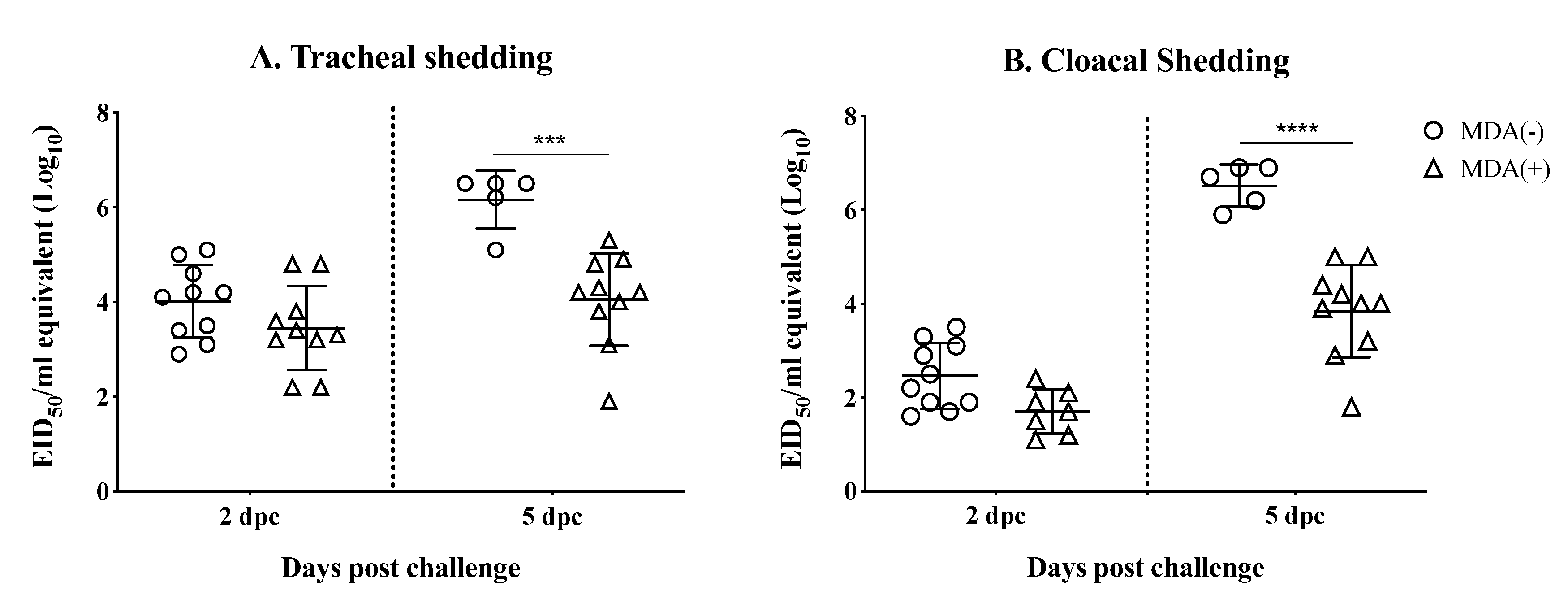
3.4. MDAs Lead to Reduced Virus Shedding after Challenge
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- OIE. Avian Influenza (Infection with Avian Influenza Viruses). In Manual of Diagnostic Tests and Vaccines for Terrestrial Animals 2018; World Organization for Animal Health: Paris, France, 2015. [Google Scholar]
- Berhane, Y.; Hisanaga, T.; Kehler, H.; Neufeld, J.; Manning, L.; Argue, C.; Handel, K.; Hooper-McGrevy, K.; Jonas, M.; Robinson, J.; et al. Highly pathogenic avian influenza virus A (H7N3) in domestic poultry, Saskatchewan, Canada, 2007. Emerg. Infect. Dis. 2009, 15, 1492–1495. [Google Scholar] [CrossRef]
- Max, V.; Herrera, J.; Moreira, R.; Rojas, H. Avian Influenza in Chile: A Successful Experience. Avian Dis. 2007, 51, 363–365. [Google Scholar] [CrossRef]
- Pasick, J.; Berhane, Y.; Hooper-McGrevy, K. Avian influenza: The Canadian experience. Revue Scientifique Technique 2009, 28, 349–358. [Google Scholar] [CrossRef]
- FAO. Highly Pathogenic Avian Influenza in Mexico (H7N3)—A Significant Threat to Poultry Production not to Be Underestimated; EMPRESWATCH: Rome, Italy, 2012. [Google Scholar]
- OIE. Immediate Notifications and Follow-Up Reports of Highly Pathogenic Avian Influenza (Types H5 and H7); Avian Influenza portal Paris; World Organization for Animal Health: Paris, France, 2019. [Google Scholar]
- Criado, M.F.; Bertran, K.; Lee, D.-H.; Killmaster, L.; Stephens, C.B.; Spackman, E.; E Silva, M.S.; Atkins, E.; Mebatsion, T.; Widener, J.; et al. Efficacy of novel recombinant fowlpox vaccine against recent Mexican H7N3 highly pathogenic avian influenza virus. Vaccine 2019, 37, 2232–2243. [Google Scholar] [CrossRef] [PubMed]
- Youk, S.-S.; Lee, D.-H.; Leyson, C.M.; Smith, D.; Criado, M.F.; DeJesus, E.; Swayne, D.E.; Pantin-Jackwood, M.J. Loss of Fitness of Mexican H7N3 Highly Pathogenic Avian Influenza Virus in Mallards after Circulating in Chickens. J. Virol. 2019, 93. [Google Scholar] [CrossRef] [PubMed]
- Schat, K.A.; Kaspers, B.; Kaiser, P. Avian Immunology, 2nd ed.; Acedemic Press: San Diego, CA, USA, 2014. [Google Scholar]
- Leslie, G.A.; Clem, L.W. Phylogen of immunoglobulin structure and function. 3. Immunoglobulins of the chicken. J. Exp. Med. 1969, 130, 1337–1352. [Google Scholar] [CrossRef] [PubMed]
- Lebacq-Verheyden, A.-M.; Vaerman, J.P.; Heremans, J.F. A possible homologue of mammalian IgA in chicken serum and secretions. Immunology 1972, 22, 165–175. [Google Scholar]
- Orlans, E. An IgA-like immunoglobulin in the fowl. Mol. Immunol. 1972, 9, 833–836. [Google Scholar] [CrossRef]
- Patterson, R.; Youngner, J.S.; O Weigle, W.; Dixon, F.J. Antibody production and transfer to egg yolk in chickens. J. Immunol. 1962, 89, 272–278. [Google Scholar]
- Cutting, J.A.; Roth, T.F. Changes in specific sequestration of protein during transport into the developing oocyte of the chicken. Biochim. Biophys. Acta (BBA) Biomembr. 1973, 298, 951–955. [Google Scholar] [CrossRef]
- Rose, M.E.; Orlans, E. Immunoglobulins in the egg, embryo and young chick. Dev. Comp. Immunol. 1981, 5, 15–20. [Google Scholar] [CrossRef]
- Loeken, M.R.; Roth, T.F. Analysis of maternal IgG subpopulations which are transported into the chicken oocyte. Immunology 1983, 49, 21–28. [Google Scholar] [PubMed]
- Morrison, S.L.; Mohammed, M.S.; Wims, L.A.; Trinh, R.; Etches, R. Sequences in antibody molecules important for receptor-mediated transport into the chicken egg yolk. Mol. Immunol. 2002, 38, 619–625. [Google Scholar] [CrossRef]
- Rose, M.E.; Orlans, E.; Buttress, N. Immunoglobulin classes in the hen’s egg: Their segregation in yolk and white. Eur. J. Immunol. 1974, 4, 521–523. [Google Scholar] [CrossRef] [PubMed]
- Brierley, J.; Hemmings, W.A. The Selective Transport of Antibodies from the Yolk Sac to the Circulation of the Chick. J. Embryol. Exp. Morph. 1956, 4, 34–41. [Google Scholar]
- Tressler, R.L.; Roth, T.F. IgG receptors on the embryonic chick yolk sac. J. Boil. Chem. 1987, 262, 15406–15412. [Google Scholar]
- Tesar, D.B.; Cheung, E.J.; Bjorkman, P.J. The Chicken Yolk Sac IgY Receptor, a Mammalian Mannose Receptor Family Member, Transcytoses IgY across Polarized Epithelial Cells. Mol. Biol. Cell 2008, 19, 1587–1593. [Google Scholar] [CrossRef]
- Patterson, R.; Youngner, J.S.; Weigle, W.O.; Dixon, F.J. The Metabolism of Serum Proteins in the Hen and Chick and Secretion of Serum Proteins by the Ovary of the Hen. J. Gen. Physiol. 1962, 45, 501–513. [Google Scholar] [CrossRef]
- Kramer, T.T.; Cho, H.C. Transfer of immunoglobulins and antibodies in the hen’s egg. Immunology 1970, 19, 157–167. [Google Scholar]
- Kowalczyk, K.; Daiss, J.; Halpern, J.; Roth, T.F. Quantitation of maternal-fetal IgG transport in the chicken. Immunology 1985, 54, 755–762. [Google Scholar]
- Mondal, S.P.; Naqi, S.A. Maternal antibody to infectious bronchitis virus: Its role in protection against infection and development of active immunity to vaccine. Vet. Immunol. Immunopathol. 2001, 79, 31–40. [Google Scholar] [CrossRef]
- Maas, R.; Rosema, S.; Van Zoelen, D.; Venema, S. Maternal immunity against avian influenza H5N1 in chickens: Limited protection and interference with vaccine efficacy. Avian Pathol. 2011, 40, 87–92. [Google Scholar] [CrossRef] [PubMed]
- De Vriese, J.; Steensels, M.; Palya, V.; Tardin, Y.; Dorsey, K.M.; Lambrecht, B.; Van Borm, S.; Berg, T.V.D. Passive Protection Afforded by Maternally-Derived Antibodies in Chickens and the Antibodies’ Interference with the Protection Elicited by Avian Influenza–Inactivated Vaccines in Progeny. Avian Dis. Dig. 2010, 5, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Rajao, D.S.; Sandbulte, M.R.; Gauger, P.C.; Kitikoon, P.; Platt, R.; Roth, J.A.; Perez, D.R.; Loving, C.L.; Vincent, A.L. Heterologous challenge in the presence of maternally-derived antibodies results in vaccine-associated enhanced respiratory disease in weaned piglets. Virology 2016, 491, 79–88. [Google Scholar] [CrossRef]
- Abdelwhab, E.; Grund, C.; Aly, M.M.; Beer, M.; Harder, T.C.; Hafez, H.M. Influence of maternal immunity on vaccine efficacy and susceptibility of one day old chicks against Egyptian highly pathogenic avian influenza H5N1. Vet. Microbiol. 2012, 155, 13–20. [Google Scholar] [CrossRef]
- Gharaibeh, S.; Mahmoud, K. Decay of maternal antibodies in broiler chickens. Poult. Sci. 2013, 92, 2333–2336. [Google Scholar] [CrossRef]
- Hamal, K.R.; Burgess, S.C.; Pevzner, I.Y.; Erf, G.F. Maternal antibody transfer from dams to their egg yolks, egg whites, and chicks in meat lines of chickens. Poult. Sci. 2006, 85, 1364–1372. [Google Scholar] [CrossRef]
- De Wit, E.; Munster, V.J.; Spronken, M.I.J.; Bestebroer, T.M.; Baas, C.; Beyer, W.E.P.; Rimmelzwaan, G.F.; Osterhaus, A.D.M.E.; Fouchier, R.A.M. Protection of Mice against Lethal Infection with Highly Pathogenic H7N7 Influenza A Virus by Using a Recombinant Low-Pathogenicity Vaccine Strain. J. Virol. 2005, 79, 12401–12407. [Google Scholar] [CrossRef][Green Version]
- De Groot, A.S.; Ardito, M.; Terry, F.; Levitz, L.; Ross, T.; Moise, L.; Martin, W. Low immunogenicity predicted for emerging avian-origin H7N9: Implication for influenza vaccine design. Hum. Vaccines Immunother. 2013, 9, 950–956. [Google Scholar] [CrossRef]
- De Groot, A.S.; Moise, L.; Liu, R.; Gutierrez, A.H.; Terry, F.; Koita, O.A.; Ross, T.M.; Martin, W. Cross-conservation of T-cell epitopes: Now even more relevant to (H7N9) influenza vaccine design. Hum. Vaccines Immunother. 2014, 10, 256–262. [Google Scholar] [CrossRef]
- Blanchfield, K.; Kamal, R.P.; Tzeng, W.-P.; Music, N.; Wilson, J.R.; Stevens, J.; Lipatov, A.S.; Katz, J.M.; York, I.A. Recombinant influenza H7 hemagglutinins induce lower neutralizing antibody titers in mice than do seasonal hemagglutinins. Influ. Other Respir. Viruses 2014, 8, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Swayne, D.E.; Suarez, D.L.; Schultz-Cherry, S.; Tumpey, T.M.; King, D.J.; Nakaya, T.; Palese, P.; Garcia-Sastre, A. Recombinant Paramyxovirus Type 1-Avian Influenza-H7 Virus as a Vaccine for Protection of Chickens Against Influenza and Newcastle Disease. Avian Dis. 2003, 47, 1047–1050. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Nieto, G.R.; Perez, D.R. A New Generation of Modified Live-Attenuated Avian Influenza Viruses Using a Two-Strategy Combination as Potential Vaccine Candidates. J. Virol. 2007, 81, 9238–9248. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Mena, I.; Ma, J.; Bawa, B.; Krammer, F.; Lyoo, Y.S.; Lang, Y.; Morozov, I.; Mahardika, G.N.; Ma, W.; et al. Newcastle Disease Virus-Vectored H7 and H5 Live Vaccines Protect Chickens from Challenge with H7N9 or H5N1 Avian Influenza Viruses. J. Virol. 2015, 89, 7401–7408. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.; Elaish, M.; Kc, M.; Abundo, M.C.; Ghorbani, A.; Ngunjiri, J.M.; Lee, C.-W. Efficacy and synergy of live-attenuated and inactivated influenza vaccines in young chickens. PLoS ONE 2018, 13, e0195285. [Google Scholar] [CrossRef] [PubMed]
- Perez, D.R.; Angel, M.; Gonzalez-Reiche, A.S.; Santos, J.; Obadan, A.; Martinez-Sobrido, L. Plasmid-Based Reverse Genetics of Influenza A Virus. Breast Cancer 2017, 1602, 251–273. [Google Scholar]
- Bertran, K.; Lee, D.-H.; Criado, M.F.; Balzli, C.L.; Killmaster, L.F.; Kapczynski, D.R.; Swayne, D.E. Maternal antibody inhibition of recombinant Newcastle disease virus vectored vaccine in a primary or booster avian influenza vaccination program of broiler chickens. Vaccine 2018, 36, 6361–6372. [Google Scholar] [CrossRef]
- Liu, R.; Moise, L.; Tassone, R.; Gutiérrez, A.H.; Terry, F.E.; Sangare, K.; Ardito, M.T.; Martin, W.D.; De Groot, A.S. H7N9 T-cell epitopes that mimic human sequences are less immunogenic and may induce Treg-mediated tolerance. Hum. Vaccines Immunother. 2015, 11, 2241–2252. [Google Scholar] [CrossRef]
- Kamal, R.P.; Blanchfield, K.; Belser, J.A.; Music, N.; Tzeng, W.-P.; Holiday, C.; Burroughs, A.; Sun, X.; Maines, T.R.; Levine, M.Z.; et al. Inactivated H7 Influenza Virus Vaccines Protect Mice despite Inducing Only Low Levels of Neutralizing Antibodies. J. Virol. 2017, 91, e01202–e01217. [Google Scholar] [CrossRef]
- Maas, R.; Tacken, M.; Van Zoelen, D.; Oei, H. Dose response effects of avian influenza (H7N7) vaccination of chickens: Serology, clinical protection and reduction of virus excretion. Vaccine 2009, 27, 3592–3597. [Google Scholar] [CrossRef]
- Angeletti, D.; Yewdell, J.W. Is It Possible to Develop a “Universal” Influenza Virus Vaccine? Outflanking Antibody Immunodominance on the Road to Universal Influenza Vaccination. Cold Spring Harb. Perspect. Biol. 2018, 10, a028852. [Google Scholar] [CrossRef] [PubMed]
- Cox, R.J. Correlates of protection to influenza virus, where do we go from here? Hum. Vaccines Immunother. 2013, 9, 405–408. [Google Scholar] [CrossRef] [PubMed]
- Krammer, F.; Cox, R.J. The emergence of H7N9 viruses: A chance to redefine correlates of protection for influenza virus vaccines. Expert Rev. Vaccines 2013, 12, 1369–1372. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hobson, D.; Curry, R.L.; Beare, A.S.; Ward-Gardner, A. The role of serum haemagglutination-inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. Epidemiol. Infect. 1972, 70, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Yewdell, J.W.; Frank, E.; Gerhard, W. Expression of influenza A virus internal antigens on the surface of infected P815 cells. J. Immunol. 1981, 126, 1814–1819. [Google Scholar] [PubMed]
- Carragher, D.M.; Kaminski, D.A.; Moquin, A.; Hartson, L.; Randall, T.D. A novel role for non-neutralizing antibodies against nucleoprotein in facilitating resistance to influenza virus. J. Immunol. 2008, 181, 4168–4176. [Google Scholar] [CrossRef] [PubMed]
- Christensen, S.R.; Toulmin, S.A.; Griesman, T.; Lamerato, L.E.; Petrie, J.G.; Martin, E.T.; Monto, A.S.; Hensley, S.E. Assessing the Protective Potential of H1N1 Influenza Virus Hemagglutinin Head and Stalk Antibodies in Humans. J. Virol. 2019, 93, e02134-18. [Google Scholar] [CrossRef]
- Park, J.-K.; Han, A.; Czajkowski, L.; Reed, S.; Athota, R.; Bristol, T.; Rosas, L.A.; Cervantes-Medina, A.; Taubenberger, J.K.; Memoli, M.J. Evaluation of Preexisting Anti-Hemagglutinin Stalk Antibody as a Correlate of Protection in a Healthy Volunteer Challenge with Influenza A/H1N1pdm Virus. MBio 2018, 9, e02284-17. [Google Scholar] [CrossRef]
- Chen, Y.-Q.; Wohlbold, T.J.; Zheng, N.-Y.; Huang, M.; Huang, Y.; Neu, K.E.; Lee, J.; Wan, H.; Rojas, K.T.; Kirkpatrick, E.; et al. Influenza Infection in Humans Induces Broadly Cross-Reactive and Protective Neuraminidase-Reactive Antibodies. Cell 2018, 173, 417–429.e10. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardenas-Garcia, S.; Ferreri, L.; Wan, Z.; Carnaccini, S.; Geiger, G.; Obadan, A.O.; Hofacre, C.L.; Rajao, D.; Perez, D.R. Maternally-Derived Antibodies Protect against Challenge with Highly Pathogenic Avian Influenza Virus of the H7N3 Subtype. Vaccines 2019, 7, 163. https://doi.org/10.3390/vaccines7040163
Cardenas-Garcia S, Ferreri L, Wan Z, Carnaccini S, Geiger G, Obadan AO, Hofacre CL, Rajao D, Perez DR. Maternally-Derived Antibodies Protect against Challenge with Highly Pathogenic Avian Influenza Virus of the H7N3 Subtype. Vaccines. 2019; 7(4):163. https://doi.org/10.3390/vaccines7040163
Chicago/Turabian StyleCardenas-Garcia, Stivalis, Lucas Ferreri, Zhimin Wan, Silvia Carnaccini, Ginger Geiger, Adebimpe O. Obadan, Charles L. Hofacre, Daniela Rajao, and Daniel R. Perez. 2019. "Maternally-Derived Antibodies Protect against Challenge with Highly Pathogenic Avian Influenza Virus of the H7N3 Subtype" Vaccines 7, no. 4: 163. https://doi.org/10.3390/vaccines7040163
APA StyleCardenas-Garcia, S., Ferreri, L., Wan, Z., Carnaccini, S., Geiger, G., Obadan, A. O., Hofacre, C. L., Rajao, D., & Perez, D. R. (2019). Maternally-Derived Antibodies Protect against Challenge with Highly Pathogenic Avian Influenza Virus of the H7N3 Subtype. Vaccines, 7(4), 163. https://doi.org/10.3390/vaccines7040163







