Advances in RNA Vaccines for Preventive Indications: A Case Study of a Vaccine against Rabies
Abstract
1. Introduction
1.1. Innovative Approaches with Recombinant Technology
1.2. DNA Vaccines
1.3. mRNA Vaccines
2. Application of mRNA Technology to Rabies
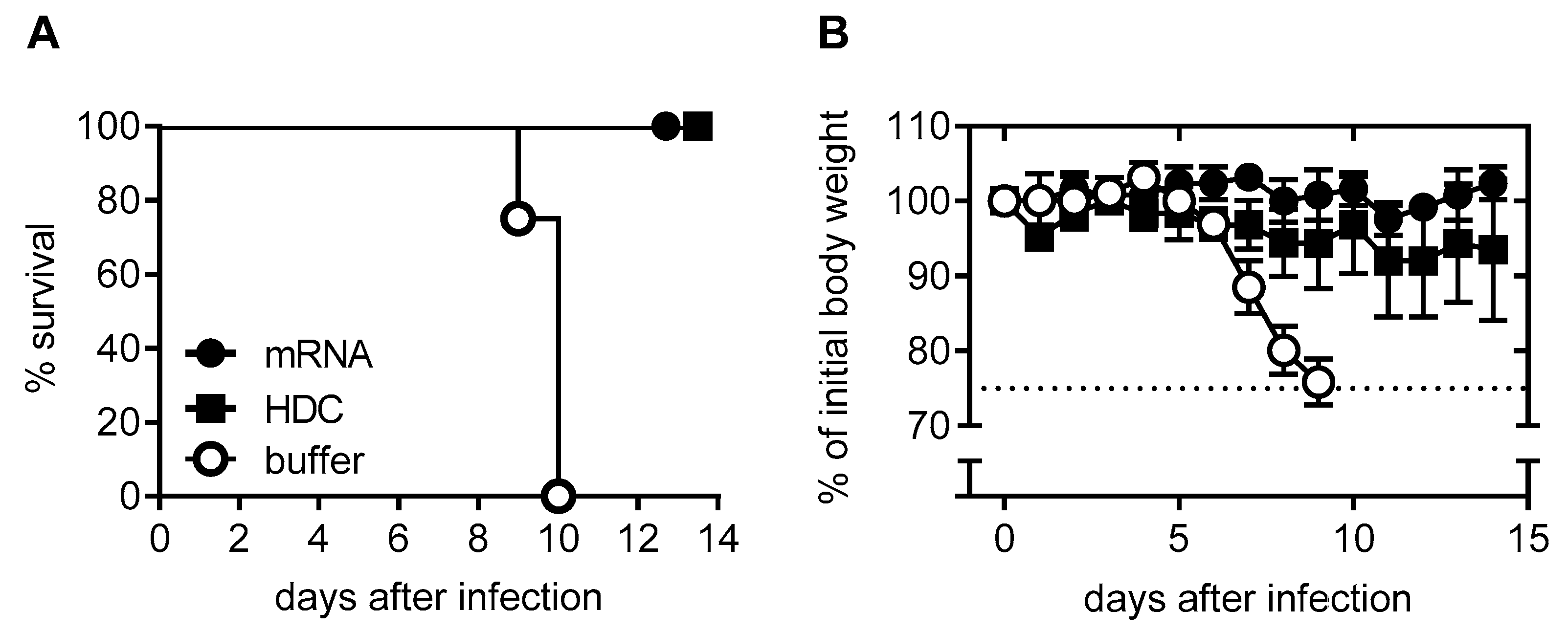
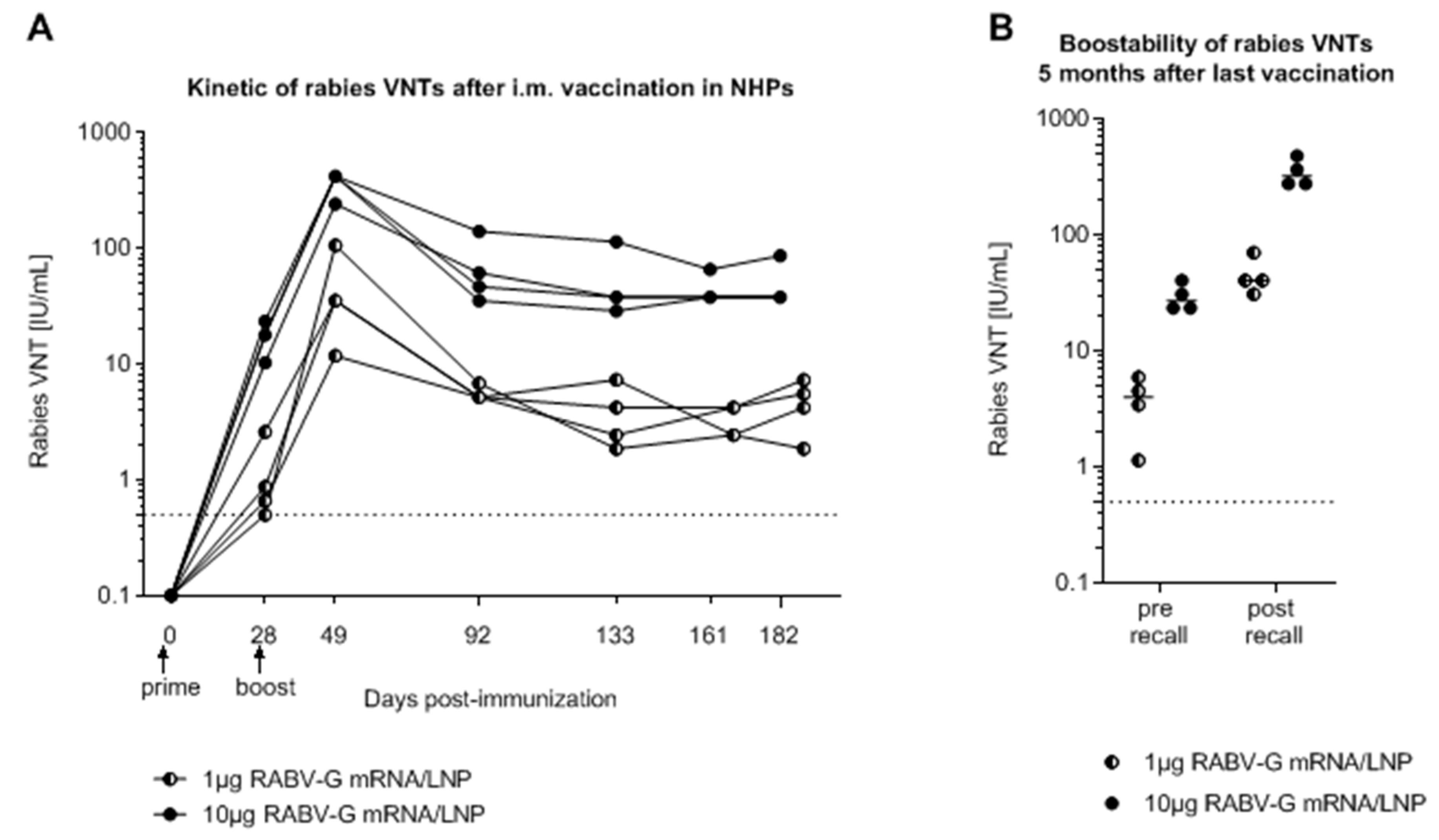
2.1. mRNA Vaccine Candidate CV7201: Preclinical Studies
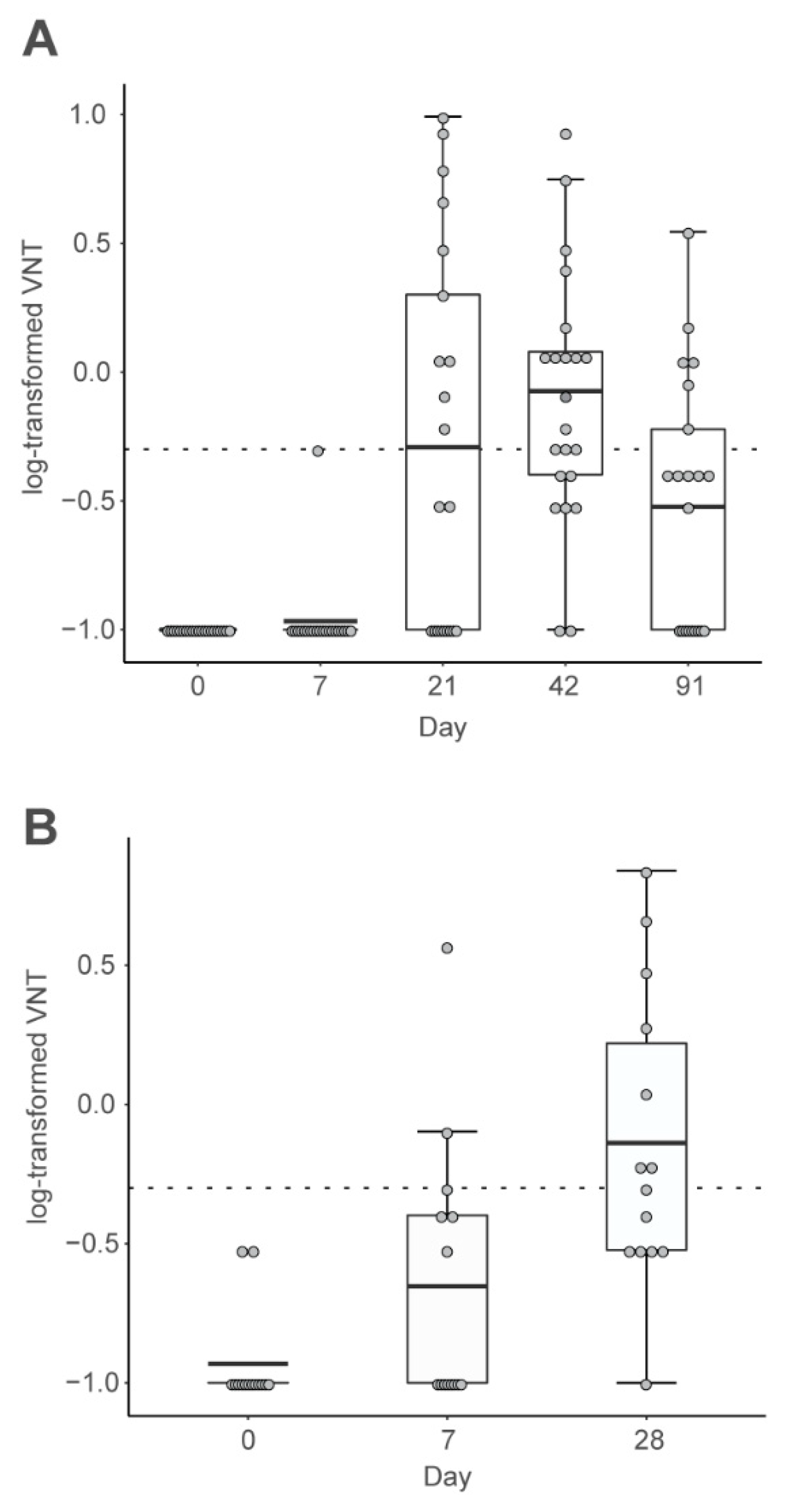
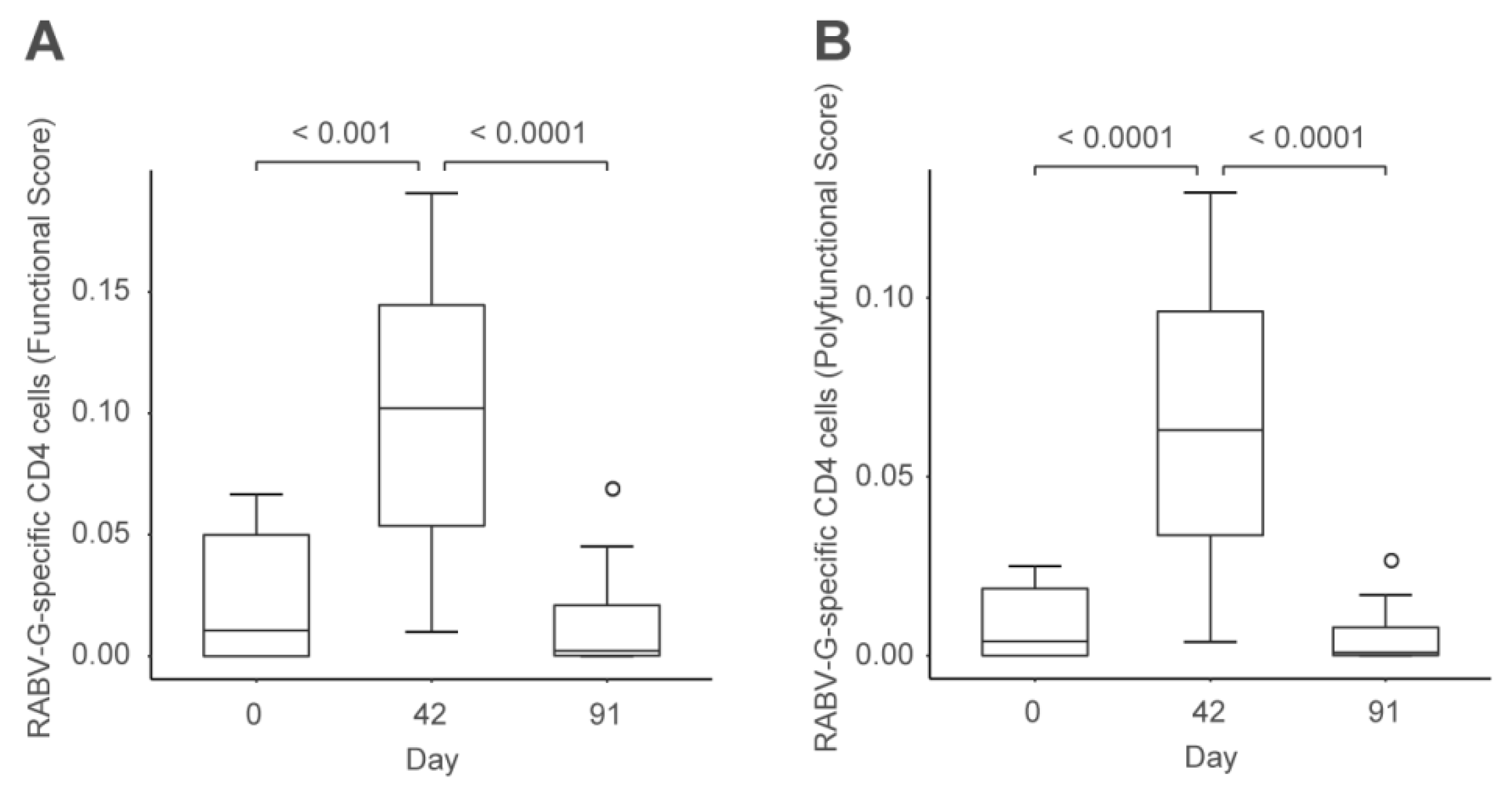
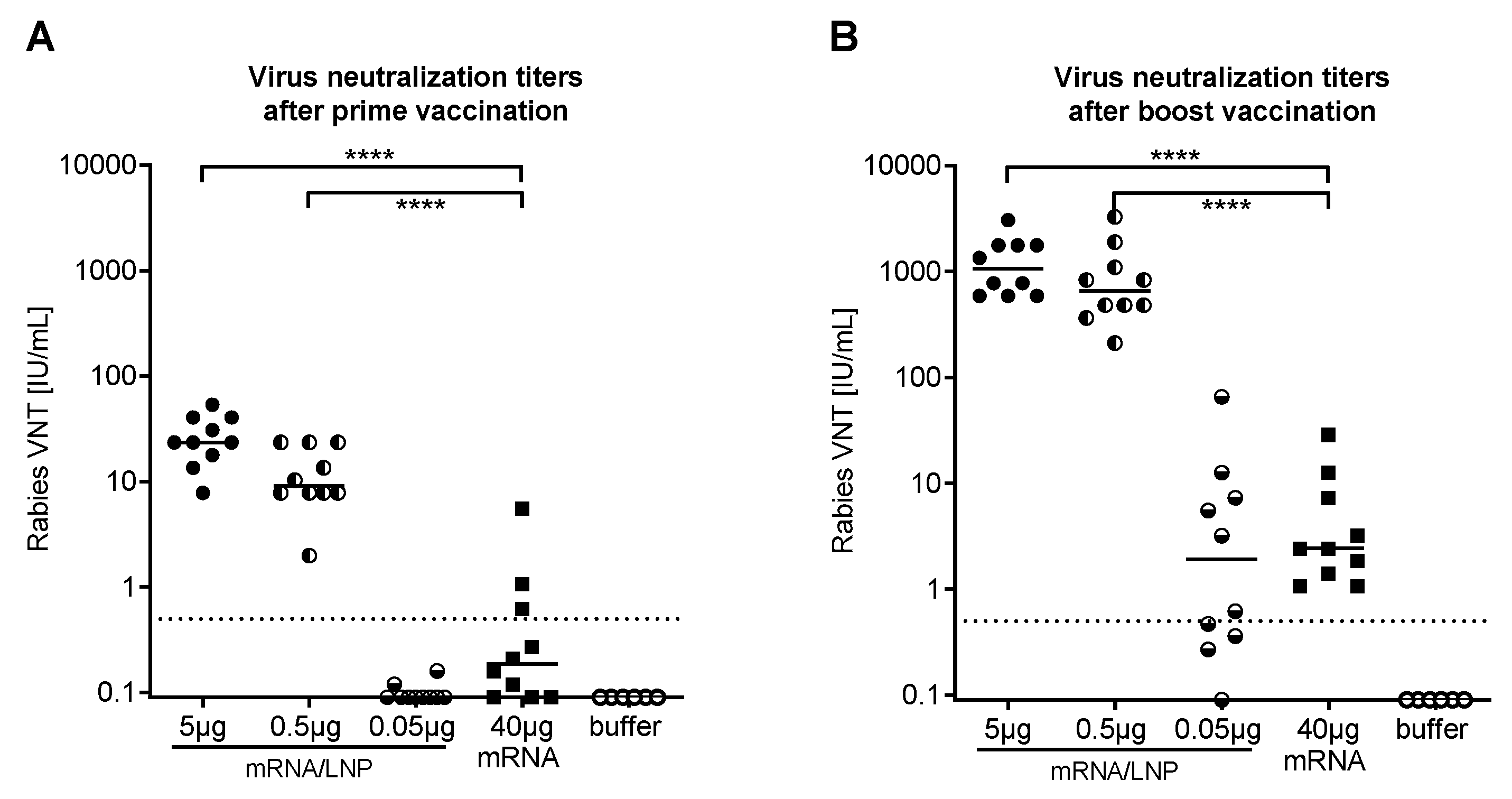
2.2. CV7201 in Humans
3. Formulation of Lipid Nanoparticle (LNP) mRNA
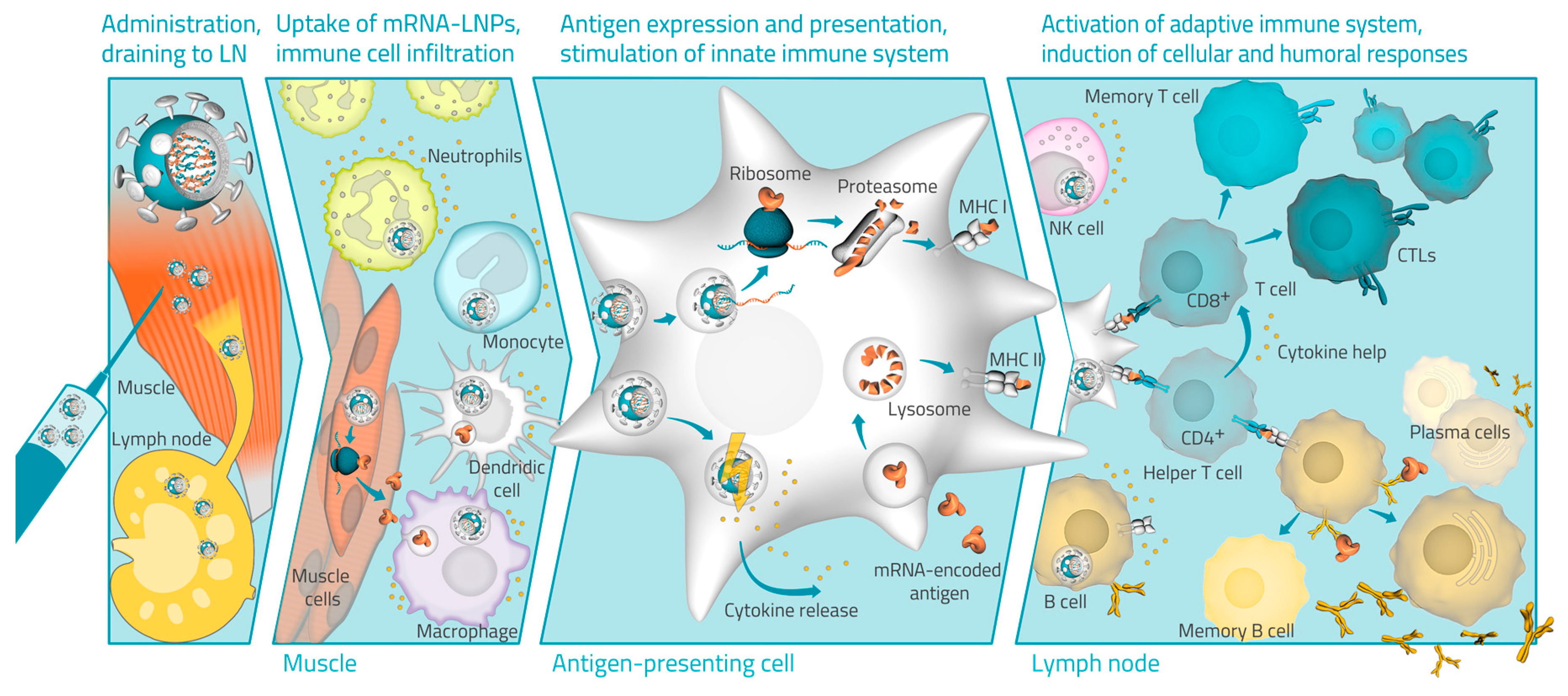
4. Hypothesized Mode of Action of LNP mRNA
5. Conclusions
Funding
Conflicts of Interest
References
- Fahrion, A.S.; Mikhailov, A.; Abela-Ridder, B.; Giacinti, J.; Harries, J. Human rabies transmitted by dogs: Current status of global data, 2015. Wkly. Epidemiol. Rec. 2016, 91, 13–20. [Google Scholar]
- Velasco-Villa, A.; Mauldin, M.R.; Shi, M. The history of rabies in the Western Hemisphere. Antivir. Res. 2017, 146, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Hankins, D.G.; Rosekrans, J.A. Overview, prevention, and treatment of rabies. Mayo Clin. Proc. 2004, 79, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Taylor, L.H.; Hampson, K.; Fahrion, A.; Abela-Ridder, B.; Nela, L.H. Difficulties in estimating the human burden of canine rabies. Acta Trop. 2017, 165, 133–140. [Google Scholar] [CrossRef]
- Hampson, K.; Coudeville, L.; Lembo, T. Global Alliance for Rabies Control Partners for Rabies Prevention. Estimating the global burden of endemic canine rabies. PLoS Negl. Trop. Dis. 2015, 9, e0003786. [Google Scholar]
- Pasteur, L. Méthode pour prévenir la rage après morsure. L’Academie Sci. 1885, 101, 765–772. [Google Scholar]
- Hicks, D.J.; Fooks, A.R.; Johnson, N. Developments in rabies vaccines. Clin. Exp. Immunol. 2012, 169, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Smith, T.G.; Rupprecht, C.E. From brain passage to cell adaptation: The road of human rabies vaccine development. Exp. Rev. Vaccines 2011, 10, 1597–1608. [Google Scholar] [CrossRef]
- Javier, R.S.; Kunishita, T.; Koike, F.; Tabira, T. Semple rabies vaccine: Presence of myelin basic protein and proteolipid protein and its activity in experimental allergic encephalomyelitis. J. Neurol. Sci. 1989, 93, 221–230. [Google Scholar] [CrossRef]
- Diallo, M.K.; Diallo, A.O.; Dicko, A.; Richard, V.; Espié, E. Human rabies post exposure prophylaxis at the Pasteur Institute of Dakar, Senegal: Trends and risk factors. BMC Infect. Dis. 2019, 19, 321. [Google Scholar] [CrossRef]
- WHO. Rabies vaccines: WHO position paper. Wkly. Epidemiol. Rec. 2010, 85, 309–320. [Google Scholar]
- WHO Guide for Rabies Pre and Post Exposure Prophylaxis in Humans. Available online: https://www.who.int/rabies/PEP_Prophylaxis_guideline_15_12_2014.pdf (accessed on 10 May 2019).
- Quiambao, B.P.; Dy-Tioco, H.Z.; Dizon, R.M.; Crisostomo, M.E.; Teuwen, D.E. Rabies post-exposure prophylaxis with purified equine rabies immunoglobulin: One-year follow-up of patients with laboratory-confirmed category III rabies exposure in the Philippines. Vaccine 2009, 27, 7162–7166. [Google Scholar] [CrossRef] [PubMed]
- Quiambao, B.P.; Dimaano, E.M.; Ambas, C.; Davis, R.; Banzhoff, A.; Malerczyk, C. Reducing the cost of post-exposure rabies prophylaxis: Efficacy of 0.1 ml PCEC rabies vaccine administered intradermally using the Thai Red Cross post-exposure regimen in patients severely exposed to laboratory-confirmed rabid animals. Vaccine 2005, 23, 1709–1714. [Google Scholar] [CrossRef] [PubMed]
- Tordo, N.; Poch, O.; Ermine, A.; Keith, G.; Rougeon, F. Completion of the rabies virus genome sequence determination: Highly conserved domains among the L (polymerase) proteins of unsegmented negative-strand RNA viruses. Virology 1988, 165, 565–576. [Google Scholar] [CrossRef]
- Baer, G.M. Animal models in the pathogenesis and treatment of rabies. Rev. Infect. Dis. 1988, 10 (Suppl. 4), S739–S750. [Google Scholar] [CrossRef] [PubMed]
- Astray, R.M.; Jorge, S.A.; Pereira, C.A. Rabies vaccine development by expression of recombinant viral glycoprotein. Arch. Virol. 2017, 162, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Fooks, A.R.; Banyard, A.C.; Ertl, H.C.J. New human rabies vaccines in the pipeline. Vaccine 2018. [Google Scholar] [CrossRef] [PubMed]
- Ullas, P.T.; Desai, A.; Madhusudana, S.N. Immunogenicity and efficacy of a plasmid DNA rabies vaccine incorporating Myd88 as a genetic adjuvant. Clin. Exp. Vaccine Res. 2014, 3, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Maruggi, G.; Zhnag, C.; Li, J.; Ulmer, J.B.; Yu, D. mRNA as a transformative technology for vaccine development to control infectious diseases. Mol. Ther. 2019, 27, 757–772. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef]
- Hassett, K.J.; Benenato, K.E.; Jacquinet, E. Optimization of lipid nanoparticles for intramuscular administration of mRNA vaccines. Mol. Ther. Nucleic Acids 2019, 15, 1–11. [Google Scholar] [CrossRef]
- Zhang, C.; Maruggi, G.; Shan, H.; Li, J. Advances in mRNA vaccines for infectious diseases. Front. Immunol. 2019, 10, 594. [Google Scholar] [CrossRef] [PubMed]
- Minor, P. Vaccine-derived poliovirus (VDPV): Impact on poliomyelitis eradication. Vaccine 2009, 27, 2649–2652. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Global Action Plan to Minimize Poliovirus Facility-Associated Risk After Type-Specific Eradication of Wild Polioviruses and Sequential Cessation of Oral Polio Vaccine Use (GAPIII)—2015. WHO/POLIO/15.05. Available online: http://polioeradication.org/wp-content/uploads/2016/12/GAPIII_2014.pdf (accessed on 26 June 2019).
- Fotin-Mleczek, M.; Duchardt, K.M.; Lorenz, C. Messenger RNA-based vaccines with dual activity induce balanced TLR-7 dependent adaptive immune responses and provide antitumor activity. J. Immunother. 2011, 34, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Petsch, B.; Schnee, M.; Vogel, A.B. Protective efficacy of in vitro synthesized, specific mRNA vaccines against influenza A virus infection. Nat. Biotechnol. 2012, 30, 1210–1216. [Google Scholar] [CrossRef]
- Schlake, T.; Thess, A.; Fotin-Mleczek, M.; Kallen, K.J. Developing mRNA-vaccine technologies. RNA Biol. 2012, 9, 1319–1330. [Google Scholar] [CrossRef] [PubMed]
- Wiktor, T.J.; György, E.; Schlumberger, H.D.; Sokol, F.; Koprowski, H. Antigenic properties of rabies virus components. J. Immunol. 1973, 110, 269–276. [Google Scholar]
- Barth, R.; Gruschkau, H.; Bijok, U.; Hilfenhaus, J.; Hinz, J.; Milcke, L.; Moser, H.; Jaeger, O.; Ronneberger, H.; Weinmann, E. A new inactivated tissue culture rabies vaccine for use in man. Evaluation of PCEC-vaccine by laboratory tests. J. Biol. Stand. 1984, 12, 29–46. [Google Scholar] [CrossRef]
- Schnee, M.; Vogel, A.B.; Voss, D. An mRNA vaccine encoding rabies virus glycoprotein induces protection against lethal infection in mice and correlates of protection in adult and newborn pigs. PLoS Negl. Trop. Dis. 2016, 10, e0004746. [Google Scholar] [CrossRef]
- Watanabe, H.; Numata, K.; Ito, T.; Takagi, K.; Matsukawa, A. Innate immune response in Th1- and Th2-dominant mouse strains. Shock 2004, 22, 460–466. [Google Scholar] [CrossRef]
- Meslin, F.-X.; Kaplan, M.M.; Koprowski, H. Laboratory Techniques in Rabies, 4th ed.; WHO: Geneva, Switzerland, 1996. [Google Scholar]
- Alberer, M.; Gnad-Vogt, U.; Hong, H.S. Safety and immunogenicity of a mRNA rabies vaccine in healthy adults: An open-label, non-randomised, prospective, first-in-human phase 1 clinical trial. Lancet 2017, 390, 1511–1520. [Google Scholar] [CrossRef]
- WHO. WHO Expert Consultation on Rabies First Report 2004. Available online: http://www.who.int/rabies/trs931_%2006_05.pdf (accessed on 10 September 2019).
- Lin, L.; Finak, G.; Ushey, K. COMPASS identifies T-cell subsets correlated with clinical outcomes. Nat. Biotechnol. 2015, 33, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Gerdts, V.; Jöns, A.; Makoschey, B.; Visser, N.; Mettenleiter, T.C. Protection of pigs against Aujeszky’s disease by DNA vaccination. J. Gen. Virol. 1997, 78, 2139–2146. [Google Scholar] [CrossRef] [PubMed]
- Schrijver, R.S.; Langedijk, J.P.; Keil, G.M. Comparison of DNA application methods to reduce BRSV shedding in cattle. Vaccine 1998, 16, 130–134. [Google Scholar] [CrossRef]
- Mooij, P.; Grødeland, G.; Koopman, G. Needle-free delivery of DNA: Targeting of hemagglutinin to MHC class II molecules protects rhesus macaques against H1N1 influenza. Vaccine 2019, 37, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Geall, A.J.; Verma, A.; Otten, G.R. Nonviral delivery of self-amplifying RNA vaccines. Proc. Natl. Acad. Sci. USA 2012, 109, 14604–14609. [Google Scholar] [CrossRef] [PubMed]
- Lutz, J.; Lazzaro, S.; Habbeddine, M. Unmodified mRNA in LNPs constitutes a competitive technology for prophylactic vaccines. NPJ Vaccines 2017, 2, 29. [Google Scholar] [CrossRef]
- Esche, C.; Stellato, C.; Beck, L.A. Chemokines: Key players in innate and adaptive immunity. J. Investig. Dermatol. 2005, 125, 615–628. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, P.S.; Rudra, A.; Miao, L.; Anderson, D.G. Delivering the messenger: Advances in technologies for therapeutic mRNA delivery. Mol. Ther. 2019, 27, 710–728. [Google Scholar] [CrossRef]
- Rauch, S.; Jasny, E.; Schmidt, K.E.; Petsch, B. New vaccine technologies to combat outbreak situations. Front. Immunol. 2018, 9, 1963. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Armbruster, N.; Jasny, E.; Petsch, B. Advances in RNA Vaccines for Preventive Indications: A Case Study of a Vaccine against Rabies. Vaccines 2019, 7, 132. https://doi.org/10.3390/vaccines7040132
Armbruster N, Jasny E, Petsch B. Advances in RNA Vaccines for Preventive Indications: A Case Study of a Vaccine against Rabies. Vaccines. 2019; 7(4):132. https://doi.org/10.3390/vaccines7040132
Chicago/Turabian StyleArmbruster, Nicole, Edith Jasny, and Benjamin Petsch. 2019. "Advances in RNA Vaccines for Preventive Indications: A Case Study of a Vaccine against Rabies" Vaccines 7, no. 4: 132. https://doi.org/10.3390/vaccines7040132
APA StyleArmbruster, N., Jasny, E., & Petsch, B. (2019). Advances in RNA Vaccines for Preventive Indications: A Case Study of a Vaccine against Rabies. Vaccines, 7(4), 132. https://doi.org/10.3390/vaccines7040132



