Dog Ecology, Bite Incidence, and Disease Awareness: A Cross-Sectional Survey among a Rabies-Affected Community in the Democratic Republic of the Congo
Abstract
1. Introduction
2. Methods
2.1. Study Site
2.2. Data Collection
2.3. Sample Size Calculation and Household Sampling Procedure
2.4. Data Analysis
2.5. Ethical Approval
3. Results
3.1. Household and Respondent Characteristics
3.2. Dog Ownership and Dog-Keeping Practices
Factors Associated with Dog Ownership
3.3. Dog Population Characterisics
Dog Vaccination and Accessibility for Parenteral Vaccination
3.4. Animal Bite Incidents
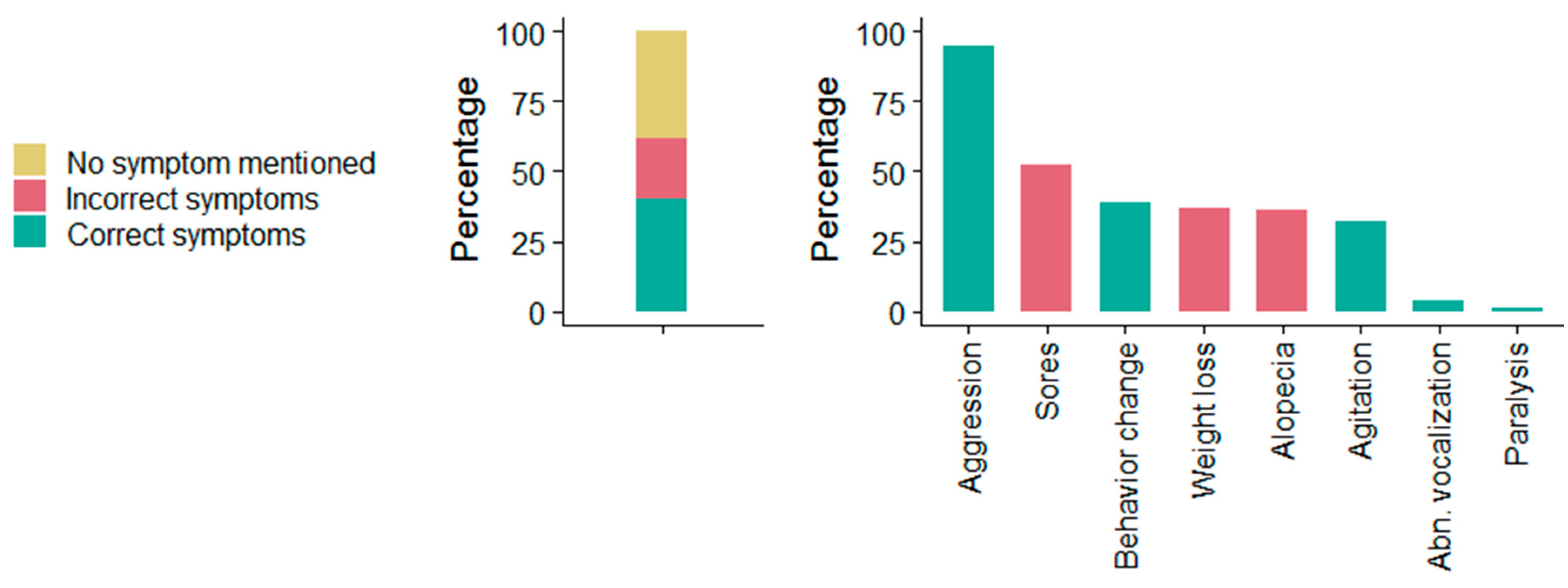
3.5. Community Rabies Knowledge
3.5.1. Factors Associated with Rabies Knowledge
3.5.2. Practices towards Suspected Rabid Animals
3.5.3. Health Seeking Behavior
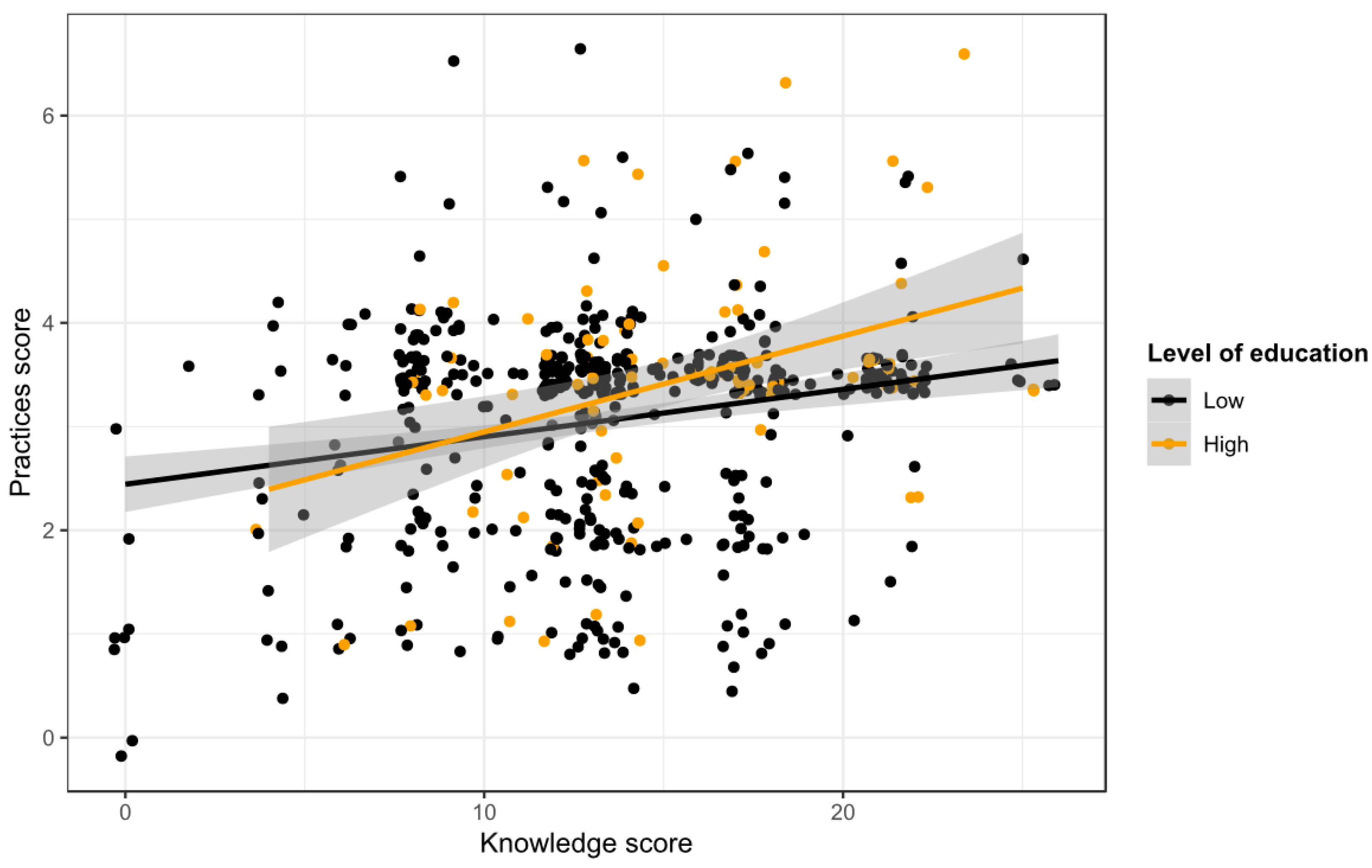
3.5.4. Relationship between Rabies Knowledge and Practices
4. Discussion
4.1. Dog Ownership and Dog-Keeping Practices
4.2. Dog Vaccination and Accessibility
4.3. Dog Population Characteristics
4.4. Animal Bite Incidents
4.5. Community Knowledge
4.6. Community Practices
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hampson, K.; Coudeville, L.; Lembo, T.; Sambo, M.; Kieffer, A.; Attlan, M.; Barrat, J.; Blanton, J.D.; Briggs, D.J.; Cleaveland, S.; et al. Estimating the global burden of endemic canine rabies. PLoS Negl. Trop. Dis. 2015, 9, e0003709. [Google Scholar] [CrossRef]
- World Health Organization; Food and Agriculture Organization of the United Nations; World Organisation for Animal Health; Global Alliance for Rabies Control. Zero by 30: The Global Strategic Plan to End Human Deaths from Dog-Mediated Rabies by 2030; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Coleman, P.G.; Dye, C. Immunization coverage required to prevent outbreaks of dog rabies. Vaccine 1996, 14, 185–186. [Google Scholar] [CrossRef]
- Hampson, K.; Dushoff, J.; Cleaveland, S.; Haydon, D.T.; Kaare, M.; Packer, C.; Dobson, A. Transmission dynamics and prospects for the elimination of canine rabies. PLoS Biol. 2009, 7, e53. [Google Scholar] [CrossRef] [PubMed]
- Zinsstag, J.; Lechenne, M.; Laager, M.; Mindekem, R.; Naissengar, S.; Oussiguere, A.; Bidjeh, K.; Rives, G.; Tessier, J.; Madjaninan, S.; et al. Vaccination of dogs in an African city interrupts rabies transmission and reduces human exposure. Sci. Transl. Med. 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Expert Consultation on Rabies: Third Report; WHO Technical Report Series, No. 1012; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Laager, M.; Mbilo, C.; Madaye, E.A.; Naminou, A.; Lechenne, M.; Tschopp, A.; Naissengar, S.K.; Smieszek, T.; Zinsstag, J.; Chitnis, N. The importance of dog population contact network structures in rabies transmission. PLoS Negl. Trop. Dis. 2018, 12, e0006680. [Google Scholar] [CrossRef] [PubMed]
- Davlin, S.L.; Vonville, H.M. Canine rabies vaccination and domestic dog population characteristics in the developing world: A systematic review. Vaccine 2012, 30, 3492–3502. [Google Scholar] [CrossRef] [PubMed]
- Obrist, B.; Iteba, N.; Lengeler, C.; Makemba, A.; Mshana, C.; Nathan, R.; Alba, S.; Dillip, A.; Hetzel, M.W.; Mayumana, I.; et al. Access to Health Care in Contexts of Livelihood Insecurity: A Framework for Analysis and Action. PLoS Med. 2007, 4, e308. [Google Scholar] [CrossRef]
- Muthiani, Y.; Traoré, A.; Mauti, S.; Zinsstag, J.; Hattendorf, J. Low coverage of central point vaccination against dog rabies in Bamako, Mali. Prev. Vet. Med. 2015, 120, 203–209. [Google Scholar] [CrossRef]
- Kaare, M.; Lembo, T.; Hampson, K.; Ernest, E.; Estes, A.; Mentzel, C.; Cleaveland, S. Rabies control in rural Africa: Evaluating strategies for effective domestic dog vaccination. Vaccine 2009, 27, 152–160. [Google Scholar] [CrossRef]
- Zinsstag, J.; Schelling, E.; Waltner-Toews, D.; Tanner, M. From “one medicine” to “one health” and systemic approaches to health and well-being. Prev. Vet. Med. 2011, 101, 148–156. [Google Scholar] [CrossRef]
- Mosimann, L.; Traore, A.; Mauti, S.; Lechenne, M.; Obrist, B.; Veron, R.; Hattendorf, J.; Zinsstag, J. A mixed methods approach to assess animal vaccination programmes: The case of rabies control in Bamako, Mali. Acta Trop. 2017, 165, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Lechenne, M.; Oussiguere, A.; Naissengar, K.; Mindekem, R.; Mosimann, L.; Rives, G.; Hattendorf, J.; Moto, D.D.; Alfaroukh, I.O.; Zinsstag, J.; et al. Operational performance and analysis of two rabies vaccination campaigns in N’Djamena, Chad. Vaccine 2016, 34, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Lembo, T.; Hampson, K.; Kaare, M.T.; Ernest, E.; Knobel, D.; Kazwala, R.R.; Haydon, D.T.; Cleaveland, S. The Feasibility of Canine Rabies Elimination in Africa: Dispelling Doubts with Data. PLoS Negl. Trop. Dis. 2010, 4, e626. [Google Scholar] [CrossRef] [PubMed]
- Mpolya, E.A.; Lembo, T.; Lushasi, K.; Mancy, R.; Mbunda, E.M.; Makungu, S.; Maziku, M.; Sikana, L.; Jaswant, G.; Townsend, S.; et al. Toward Elimination of Dog-Mediated Human Rabies: Experiences from Implementing a Large-scale Demonstration Project in Southern Tanzania. Front. Vet. Sci. 2017, 4, 21. [Google Scholar] [CrossRef] [PubMed]
- LeRoux, K.; Stewart, D.; Perrett, K.D.; Nel, L.H.; Kessels, J.A.; Abela-Ridder, B. Rabies control in KwaZulu-Natal, South Africa. Bull. World Health Organ. 2018, 96, 360–365. [Google Scholar] [CrossRef]
- Cleaveland, S.; Kaare, M.; Tiringa, P.; Mlengeya, T.; Barrat, J. A dog rabies vaccination campaign in rural Africa: Impact on the incidence of dog rabies and human dog-bite injuries. Vaccine 2003, 21, 1965–1973. [Google Scholar] [CrossRef]
- Cleaveland, S.; Fèvre, E.M.; Kaare, M.; Coleman, P.G. Estimating human rabies mortality in the United Republic of Tanzania from dog bite injuries. Bull. World Health Organ. 2002, 80, 304–310. [Google Scholar]
- Dhand, N.K.; Gyeltshen, T.; Firestone, S.; Zangmo, C.; Dema, C.; Gyeltshen, R.; Ward, M.P. Dog Bites in Humans and Estimating Human Rabies Mortality in Rabies Endemic Areas of Bhutan. PLoS Negl. Trop. Dis. 2011, 5, e1391. [Google Scholar] [CrossRef]
- Ly, S.; Buchy, P.; Heng, N.Y.; Ong, S.; Chhor, N.; Bourhy, H.; Vong, S. Rabies situation in Cambodia. PLoS Negl. Trop. Dis. 2009, 3, e511. [Google Scholar] [CrossRef]
- Taylor, L.H.; Hampson, K.; Fahrion, A.; Abela-Ridder, B.; Nel, L.H. Difficulties in estimating the human burden of canine rabies. Acta Trop. 2015. [Google Scholar] [CrossRef]
- Courtois, G.; Ninane, G.; Thys, A. On cases of rabies diagnosed in the laboratory at stanleyville from 1939 to 1958. Ann. Soc. Belg. Med. Trop. Parasitol. Mycol. 1964, 44, 405–414. [Google Scholar]
- Food and Agriculture Organization of the United Nations. FAO Taking Steps to Address Rabies Situation in DRC. Available online: http://www.fao.org/ag/againfo/home/en/news_archive/AGA_in_action/2012_FAO_address_rabies_situation_in_DRC.html (accessed on 22 July 2019).
- Muyila, D.I.; Aloni, M.N.; Lose-Ekanga, M.J.; Nzita, J.M.; Kalala-Mbikay, A.; Bongo, H.L.; Esako, M.N.; Malonga-Biapi, J.P.; Mputu-Dibwe, B.; Aloni, M.L.; et al. Human rabies: A descriptive observation of 21 children in Kinshasa, the Democratic Republic of Congo. Pathog. Glob. Health 2014, 108, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Global Alliance for Rabies Control. Rabies in the Democratic Republic of Congo. Available online: https://rabiesalliance.org/resource/rabies-democratic-republic-congo (accessed on 24 August 2019).
- Twabela, A.T.; Mweene, A.S.; Masumu, J.M.; Muma, J.B.; Lombe, B.P.; Hankanga, C. Overview of Animal Rabies in Kinshasa Province in the Democratic Republic of Congo. PLoS ONE 2016, 11, e0150403. [Google Scholar] [CrossRef] [PubMed]
- Kazadi, E.K.; Tshilenge, G.M.; Mbao, V.; Njoumemi, Z.; Masumu, J. Determinants of dog owner-charged rabies vaccination in Kinshasa, Democratic Republic of Congo. PLoS ONE 2017, 12, e0186677. [Google Scholar] [CrossRef] [PubMed]
- Durr, S.; Meltzer, M.I.; Mindekem, R.; Zinsstag, J. Owner valuation of rabies vaccination of dogs, Chad. Emerg. Infect. Dis. 2008, 14, 1650–1652. [Google Scholar] [CrossRef]
- Kazadi, E.K.; Marcotty, T.; Muylkens, B.; Antoine-Moussiaux, N.; van Gucht, S.; Mulumba, L.; Kirschvink, N. Factors of rabies maintenance in dog population in Kinshasa, Democratic Republic of Congo (DRC). Int. J. Infect. Dis. 2019, 79, 56. [Google Scholar] [CrossRef]
- Ministère du Plan et Suivi de la Mise en oeuvre de la Révolution de la Modernité (MPSMRM); Ministère de la Santé Publique (MSP); ICF International. Enquête Démographique et de Santé en République Démocratique du Congo 2013–2014; MPSMRM, MSP et ICF International: Rockville, MD, USA, 2014. [Google Scholar]
- World Health Organization; Veterinary Public Health Unit. Report of WHO Consultation on Dog Ecology Studies Related to Rabies Control. In Proceedings of the WHO Consultation on Dog Ecology Studies Related to Rabies Control, Geneva, Switzerland, 22–25 February 1988. [Google Scholar]
- World Health Organization; Veterinary Public Health Unit. Guide Pour la Gestion des Populations Canines; World Health Organization: Geneva, Switzerland, 1990. [Google Scholar]
- Schelling, E.; Hattendorf, J. One Healh Study Designs. In One Health: The Theory and Practice of Integrated Health Approaches, 1st ed.; Zinsstag, J., Schelling, E., Waltner-Toews, D., Whittaker, M., Tanner, M., Eds.; CAB International: Wallingford, UK, 2015. [Google Scholar]
- R Development Core Team R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2010.
- Mindekem, R.; Kayali, U.; Yemadji, N.; Ndoutamia, A.G.; Zinsstag, J. Impact of canine demography on rabies transmission in N’djamena, Chad. Med. Trop. Rev. Corps Sante Colon. 2005, 65, 53–58. [Google Scholar]
- Gsell, A.S.; Knobel, D.L.; Kazwala, R.R.; Vounatsou, P.; Zinsstag, J. Domestic dog demographic structure and dynamics relevant to rabies control planning in urban areas in Africa: The case of Iringa, Tanzania. BMC Vet. Res. 2012, 8, 236. [Google Scholar] [CrossRef]
- Mauti, S.; Traore, A.; Sery, A.; Bryssinckx, W.; Hattendorf, J.; Zinsstag, J. First study on domestic dog ecology, demographic structure and dynamics in Bamako, Mali. Prev. Vet. Med. 2017, 146, 44–51. [Google Scholar] [CrossRef]
- Mickey, R.M.; Greenland, S. The impact of confounder selection criteria on effect estimation. Am. J. Epidemiol. 1989, 129, 125–137. [Google Scholar] [CrossRef]
- World Health Organization; UNICEF. Progress on Sanitation and Drinking-Water. Available online: https://www.who.int/iris/bitstream/10665/81245/1/9789241505390_eng.pdf?ua=1 (accessed on 4 July 2019).
- Velasco-Villa, A.; Escobar, L.E.; Sanchez, A.; Shi, M.; Streicker, D.G.; Gallardo-Romero, N.F.; Vargas-Pino, F.; Gutierrez-Cedillo, V.; Damon, I.; Emerson, G. Successful strategies implemented towards the elimination of canine rabies in the Western Hemisphere. Antivir. Res. 2017, 143, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mindekem, R.; Lechenne, M.S.; Naissengar, K.S.; Oussiguere, A.; Kebkiba, B.; Moto, D.D.; Alfaroukh, I.O.; Ouedraogo, L.T.; Salifou, S.; Zinsstag, J. Cost Description and Comparative Cost Efficiency of Post-Exposure Prophylaxis and Canine Mass Vaccination against Rabies in N’Djamena, Chad. Front. Vet. Sci. 2017, 4, 38. [Google Scholar] [CrossRef] [PubMed]
- Knobel, D.L.; Laurenson, M.K.; Kazwala, R.R.; Boden, L.A.; Cleaveland, S. A cross-sectional study of factors associated with dog ownership in Tanzania. BMC Vet. Res. 2008, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- De Balogh, K.K.; Wandeler, A.I.; Meslin, F.X. A dog ecology study in an urban and a semi-rural area of Zambia. Onderstepoort J. Vet. Res. 1993, 60, 437–443. [Google Scholar] [PubMed]
- Conan, A.; Akerele, O.; Simpson, G.; Reininghaus, B.; van Rooyen, J.; Knobel, D. Population Dynamics of Owned, Free-Roaming Dogs: Implications for Rabies Control. PLoS Negl. Trop. Dis. 2015, 9, e0004177. [Google Scholar] [CrossRef] [PubMed]
- Tschopp, R.; Bekele, S.; Aseffa, A. Dog Demography, Animal Bite Management and Rabies Knowledge-Attitude and Practices in the Awash Basin, Eastern Ethiopia. PLoS Negl. Trop. Dis. 2016, 10, e0004471. [Google Scholar] [CrossRef] [PubMed]
- Ratsitorahina, M.; Rasambainarivo, J.H.; Raharimanana, S.; Rakotonandrasana, H.; Andriamiarisoa, M.P.; Rakalomanana, F.A.; Richard, V. Dog ecology and demography in Antananarivo, 2007. BMC Vet. Res. 2009, 5, 21. [Google Scholar] [CrossRef] [PubMed]
- Hambolu, S.E.; Dzikwi, A.A.; Kwaga, J.K.; Kazeem, H.M.; Umoh, J.U.; Hambolu, D.A. Dog ecology and population studies in Lagos State, Nigeria. Glob. J. Health Sci. 2014, 6, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Knobel, D.L.; Cleaveland, S.; Coleman, P.G.; Fevre, E.M.; Meltzer, M.I.; Miranda, M.E.; Shaw, A.; Zinsstag, J.; Meslin, F.X. Re-evaluating the burden of rabies in Africa and Asia. Bull. World Health Organ. 2005, 83, 360–368. [Google Scholar]
- Mshelbwala, P.P.; Akinwolemiwa, D.K.; Maikai, B.V.; Otolorin, R.G.; Maurice, N.A.; Weese, J.S. Dog ecology and its implications for rabies control in Gwagwalada, Federal Capital Territory, Abuja, Nigeria. Zoonoses Public Health 2018, 65, 168–176. [Google Scholar] [CrossRef]
- Sambo, M.; Hampson, K.; Changalucha, J.; Cleaveland, S.; Lembo, T.; Lushasi, K.; Mbunda, E.; Mtema, Z.; Sikana, L.; Johnson, P.C.D. Estimating the Size of Dog Populations in Tanzania to Inform Rabies Control. Vet. Sci. 2018, 5, 77. [Google Scholar] [CrossRef] [PubMed]
- Jibat, T.; Hogeveen, H.; Mourits, M.C. Review on dog rabies vaccination coverage in Africa: A question of dog accessibility or cost recovery? PLoS Negl. Trop. Dis. 2015, 9, e0003447. [Google Scholar] [CrossRef] [PubMed]
- Otolorin, G.R.; Umoh, J.U.; Dzikwi, A.A. Demographic and ecological survey of dog population in aba, abia state, Nigeria. ISRN Vet. Sci. 2014, 2014, 806849. [Google Scholar] [CrossRef] [PubMed]
- Bureau Provincial de la Production et Sante Animales (PSA). Rapport de la Journee Mondiale Contre la Rage; Inspection Provinciale de l’Agriculture, Peche et Elevage: Matadi, Democratic Republic of the Congo, 2017. [Google Scholar]
- The World Bank. The World Bank in DRC. Available online: https://www.worldbank.org/en/country/drc/overview (accessed on 16 July 2019).
- Hanemann, M.; Loomis, J.; Kanninen, B. Statistical Efficiency of Double-Bounded Dichotomous Choice Contingent Valuation. Am. J. Agric. Econ. 1991, 73, 1255–1263. [Google Scholar] [CrossRef]
- Anyiam, F.; Lechenne, M.; Mindekem, R.; Oussigere, A.; Naissengar, S.; Alfaroukh, I.O.; Mbilo, C.; Moto, D.D.; Coleman, P.G.; Probst-Hensch, N.; et al. Cost-estimate and proposal for a development impact bond for canine rabies elimination by mass vaccination in Chad. Acta Trop. 2016. [Google Scholar] [CrossRef] [PubMed]
- Morters, M.K.; McKinley, T.J.; Restif, O.; Conlan, A.J.; Cleaveland, S.; Hampson, K.; Whay, H.R.; Damriyasa, I.M.; Wood, J.L. The demography of free-roaming dog populations and applications to disease and population control. J. Appl. Ecol. 2014, 51, 1096–1106. [Google Scholar] [CrossRef]
- Brooks, R. Survey of the dog population of Zimbabwe and its level of rabies vaccination. Vet. Rec. 1990, 127, 592–596. [Google Scholar] [PubMed]
- Kitala, P.; McDermott, J.; Kyule, M.; Gathuma, J.; Perry, B.; Wandeler, A. Dog ecology and demography information to support the planning of rabies control in Machakos District, Kenya. Acta Trop. 2001, 78, 217–230. [Google Scholar] [CrossRef]
- Czupryna, A.M.; Brown, J.S.; Bigambo, M.A.; Whelan, C.J.; Mehta, S.D.; Santymire, R.M.; Lankester, F.J.; Faust, L.J. Ecology and Demography of Free-Roaming Domestic Dogs in Rural Villages near Serengeti National Park in Tanzania. PLoS ONE 2016, 11, e0167092. [Google Scholar] [CrossRef]
- Fevre, E.M.; Kaboyo, R.W.; Persson, V.; Edelsten, M.; Coleman, P.G.; Cleaveland, S. The epidemiology of animal bite injuries in Uganda and projections of the burden of rabies. Trop. Med. Int. Health TM IH 2005, 10, 790–798. [Google Scholar] [CrossRef]
- Salomão, C.; Nacima, A.; Cuamba, L.; Gujral, L.; Amiel, O.; Baltazar, C.; Cliff, J.; Gudo, E.S. Epidemiology, clinical features and risk factors for human rabies and animal bites during an outbreak of rabies in Maputo and Matola cities, Mozambique, 2014: Implications for public health interventions for rabies control. PLoS Negl. Trop. Dis. 2017, 11, e0005787. [Google Scholar] [CrossRef] [PubMed]
- Mustiana, A.; Toribio, J.A.; Abdurrahman, M.; Suadnya, I.W.; Hernandez-Jover, M.; Putra, A.A.; Ward, M.P. Owned and unowned dog population estimation, dog management and dog bites to inform rabies prevention and response on Lombok Island, Indonesia. PLoS ONE 2015, 10, e0124092. [Google Scholar] [CrossRef] [PubMed]
- Frey, J.; Mindekem, R.; Kessely, H.; Doumagoum Moto, D.; Naissengar, S.; Zinsstag, J.; Schelling, E. Survey of animal bite injuries and their management for an estimate of human rabies deaths in N’Djamena, Chad. Trop. Med. Int. Health 2013, 18, 1555–1562. [Google Scholar] [CrossRef] [PubMed]
- Sudarshan, M.K.; Mahendra, B.J.; Madhusudana, S.N.; Ashwath Narayana, D.H.; Rahman, A.; Rao, N.S.N.; Meslin, F.X.; Lobo, D.; Ravikumar, K.; Gangaboraiah. An epidemiological study of animal bites in India: Results of a who sponsored national multi-centric rabies survey. J. Commun. Dis. 2006, 38, 32–39. [Google Scholar] [PubMed]
- Overall, K.L.; Love, M. Dog bites to humans—Demography, epidemiology, injury, and risk. J. Am. Vet. Med. Assoc. 2001, 218, 1923–1934. [Google Scholar] [CrossRef]
- Rosado, B.; Garcia-Belenguer, S.; Leon, M.; Palacio, J. A comprehensive study of dog bites in Spain, 1995–2004. Vet. J. 2009, 179, 383–391. [Google Scholar] [CrossRef]
- Westgarth, C.; Brooke, M.; Christley, R.M. How many people have been bitten by dogs? A cross-sectional survey of prevalence, incidence and factors associated with dog bites in a UK community. J. Epidemiol. Community Health 2018, 72, 331–336. [Google Scholar] [CrossRef]
- Esmaeilzadeh, F.; Rajabi, A.; Vahedi, S.; Shamsadiny, M.; Ghelichi Ghojogh, M.; Hatam, N. Epidemiology of Animal Bites and Factors Associated with Delays in Initiating Post-exposure Prophylaxis for Rabies Prevention among Animal Bite Cases: A Population-based Study. J. Prev. Med. Public Health 2017, 50, 210–216. [Google Scholar] [CrossRef]
- World Health Organization. Rabies Fact Sheet. Available online: https://www.who.int/news-room/fact-sheets/detail/rabies (accessed on 16 July 2019).
- Shen, J.; Rouse, J.; Godbole, M.; Wells, H.L.; Boppana, S.; Schwebel, D.C. Systematic Review: Interventions to Educate Children about Dog Safety and Prevent Pediatric Dog-Bite Injuries: A Meta-Analytic Review. J. Pediatr. Psychol. 2017, 42, 779–791. [Google Scholar] [CrossRef]
- Duperrex, O.; Blackhall, K.; Burri, M.; Jeannot, E. Education of children and adolescents for the prevention of dog bite injuries. Cochrane Database Syst. Rev. 2009. [Google Scholar] [CrossRef]
- Auplish, A.; Clarke, A.S.; Van Zanten, T.; Abel, K.; Tham, C.; Bhutia, T.N.; Wilks, C.R.; Stevenson, M.A.; Firestone, S.M. Estimating the intra-cluster correlation coefficient for evaluating an educational intervention program to improve rabies awareness and dog bite prevention among children in Sikkim, India: A pilot study. Acta Trop. 2017, 169, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Burdon Bailey, J.L.; Gamble, L.; Gibson, A.D.; Bronsvoort, B.M.D.; Handel, I.G.; Mellanby, R.J.; Mazeri, S. A rabies lesson improves rabies knowledge amongst primary school children in Zomba, Malawi. PLoS Negl. Trop. Dis. 2018, 12, e0006293. [Google Scholar] [CrossRef] [PubMed]
- Deray, R.; Rivera, C.; Gripon, S.; Ulanday, C.; Roces, M.C.; Amparo, A.C.; Attlan, M.; Demont, C.; Kieffer, A.; Miranda, M.E. Protecting children from rabies with education and pre-exposure prophylaxis: A school-based campaign in El Nido, Palawan, Philippines. PLoS ONE 2018, 13, e0189596. [Google Scholar] [CrossRef] [PubMed]
- Keita, Z.G.F.; Léchenne, M.; Thiero, O.; Hattendorf, J.; Zinsstag, J.; Traoré, A.; Traoré, A.K. Burden of Rabies in Mali. 2019; submitted to Acta Trop. [Google Scholar]
- Kallo, V. Estimation of Dog Population and Exposure to Bites in Côte D’Ivoire. 2019; submitted to Acta Trop. [Google Scholar]
- Rumana, R.; Sayeed, A.A.; Basher, A.; Islam, Z.; Rahman, M.R.; Faiz, M.A. Perceptions and treatment seeking behavior for dog bites in rural Bangladesh. Southeast Asian J. Trop. Med. Public Health 2013, 44, 244–248. [Google Scholar] [PubMed]
- Ponsich, A.; Goutard, F.; Sorn, S.; Tarantola, A. A prospective study on the incidence of dog bites and management in a rural Cambodian, rabies-endemic setting. Acta Trop. 2016, 160, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Agarwal, A.; Khan, A.M.; Ingle, G.K. Prevalence of Dog Bites in Rural and Urban Slums of Delhi: A Community-based Study. Ann. Med. Health Sci. Res. 2016, 6, 115–119. [Google Scholar] [PubMed]
- Murugan, V.; Amol, D.; Kalaiselvan, G. A Community based cross sectional study of dog bites in children in a rural district of Tamil Nadu. Int. J. Med. Sci. Public Health 2016, 6, 109–112. [Google Scholar]
- Digafe, R.T.; Kifelew, L.G.; Mechesso, A.F. Knowledge, attitudes and practices towards rabies: Questionnaire survey in rural household heads of Gondar Zuria District, Ethiopia. BMC Res. Notes 2015, 8, 400. [Google Scholar] [CrossRef]
- Hergert, M.; Le Roux, K.; Nel, L.H. Risk factors associated with nonvaccination rabies status of dogs in KwaZulu-Natal, South Africa. Vet. Med.-Res. Rep. 2016, 7, 75–83. [Google Scholar] [CrossRef]
- Tiembre, I.; Vroh Benie Bi, J.; Kouassi, P.; Attoh-Toure, H.; Ekra, K.D.; Diane, A.; Dagnan, N.S.; Tagliante-Saracino, J. Knowledge, attitudes and practices of household heads regarding rabies in the Abobo district (Abidjan, Cote d’Ivoire) in 2008. Sante Publique 2014, 26, 547–553. [Google Scholar]
- Mbilo, C.; Lechenne, M.; Hattendorf, J.; Madjadinan, S.; Anyiam, F.; Zinsstag, J. Rabies awareness and dog ownership among rural northern and southern Chadian communities-Analysis of a community-based, cross-sectional household survey. Acta Trop. 2017, 175, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Mallewa, M.; Fooks, A.R.; Banda, D.; Chikungwa, P.; Mankhambo, L.; Molyneux, E.; Molyneux, M.E.; Solomon, T. Rabies encephalitis in malaria-endemic area, Malawi, Africa. Emerg. Infect. Dis. 2007, 13, 136–139. [Google Scholar] [CrossRef] [PubMed]
- Barbosa Costa, G.; Gilbert, A.; Monroe, B.; Blanton, J.; Ngam Ngam, S.; Recuenco, S.; Wallace, R. The influence of poverty and rabies knowledge on healthcare seeking behaviors and dog ownership, Cameroon. PLoS ONE 2018, 13, e0197330. [Google Scholar] [CrossRef] [PubMed]
- Sambo, M.; Lembo, T.; Cleaveland, S.; Ferguson, H.M.; Sikana, L.; Simon, C.; Urassa, H.; Hampson, K. Knowledge, attitudes and practices (KAP) about rabies prevention and control: A community survey in Tanzania. PLoS Negl. Trop. Dis. 2014, 8, e3310. [Google Scholar] [CrossRef] [PubMed]
- Hergert, M.; Nel, L.H. Dog Bite Histories and Response to Incidents in Canine Rabies-Enzootic KwaZulu-Natal, South Africa. PLoS Negl. Trop. Dis. 2013, 7, e2059. [Google Scholar] [CrossRef]
- World Health Organization. Rabies vaccines: WHO position paper-Recommendations. Vaccine 2010, 28, 7140–7142. [Google Scholar]
- Jemberu, W.T.; Molla, W.; Almaw, G.; Alemu, S. Incidence of rabies in humans and domestic animals and people’s awareness in North Gondar Zone, Ethiopia. PLoS Negl. Trop. Dis. 2013, 7, e2216. [Google Scholar] [CrossRef] [PubMed]
- Kenu, E.; Ganu, V.; Noora, C.L.; Adanu, R.; Lartey, M. Management of dog bites by frontline service providers in primary healthcare facilities in the Greater Accra Region of Ghana, 2014–2015. Infect. Dis. Poverty 2018, 7, 18. [Google Scholar] [CrossRef]

| Variable | Overall (n = 1056) |
|---|---|
| Sex of respondent | |
| Female | 669 (63.4%) |
| Male | 387 (36.6%) |
| Age of respondent (years) | |
| 16-29 | 259 (24.5%) |
| 30-39 | 281 (26.6%) |
| >40 | 516 (48.9%) |
| Level of education of respondent | |
| None | 23 (2.2%) |
| Primary | 85 (8.0%) |
| Secondary | 818 (77.5%) |
| Tertiary | 130 (12.3%) |
| Socioeconomic status | |
| Middle | 920 (87.1%) |
| Low | 136 (12.9%) |
| Position of respondent in the household | |
| Wife of head of household | 464 (43.9%) |
| Head of household | 317 (30.0%) |
| Child of head of household | 211 (20.0%) |
| Other relative of head of household | 64 (6.1%) |
| Occupation of respondent | |
| Private sector | 603 (57.1%) |
| Public sector | 316 (29.9%) |
| Unemployed | 101 (9.6%) |
| Retired | 36 (3.4%) |
| Dwelling ownership status | |
| Owner | 629 (59.6%) |
| Tenant | 427 (40.4%) |
| Source of water | |
| Improved | 893 (84.6%) |
| Unimproved | 163 (15.4%) |
| Waste disposal | |
| Open | 707 (67.0%) |
| Closed | 349 (33.0%) |
| Livestock ownership | |
| No | 769 (72.8%) |
| Yes | 287 (27.2%) |
| Residence | |
| Peri-urban | 557 (52.7%) |
| Urban | 499 (47.3%) |
| History of bite incident | |
| No | 964 (91.3%) |
| Yes | 92 (8.7%) |
| Dog ownership | |
| No | 956 (90.5%) |
| Yes | 100 (9.5%) |
| Variables | % (Npos) | OR | 95% CI | p-value | Adj OR | 95% CI | p-value | |
|---|---|---|---|---|---|---|---|---|
| Socioeconomic status | Middle | 10% (90/920) | reference | |||||
| Low | 7% (10/136) | 0.73 | 0.37–1.44 | 0.366 | ||||
| Dwelling ownership status | Tenant | 5% (21/427) | reference | reference | ||||
| Owner | 13% (79/629) | 2.78 | 1.65–4.68 | <0.001 | 2.37 | 1.36–4.15 | 0.002 | |
| Livestock ownership | No | 7% (55/769) | reference | reference | ||||
| Yes | 16% (45/287) | 2.41 | 1.53–3.8 | <0.001 | 2.01 | 1.25–3.23 | 0.004 | |
| Residence | Peri-urban | 10% (56/557) | reference | |||||
| Urban | 9% (44/499) | 0.87 | 0.54–1.39 | 0.55 |
| Variable | % (Npos) | OR | 95% CI | p-value | Adj OR | 95% CI | p-value | |
|---|---|---|---|---|---|---|---|---|
| Sex | Female | 29% (86/299) | reference | reference | ||||
| Male | 41% (103/252) | 1.71 | 1.25–2.34 | 0.001 | 1.44 | 1.02-2.03 | 0.038 | |
| Age | 16-29 | 24% (28/118) | reference | reference | ||||
| 30-39 | 31% (39/128) | 1.41 | 0.78–2.53 | 0.252 | 1.63 | 0.85–3.11 | 0.14 | |
| >40 | 40% (122/305) | 2.14 | 1.28–3.58 | 0.004 | 2.21 | 1.29–3.78 | 0.004 | |
| Level of education | Low | 32% (149/466) | reference | reference | ||||
| High | 47% (40/85) | 1.89 | 1.19–3.01 | 0.007 | 1.87 | 1.22–2.87 | 0.004 | |
| Socioeconomic status | Middle | 36% (176/493) | reference | |||||
| Low | 22% (13/58) | 0.52 | 0.24–1.15 | 0.107 | 0.49 | 0.22–1.1 | 0.084 | |
| Livestock ownership | No | 32% (126/392) | reference | reference | ||||
| Yes | 40% (63/159) | 1.39 | 0.87–2.21 | 0.17 | 1.14 | 0.71–1.83 | 0.599 | |
| Residence | Peri-urban | 41% (126/311) | reference | reference | ||||
| Urban | 26% (63/240) | 0.52 | 0.29–0.95 | 0.033 | 0.5 | 0.28–0.91 | 0.024 | |
| History of bite incident | No | 34% (170/494) | reference | reference | ||||
| Yes | 33% (19/57) | 0.95 | 0.53–1.73 | 0.874 | ||||
| Dog ownership | No | 35% (173/491) | reference | reference | ||||
| Yes | 27% (16/60) | 0.67 | 0.39–1.14 | 0.14 | 0.59 | 0.34–1.02 | 0.06 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mbilo, C.; Kabongo, J.-B.; Pyana, P.P.; Nlonda, L.; Nzita, R.W.; Luntadila, B.; Badibanga, B.; Hattendorf, J.; Zinsstag, J. Dog Ecology, Bite Incidence, and Disease Awareness: A Cross-Sectional Survey among a Rabies-Affected Community in the Democratic Republic of the Congo. Vaccines 2019, 7, 98. https://doi.org/10.3390/vaccines7030098
Mbilo C, Kabongo J-B, Pyana PP, Nlonda L, Nzita RW, Luntadila B, Badibanga B, Hattendorf J, Zinsstag J. Dog Ecology, Bite Incidence, and Disease Awareness: A Cross-Sectional Survey among a Rabies-Affected Community in the Democratic Republic of the Congo. Vaccines. 2019; 7(3):98. https://doi.org/10.3390/vaccines7030098
Chicago/Turabian StyleMbilo, Céline, Jean-Baptiste Kabongo, Pati Patient Pyana, Léon Nlonda, Raymond Williams Nzita, Bobo Luntadila, Badivé Badibanga, Jan Hattendorf, and Jakob Zinsstag. 2019. "Dog Ecology, Bite Incidence, and Disease Awareness: A Cross-Sectional Survey among a Rabies-Affected Community in the Democratic Republic of the Congo" Vaccines 7, no. 3: 98. https://doi.org/10.3390/vaccines7030098
APA StyleMbilo, C., Kabongo, J.-B., Pyana, P. P., Nlonda, L., Nzita, R. W., Luntadila, B., Badibanga, B., Hattendorf, J., & Zinsstag, J. (2019). Dog Ecology, Bite Incidence, and Disease Awareness: A Cross-Sectional Survey among a Rabies-Affected Community in the Democratic Republic of the Congo. Vaccines, 7(3), 98. https://doi.org/10.3390/vaccines7030098




