The Increase of the Magnitude of Spontaneous Viral Blips in Some Participants of Phase II Clinical Trial of Therapeutic Optimized HIV DNA Vaccine Candidate
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Vaccine
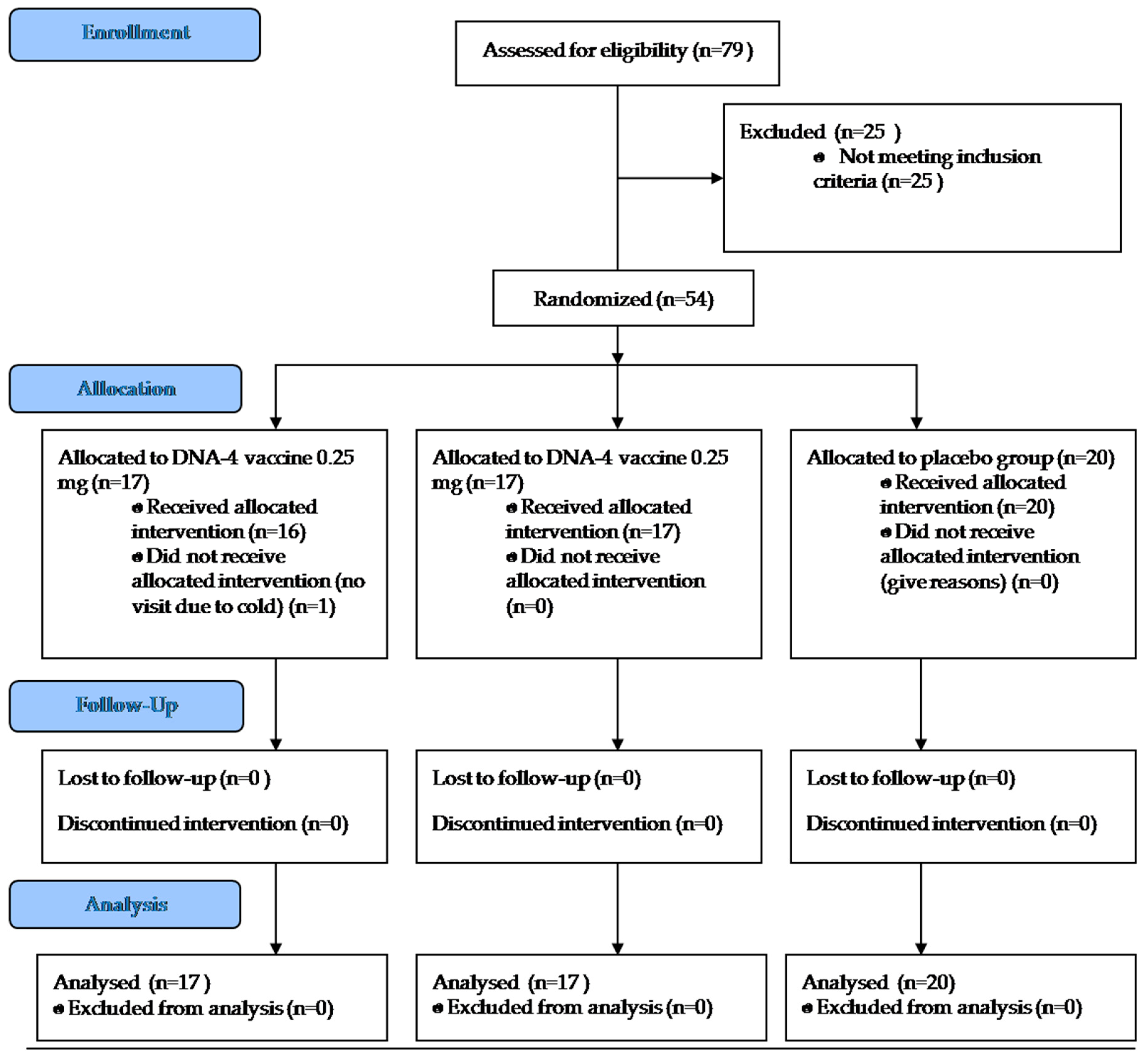
2.2. Phase II Clinical Trial Design
2.3. Ethical Compliance
3. Results
3.1. Adherence and Tolerability
3.2. Viral Load Dynamics
3.3. CD4 and CD8 T Cells Measurement
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
- Inclusion criteria:
- Informed consent to participate in the study;
- HIV positive men and women over 18 years old receiving stable first-line ART for at least 6 months and not more than 2 years;
- Stable clinical course of HIV infection (clinical stage 1 or 2 according to WHO classification);
- HIV viral load less than 50 copies/mL at screening;
- Number of CD4 T cells more than 250 cells/mm3 at screening;
- blood parameters: leukocytes—≥2900/mm3 (2.9 × 109 cells/L), absolute neutrophil count—≥1500/mm3 (1.5 × 109 cells/L), platelets—≥100,000/mm3 (100 × 109 cells/L), hemoglobin—≥9.0 g/dL, bilirubin—≤1.5 × upper limit of normal, ALT and AST—≤2.5 × upper limit of normal;
- Glomerular filtration rate (GFR)—>60 mL/min.
- Consent to use adequate contraceptive methods throughout the study (condom with spermicide).
- Exclusion criteria:
- Acute hepatitis or cirrhosis of any etiology; anti-HCV or HBsAg, at screening;
- Opportunistic infections that meet the Category C classification of the Centers for Disease Control and Prevention (CDC) of 2008, with the exception of Kaposi’s sarcoma that does not require systemic therapy;
- Tuberculosis;
- Malignant neoplasms;
- Participation in other clinical studies within 3 months before screening;
- Reception of immunomodulators (interferons, interleukins), immunosuppressive (cyclosporine), glucocorticosteroids within 3 months before screening;
- Any vaccination within 6 months before screening;
- Significant alcohol or drug addiction;
- Hypersensitivity to any component of the study vaccine;
- Severe concomitant diseases, such as disorders of the nervous, respiratory, cardiovascular, renal, hepatic, endocrine system and the gastrointestinal tract;
- Systemic autoimmune diseases or connective tissue diseases requiring treatment with systemic glucocorticosteroids, cytostatics or penicillamine;
- Pregnancy or breastfeeding. Women planning a pregnancy during a clinical trial; women who do not use adequate methods of contraception;
- The inability to read or write, failure to understand and follow research protocol procedures.
Appendix B
| Group | Participant Number | Visit | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Screening | 2 | 6 | 7 | 8 | 9 | 10 | 11 | ||
| Placebo | 01 | 20 | 440 | 20 | 39 | 20 | 20 | 80 | 20 |
| 02 | 20 | 20 | 565 | 20 | 71 | 39 | 20 | 20 | |
| 03 | 20 | 200 | 39 | 20 | 20 | 39 | 43 | 20 | |
| 04 | 20 | 55 | 20 | 47 | 52 | 20 | 20 | 20 | |
| 05 | 40 | 57 | 20 | 20 | 20 | 20 | 39 | 20 | |
| 06 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | |
| 07 | 20 | 39 | 20 | 39 | n/a | n/a | n/a | n/a | |
| 08 | 42 | 232 | 95 | 20 | n/a | n/a | n/a | n/a | |
| 09 | 20 | 20 | 39 | 20 | n/a | n/a | n/a | n/a | |
| 10 | 20 | 39 | 20 | 20 | 20 | 20 | 20 | 20 | |
| 11 | 20 | 20 | 20 | 20 | 80 | 20 | n/a | n/a | |
| 12 | 40 | 39 | 20 | 20 | 20 | 20 | n/a | n/a | |
| 13 | 40 | 20 | 39 | 20 | 20 | n/a | n/a | n/a | |
| 14 | 40 | 20 | 20 | 20 | n/a | 20 | 20 | n/a | |
| 15 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | |
| 16 | 40 | 39 | 39 | 20 | 20 | 39 | 39 | 42 | |
| 17 | 20 | 39 | 20 | 41 | 20 | 20 | 20 | 20 | |
| 18 | 40 | 39 | 39 | 39 | 50 | 20 | 20 | 20 | |
| 19 | 20 | 20 | 20 | 20 | 20 | 20 | 52 | n/a | |
| 20 | 20 | 20 | 20 | n/a | n/a | 20 | n/a | 20 | |
| N | 20 | 20 | 20 | 19 | 15 | 16 | 13 | 12 | |
| Median | 20 | 39 | 20 | 20 | 20 | 20 | 20 | 20 | |
| SD | 9.9 | 104.8 | 121.2 | 9.6 | 20.9 | 7.7 | 18.4 | 6.4 | |
| Min | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | |
| Max | 42 | 440 | 565 | 47 | 80 | 39 | 80 | 42 | |
| >50 | 0 | 5 | 2 | 0 | 3 | 0 | 2 | 0 | |
| 0.25 mg | 21 | 20 | 39 | 101 | 59 | 129 | 2800 | 39 | 20 |
| 22 | 20 | 20 | 20 | 20 | 39 | 20 | 20 | 20 | |
| 23 | 20 | 20 | 20 | 20 | 20 | 50 | 20 | 20 | |
| 24 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | |
| 25 | 20 | 46 | 20 | 20 | 20 | 20 | 20 | 20 | |
| 26 | 20 | 20 | 20 | 39 | n/a | 59 | 80 | 39 | |
| 27 | 20 | 20 | 39 | 20 | 20 | 20 | 57 | n/a | |
| 28 | 20 | 39 | 39 | 39 | 20 | n/a | n/a | n/a | |
| 29 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | |
| 30 | 20 | 49 | 39 | 20 | 20 | 20 | 20 | 20 | |
| 31 | 20 | 20 | n/a | 20 | 20 | 20 | 20 | 20 | |
| 32 | 20 | 20 | 20 | 20 | 20 | n/a | n/a | n/a | |
| 33 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | |
| 34 | 20 | 20 | 20 | n/a | 20 | 40 | 20 | 20 | |
| 35 | 20 | 20 | 20 | 20 | 39 | 39 | 20 | 20 | |
| 36 | 40 | 39 | 39 | 20 | 20 | 20 | 20 | 20 | |
| 37 | 20 | 20 | 20 | 18,000 | n/a | n/a | n/a | n/a | |
| N | 17 | 17 | 16 | 16 | 15 | 14 | 14 | 13 | |
| Median | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | |
| SD | 4.9 | 10.8 | 20.8 | 4493.7 | 28.2 | 740.9 | 18.3 | 5.3 | |
| Min | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | |
| Max | 40 | 49 | 101 | 18000 | 129 | 2800 | 80 | 39 | |
| >50 | 0 | 0 | 1 | 2 | 1 | 2 | 2 | 0 | |
| 0.5 mg | 38 | 20 | 39 | 62 | 20 | 20 | 20 | 20 | 20 |
| 39 | 20 | 20 | 39 | 20 | 20 | 20 | 20 | 20 | |
| 40 | 20 | 42 | 44 | 49 | 61 | 20 | 20 | 20 | |
| 41 | 20 | 39 | 20 | 20 | 20 | 20 | 20 | 20 | |
| 42 | 40 | 20 | 20 | 20 | 71 | 20 | 20 | 20 | |
| 43 | 20 | 20 | 20 | 20 | 709 | 20 | 20 | n/a | |
| 44 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | n/a | |
| 45 | 40 | 20 | 39 | 20 | 20 | 20 | n/a | n/a | |
| 46 | 20 | 20 | 39 | 20 | n/a | n/a | n/a | n/a | |
| 47 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | |
| 48 | 40 | 39 | 58 | 20 | 20 | 20 | 39 | 20 | |
| 49 | 20 | 20 | 20 | 39 | n/a | n/a | n/a | n/a | |
| 50 | 40 | 39 | 40 | 39 | 20 | 20 | 39 | 20 | |
| 51 | 40 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | |
| 52 | 40 | 39 | 39 | 57 | 39 | 39 | 20 | n/a | |
| 53 | 40 | 39 | 20 | 46 | 20 | 20 | 20 | 39 | |
| 54 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | |
| N | 17 | 17 | 17 | 17 | 15 | 15 | 14 | 11 | |
| Median | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | |
| SD | 10.1 | 9.9 | 14.3 | 12.8 | 176.6 | 4.9 | 6.9 | 5.7 | |
| Min | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | |
| Max | 40 | 42 | 62 | 57 | 709 | 39 | 39 | 39 | |
| >50 | 0 | 0 | 2 | 1 | 3 | 0 | 0 | 0 | |
References
- Federal Service for Surveillance on Consumer Rights Protection and Human Wellbeing. Available online: https://www.rospotrebnadzor.ru/en/ (accessed on 29 March 2019).
- Murashev, B.; Kazennova, E.; Kozlov, A.; Murasheva, I.; Dukhovlinova, E.; Galachyants, Y.; Dorofeeva, E.; Dukhovlinov, I.; Smirnova, G.; Masharsky, A.; et al. Immunogenicity of candidate DNA vaccine based on subtype A of human immunodeficiency virus type 1 predominant in Russia. Biotechnol. J. 2007, 2, 871–888. [Google Scholar] [CrossRef]
- Akulova, E.; Murashev, B.; Nazarenko, O.; Verevochkin, S.; Masharsky, A.; Krasnoselskih, T.; Lioznov, D.; Sokolovsky, E.; Kozlov, A.P. Immune Responses Induced by Candidate Optimized HIV DNA Vaccine in Phase I Clinical Trial. Madridge J. Vaccines 2017, 1, 34–43. [Google Scholar] [CrossRef]
- Murashev, B.V.; Nazarenko, O.V.; Akulova, E.B.; Artemyeva, A.K.; Verevochkin, S.V.; Shaboltas, A.V.; Skochilov, R.V.; Toussova, O.V.; Kozlov, A.P. The high frequency of HIV type 1-specific cellular immune responses in seronegative individuals with parenteral and/or heterosexual HIV type 1 exposure. AIDS Res. Hum. Retroviruses 2012, 28, 1598–1605. [Google Scholar] [CrossRef] [PubMed]
- Seddiki, N.; Levy, Y. Therapeutic HIV-1 vaccine: Time for immunomodulation and combinatorial strategies. Curr. Opin. HIV AIDS 2018, 13, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Mothe, B. Viral control induced by HIVconsv vaccines & romidepsin in early treated individuals. In Proceedings of the CROI 2017, Seattle, WA, USA, 13–16 February 2017. [Google Scholar]
- Hutter, G.; Nowak, D.; Mossner, M.; Ganepola, S.; Müssig, A.; Allers, K.; Schneider, T.; Hofmann, J.; Kücherer, C.; Blau, O.; et al. Long-Term Control of HIV by CCR5 Delta32/Delta32 Stem-Cell Transplantation. N. Engl. J. Med. 2009, 360, 692–698. [Google Scholar] [CrossRef]
- Allers, K.; Hütter, G.; Hofmann, J.; Loddenkemper, C.; Rieger, K.; Thiel, E.; Schneider, T. Evidence for the cure of HIV infection by CCR5Δ32/Δ32 stem cell transplantation. Blood 2011, 117, 2791–2799. [Google Scholar] [CrossRef]
- Gupta, R.K.; Abdul-Jawad, S.; McCoy, L.E.; Mok, H.P.; Peppa, D.; Salgado, M.; Martinez-Picado, J.; Nijhuis, M.; Wensing, A.M.J.; Lee, H.; et al. HIV-1 remission following CCR5Δ32/Δ32 haematopoietic stem-cell transplantation. Nature 2019, 568, 244–248. [Google Scholar] [CrossRef]
- Vandergeeten, C.; Fromentin, R.; Chomont, N. The role of cytokines in the establishment, persistence and eradication of the HIV reservoir. Cytokine Growth Factor Rev. 2012, 23, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Folks, T.M.; Clouse, K.A.; Justement, J.; Rabson, A.; Duh, E.; Kehrl, J.H.; Fauci, A.S. Tumor necrosis factor alpha induces expression of human immunodeficiency virus in a chronically infected T-cell clone. Proc. Natl. Acad. Sci. USA 1989, 86, 2365–2368. [Google Scholar] [CrossRef] [PubMed]
- Katlama, C.; Deeks, S.G.; Autran, B.; Martinez-Picado, J.; van Lunzen, J.; Rouzioux, C.; Miller, M.; Vella, S.; Schmitz, J.E.; Ahlers, J.; et al. Barriers to a cure for HIV: New ways to target and eradicate HIV-1 reservoirs. Lancet 2013, 381, 2109–2117. [Google Scholar] [CrossRef]
- Li, J.Z.; Brumme, C.J.; Lederman, M.M.; Brumme, Z.L.; Wang, H.; Spritzler, J.; Carrington, M.; Medvik, K.; Walker, B.D.; Schooley, R.T.; et al. Characteristics and outcomes of initial virologic suppressors during analytic treatment interruption in a therapeutic HIV-1 gag vaccine trial. PLoS ONE 2012, 7, e34134. [Google Scholar] [CrossRef] [PubMed]
- Lisziewicz, J.; Bakare, N.; Calarota, S.A.; Bánhegyi, D.; Szlávik, J.; Ujhelyi, E.; Tőke, E.R.; Molnár, L.; Lisziewicz, Z.; Autran, B.; et al. Single DermaVirimmunization: Dose-dependent expansion of precursor/memory T cells against all HIV antigens in HIV-1 infected individuals. PLoS ONE 2012, 7, e35416. [Google Scholar] [CrossRef] [PubMed]
- García, F.; Climent, N.; Assoumou, L.; Gil, C.; González, N.; Alcamí, J.; León, A.; Romeu, J.; Dalmau, J.; Martínez-Picado, J.; et al. A therapeutic dendritic cell-based vaccine for HIV-1 infection. J. Infect. Dis. 2011, 203, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Achenbach, C.J.; Assoumou, L.; Deeks, S.G.; Wilkin, T.J.; Berzins, B.; Casazza, J.P.; Lambert-Niclot, S.; Koup, R.A.; Costagliola, D.; Calvez, V.; et al. Effect of therapeutic intensification followed by HIV DNA prime and rAd5 boost vaccination on HIV-specific immunity and HIV reservoir (EraMune02): A multicentre randomised clinical trial. Lancet HIV 2015, 2, e82–e91. [Google Scholar] [CrossRef]
- Barouch, D.H.; Deeks, S.G. Immunologic strategies for HIV-1 remission and eradication. Science 2014, 345, 169–174. [Google Scholar] [CrossRef]
- Van Lint, C.; Bouchat, S.; Marcello, A. HIV-1 transcription and latency: An update. Retrovirology 2013, 10, 67. [Google Scholar] [CrossRef]
- Cohen, J. Tests identify HIV’s final redoubt. Science 2019, 363, 1260–1261. [Google Scholar] [CrossRef]
- Kuang, X.T.; Brockman, M.A. Implications of HIV-1 Nef for “Shock and Kill” Strategies to Eliminate Latent Viral Reservoirs. Viruses 2018, 10, 677. [Google Scholar] [CrossRef]

| Group | Placebo | 0.25 mg | 0.5 mg | Total |
|---|---|---|---|---|
| Number of participants randomized | 20 | 17 | 17 | 54 |
| Number of men | 9 | 5 | 9 | 23 |
| Number of women | 11 | 12 | 8 | 31 |
| Average age | 33.6 ± 6.2 | 36.9 ± 9.4 | 37.1 ± 8.5 | 35.7 ± 8.0 |
| Adverse Event | DNA-4 0.25 mg | DNA-4 0.5 mg | Placebo | |||
|---|---|---|---|---|---|---|
| Number | 17 | 17 | 20 | |||
| N | % | N | % | N | % | |
| Fever | 1 | 5.9 | 0 | 0.0 | 2 | 10.0 |
| Feeling of acid in the mouth | 0 | 0.0 | 0 | 0.0 | 1 | 5.0 |
| Toothache | 0 | 0.0 | 0 | 0.0 | 1 | 5.0 |
| Weakness | 0 | 0.0 | 0 | 0.0 | 1 | 5.0 |
| Left arm pain | 0 | 0.0 | 0 | 0.0 | 1 | 5.0 |
| Itching at the injection site | 1 | 5.9 | 0 | 0.0 | 1 | 5.0 |
| Hyperemia at the injection site | 1 | 5.9 | 0 | 0.0 | 0 | 0.0 |
| Menstrual disorders | 2 | 18.2 | 0 | 0.0 | 0 | 0.0 |
| Hypersecretion from the genital tract (subjective analysis) | 1 | 5.9 | 0 | 0.0 | 0 | 0.0 |
| Cold | 3 | 17.6 | 3 | 17.6 | 0 | 0.0 |
| Gastrointestinal infection | 1 | 5.9 | 0 | 0.0 | 0 | 0.0 |
| High blood pressure | 1 | 5.9 | 0 | 0.0 | 0 | 0.0 |
| Neutropenia | 3 | 17.6 | 1 | 5.9 | 1 | 5.0 |
| Increased bilirubin | 1 | 5.9 | 0 | 0.0 | 1 | 5.0 |
| Leukopenia | 2 | 11.8 | 2 | 11.8 | 1 | 5.0 |
| Anemia | 1 | 5.9 | 0 | 0.0 | 1 | 5.0 |
| Increase in alanine aminotransferase | 1 | 5.9 | 0 | 0.0 | 0 | 0.0 |
| Increase in gamma-glutamyl transferase | 1 | 5.9 | 0 | 0.0 | 0 | 0.0 |
| Erythropenia | 0 | 0.0 | 1 | 5.9 | 0 | 0.0 |
| Proteinuria | 0 | 0.0 | 1 | 5.9 | 0 | 0.0 |
| Irritability | 0 | 0.0 | 1 | 5.9 | 0 | 0.0 |
| Group | 0.25 mg | 0.5 mg | Placebo | |||
|---|---|---|---|---|---|---|
| The number of viral blips (>50 copies/mL) | 8/88 | 9.1% | 6/89 | 6.7% | 7/95 | 7.4% |
| The number of participants with viral blips | 4/17 | 23.5% | 6/17 | 35.3% | 6/20 | 30.0% |
| Group | Visit | |||||||
|---|---|---|---|---|---|---|---|---|
| Screening | 2 | 6 | 7 | 9 | 11 | t test | ||
| 0.25 mg | N | 17 | 16 | 17 | 15 | 15 | 13 | 0.132 |
| Mean | 0.669 | 0.593 | 0.619 | 0.722 | 0.703 | 0.756 | ||
| SD | 0.224 | 0.223 | 0.241 | 0.152 | 0.223 | 0.187 | ||
| Min | 0.289 | 0.246 | 0.288 | 0.506 | 0.354 | 0.348 | ||
| Max | 1.086 | 1.114 | 1.290 | 1.176 | 1.121 | 1.056 | ||
| 0.5 mg | N | 17 | 17 | 17 | 14 | 14 | 11 | 0.104 |
| Mean | 0.707 | 0.769 | 0.714 | 0.710 | 0.671 | 0.797 | ||
| SD | 0.259 | 0.282 | 0.278 | 0.278 | 0.255 | 0.323 | ||
| Min | 0.289 | 0.307 | 0.333 | 0.346 | 0.232 | 0.361 | ||
| Max | 1.157 | 1.281 | 1.267 | 1.275 | 1.093 | 1.196 | ||
| Placebo | N | 20 | 20 | 20 | 17 | 16 | 12 | - |
| Mean | 0.567 | 0.558 | 0.620 | 0.603 | 0.555 | 0.650 | ||
| SD | 0.197 | 0.202 | 0.183 | 0.213 | 0.127 | 0.211 | ||
| Min | 0.336 | 0.189 | 0.353 | 0.278 | 0.239 | 0.367 | ||
| Max | 1.159 | 1.063 | 1.018 | 1.009 | 0.749 | 1.009 | ||
| Group | Visit | |||||||
|---|---|---|---|---|---|---|---|---|
| Screening | 2 | 6 | 7 | 9 | 11 | t test | ||
| 0.25 mg | N | 17 | 16 | 17 | 15 | 15 | 13 | 0.306 |
| Mean | 0.926 | 0.939 | 0.937 | 1.059 | 1.014 | 0.937 | ||
| SD | 0.413 | 0.540 | 0.450 | 0.533 | 0.534 | 0.293 | ||
| Min | 0.347 | 0.312 | 0.316 | 0.301 | 0.390 | 0.352 | ||
| Max | 1.776 | 2.376 | 1.926 | 2.199 | 2.230 | 1.406 | ||
| 0.5 mg | N | 17 | 17 | 17 | 14 | 14 | 11 | 0.969 |
| Mean | 1.023 | 1.037 | 0.992 | 0.902 | 0.847 | 1.033 | ||
| SD | 0.477 | 0.479 | 0.380 | 0.276 | 0.390 | 0.451 | ||
| Min | 0.358 | 0.422 | 0.322 | 0.507 | 0.262 | 0.469 | ||
| Max | 1.871 | 2.020 | 1.870 | 1.287 | 1.635 | 2.033 | ||
| Placebo | N | 20 | 20 | 20 | 17 | 16 | 12 | - |
| Mean | 0.975 | 0.976 | 0.976 | 0.940 | 0.923 | 1.086 | ||
| SD | 0.436 | 0.549 | 0.484 | 0.408 | 0.349 | 0.500 | ||
| Min | 0.299 | 0.285 | 0.332 | 0.364 | 0.437 | 0.476 | ||
| Max | 2.169 | 2.506 | 2.059 | 1.711 | 1.604 | 2.365 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akulova, E.; Murashev, B.; Verevochkin, S.; Masharsky, A.; Al-Shekhadat, R.; Poddubnyy, V.; Zozulya, O.; Vostokova, N.; Kozlov, A.P. The Increase of the Magnitude of Spontaneous Viral Blips in Some Participants of Phase II Clinical Trial of Therapeutic Optimized HIV DNA Vaccine Candidate. Vaccines 2019, 7, 92. https://doi.org/10.3390/vaccines7030092
Akulova E, Murashev B, Verevochkin S, Masharsky A, Al-Shekhadat R, Poddubnyy V, Zozulya O, Vostokova N, Kozlov AP. The Increase of the Magnitude of Spontaneous Viral Blips in Some Participants of Phase II Clinical Trial of Therapeutic Optimized HIV DNA Vaccine Candidate. Vaccines. 2019; 7(3):92. https://doi.org/10.3390/vaccines7030092
Chicago/Turabian StyleAkulova, Ekaterina, Boris Murashev, Sergey Verevochkin, Alexey Masharsky, Ruslan Al-Shekhadat, Valeriy Poddubnyy, Olga Zozulya, Natalia Vostokova, and Andrei P. Kozlov. 2019. "The Increase of the Magnitude of Spontaneous Viral Blips in Some Participants of Phase II Clinical Trial of Therapeutic Optimized HIV DNA Vaccine Candidate" Vaccines 7, no. 3: 92. https://doi.org/10.3390/vaccines7030092
APA StyleAkulova, E., Murashev, B., Verevochkin, S., Masharsky, A., Al-Shekhadat, R., Poddubnyy, V., Zozulya, O., Vostokova, N., & Kozlov, A. P. (2019). The Increase of the Magnitude of Spontaneous Viral Blips in Some Participants of Phase II Clinical Trial of Therapeutic Optimized HIV DNA Vaccine Candidate. Vaccines, 7(3), 92. https://doi.org/10.3390/vaccines7030092





