A Survey of Vaccine-Induced Measles IgG Antibody Titer to Verify Temporal Changes in Response to Measles Vaccination in Young Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Laboratory Methods
2.3. Statistical Analysis
3. Results
3.1. Survey Participants
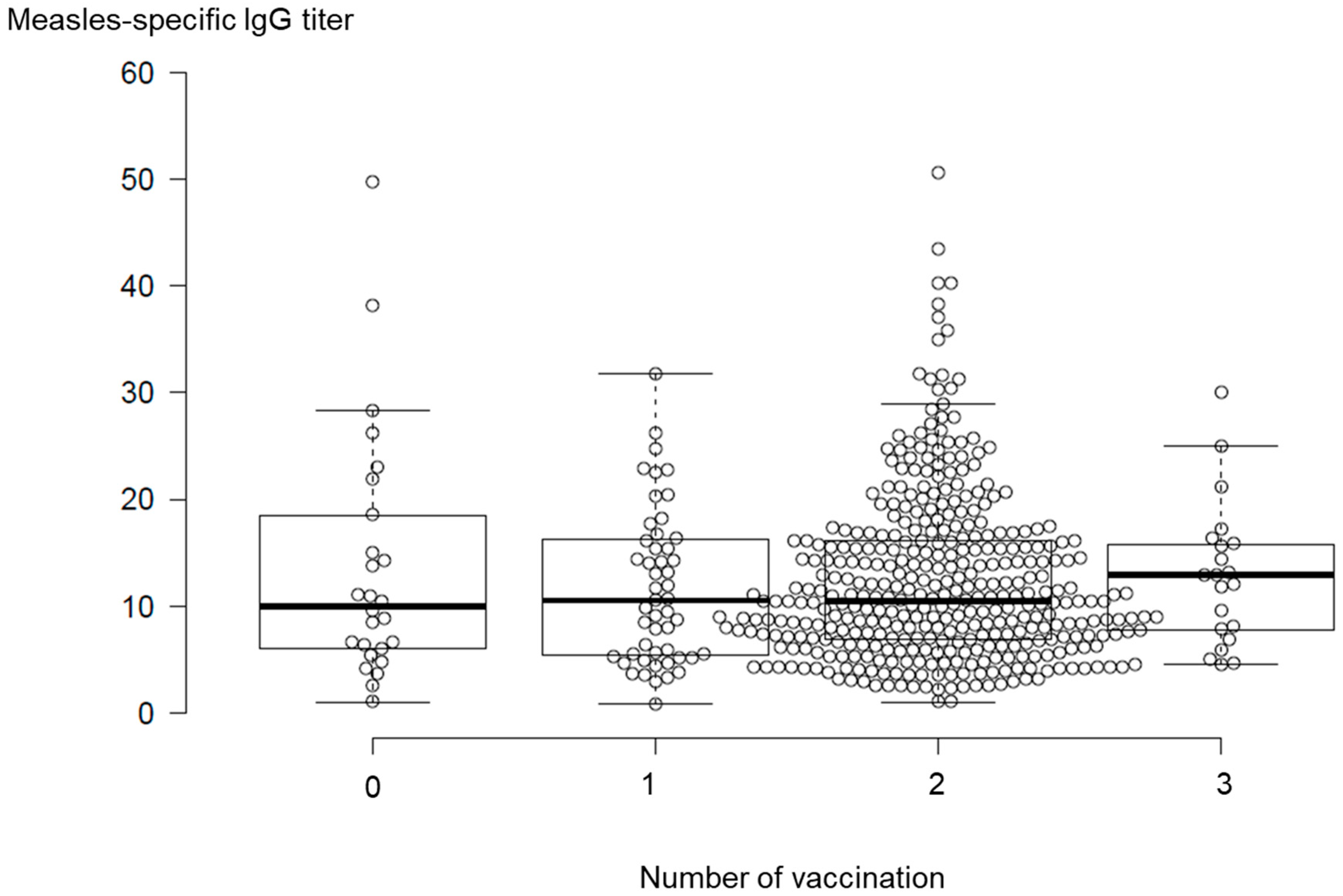
3.2. Measles-IgG Titer and Temporal Characterization
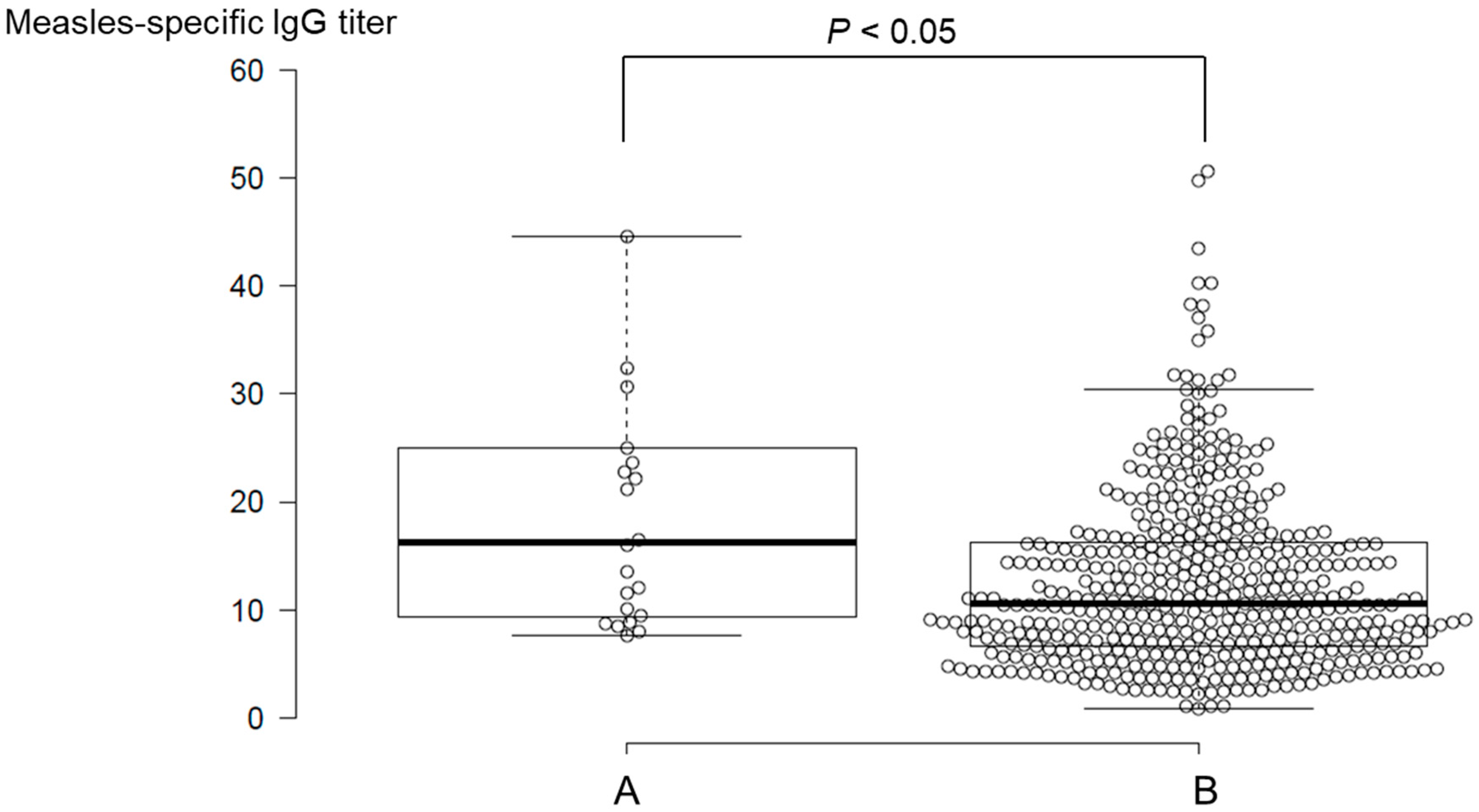
3.3. Divergence of Measles-IgG Antibody Titers by Medical History of Measles
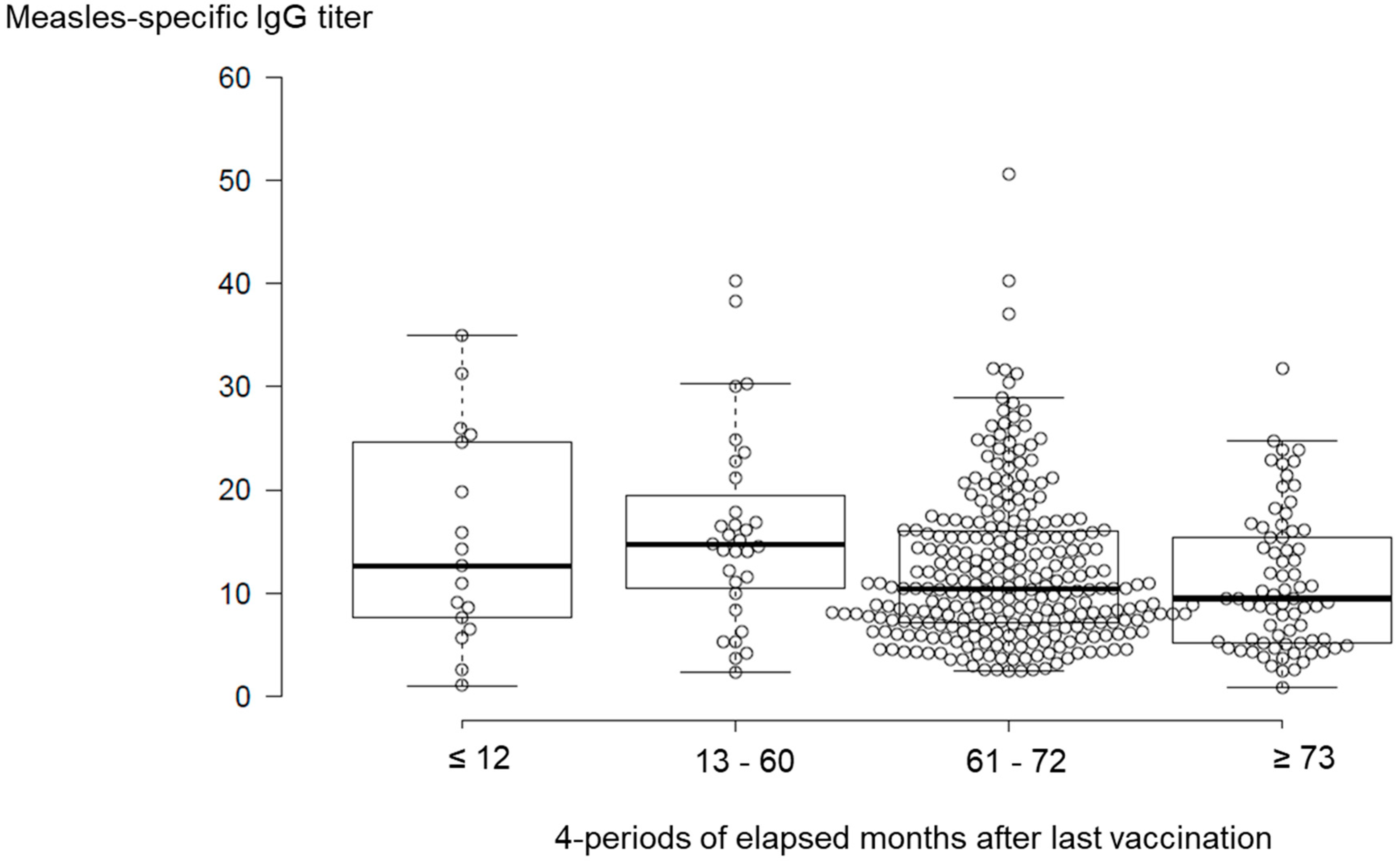
3.4. Temporal Changes in Measles-IgG Antibody Titers
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization Regional Office for the Western Pacific, BruneiDarussalam, Cambodia, Japan verified as achieving measles elimination. 2015. Available online: https://www.who.int/westernpacific/news/detail/27-03-2015-brunei-darussalam-cambodia-japan-verified-as-achieving-measles-elimination (accessed on 17 September 2019).
- National Institute of Infectious Disease, Infectious Disease Surveillance Center. Infectious Diseases Weekly Report; National Institute of Infectious Disease, Infectious Disease Surveillance Center: Shinjuku City, Japan, 2019; Volume 19, pp. 8–9. [Google Scholar]
- Demicheli, V.; Rivetti, A.; Debalini, M.G.; Di Pietrantonj, C. Vaccines for measles, mumps and rubella in children. Cochrane Database Syst. Rev. 2012, 2, CD004407. [Google Scholar] [CrossRef] [PubMed]
- Moss, W.J.; Griffin, D.E. Measles. Lancet 2012, 379, 153–164. [Google Scholar] [CrossRef]
- Ministry of Health, Labor and Welfare. Measles. Available online: https://www.mhlw.go.jp/seisakunitsuite/bunya/kenkou_iryou/kenkou/kekkaku-kansenshou/measles_eng/index.html (accessed on 17 September 2019).
- Tanaka-Taya, K. Progress towards the 2012 measles elimination goal in Japan. Uirusu 2010, 60, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health, Labor and Welfare. Available online: https://www.mhlw.go.jp/bunya/kenkou/kekkaku-kansenshou21/hashika.html (accessed on 17 September 2019).
- Durrheim, D. Measles elimination, immunity, serosurveys, and other immunity gap diagnostic tools. J. Infect. Dis. 2018, 218, 341–343. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.M.; Odahowski, C.L. Systematic review of measles and rubella serology studies. Risk Anal. 2016, 36, 1459–1486. [Google Scholar] [CrossRef] [PubMed]
- Kumakura, S.; Shibata, H.; Onoda, K.; Nishimura, N.; Matsuda, C.; Hirose, M. Seroprevalence survey on measles, mumps, rubella and varicella antibodies in healthcare workers in Japan: Sex, age, occupational-related differences and vaccine efficacy. Epidemiol. Infect. 2014, 142, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Haralambieva, I.H.; Salk, H.M.; Lambert, N.D.; Ovsyannikova, I.G.; Kennedy, R.B.; Warner, N.D.; Pankratz, V.S.; Poland, G.A. Associations between race, sex and immune response variations to rubella vaccination in two independent cohorts. Vaccine 2014, 32, 1946–1953. [Google Scholar] [CrossRef]
- Voigt, E.A.; Ovsyannikova, I.G.; Haralambieva, I.H.; Kennedy, R.B.; Larrabee, B.R.; Schaid, D.J.; Poland, G.A. Genetically defined race, but not sex, is associated with higher humoral and cellular immune responses to measles vaccination. Vaccine 2016, 34, 4913–4919. [Google Scholar] [CrossRef]
- Hatakeyama, S.; Moriya, K.; Itoyama, S.; Nukui, Y.; Uchida, M.; Shintani, Y.; Morisawa, Y.; Kimura, S. Prevalence of measles, rubella, mumps, and varicella antibodies among healthcare workers in Japan. Infect. Control Hosp Epidemiol. 2004, 25, 591–594. [Google Scholar] [CrossRef]
- Boulton, M.L.; Wang, X.; Zhang, Y.; Montgomery, J.P.; Wagner, A.L.; Carlson, B.F.; Ding, Y.; Li, X.; Gillespie, B.; Su, X. A population profile of measles susceptibility in Tianjin, China. Vaccine 2016, 34, 3037–3043. [Google Scholar] [CrossRef]
- Gohil, D.J.; Kothari, S.T.; Chaudhari, A.B.; Gunale, B.K.; Kulkarni, P.S.; Deshmukh, R.A.; Chowdhary, A.S. Seroprevalence of Measles, Mumps, and Rubella Antibodies in College Students in Mumbai, India. Viral Immunol. 2016, 29, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Takayama, N.; Saika, S.; Ichinohe, S. EIA-IgG antibody measles prevention level estimated from measles neutralizing, particle agglutination and hemagglutination-inhibition antibody titer. Kansenshogaku Zasshi 2009, 83, 519–524. [Google Scholar] [CrossRef] [PubMed][Green Version]
- World Health Organization. Disease outbreak news. Emergencies preparedness, response. Measles–Japan. Available online: https://www.who.int/csr/don/20-june-2018-measles-japan/en/ (accessed on 17 September 2019).
- Komabayashi, K.; Seto, J.; Tanaka, S.; Suzuki, Y.; Ikeda, T.; Onuki, N.; Yamada, K.; Ahiko, T.; Ishikawa, H.; Mizuta, K. The largest measles outbreak, including 38 modified measles and 22 typical measles cases in its elimination era in Yamagata, Japan in 2017. Jpn. J. Infect. Dis. 2018, 71, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, M.; Komagome, R.; Yamaguchi, H.; Ohnishi, A.; Kikuchi, M.; Ishida, S.; Nagano, H.; Okano, M. Detection of measles virus genotypes B3, D4, D5, D8, and H1 in the surveillance system in Hokkaido, Japan, 2006-2015, the last decade toward the elimination. Jpn. J. Infect. Dis. 2017, 70, 317–319. [Google Scholar] [CrossRef] [PubMed]
- Nishiura, H.; Mizumoto, K.; Asai, Y. Assessing the transmission dynamics of measles in Japan in 2016. Epidemics 2017, 20, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Kinoshita, R.; Yoshii, K.; Akhmetzhanov, A.R.; Jung, S.; Lee, H.; Nishiura, H. An investigation of a measles outbreak in Japan and China, Taiwan, China, March-May 2018. Western Pac. Surveill. Response J. 2018, 9, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Nakatani, H.; Sano, T.; Iuchi, T. Development of vaccination policy in Japan: current issues and policy directions. Jpn. J. Infect. Dis. 2002, 55, 101–111. [Google Scholar]
- Gomi, H.; Takahashi, H. Why is measles still endemic in Japan? Lancet 2004, 364, 328–329. [Google Scholar] [CrossRef]
- Papania, M.J.; Wallace, G.S.; Rota, P.A.; Icenogle, J.P.; Fiebelkorn, A.P.; Armstrong, G.L.; Reef, S.E.; Redd, S.B.; Abernathy, E.S.; Barskey, A.E.; et al. Elimination of endemic measles, rubella, and congenital rubella syndrome from the Western hemisphere: The US experience. JAMA Pediatr. 2014, 168, 148–155. [Google Scholar] [CrossRef]
- Fiebelkorn, A.P.; Redd, S.B.; Gastañaduy, P.A.; Clemmons, N.; Rota, P.A.; Rota, J.S.; Bellini, W.J.; Wallace, G.S. A comparison of postelimination measles epidemiology in the United States, 2009–2014 versus 2001–2008. J. Pediatric Infect. Dis. Soc. 2017, 6, 40–48. [Google Scholar] [CrossRef][Green Version]
- Ehresmann, K.; Banerjee, E.; Kenyon, C.; Christianson, B.; Griffith, J.; Heath, J.; Roddy, M.; MDH IDEPC Measles Outbreak Team. Response to “It could have been much worse: The Minnesota measles outbreak of 2017”. Vaccine 2019, 37, 683–684. [Google Scholar] [CrossRef] [PubMed]
- Clemmons, N.S.; Wallace, G.S.; Patel, M.; Gastañaduy, P.A. Incidence of measles in the United States, 2001-2015. JAMA 2017, 318, 1279–1281. [Google Scholar] [CrossRef] [PubMed]
- Nelson, R. US measles outbreak concentrated among unvaccinated children. Lancet Infect Dis. 2019, 19, 248. [Google Scholar] [CrossRef]
- Clifford, H.D.; Hayden, C.M.; Khoo, S.K.; Naniche, D.; Mandomando, I.M.; Zhang, G.; Richmond, P.; Le Souëf, P.N. Polymorphisms in key innate immune genes and their effects on measles vaccine responses and vaccine failure in children from Mozambique. Vaccine 2012, 30, 6180–6185. [Google Scholar] [CrossRef] [PubMed]
- Moss, W.J.; Strebel, P. Biological feasibility of measles eradication. J. Infect. Dis. 2011, 204, S47–S53. [Google Scholar] [CrossRef] [PubMed]
- Meissner, H.C.; Strebel, P.M.; Orenstein, W.A. Measles vaccines and the potential for worldwide eradication of measles. Pediatrics 2004, 114, 1065–1069. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T.; Uchiyama-Nakamura, F.; Sugata-Tsubaki, A.; Yamada, Y.; Uno, K.; Kasahara, K.; Maeda, K.; Konishi, M.; Mikasa, K. Antibody response to live attenuated vaccines in adults in Japan. Open Med. (Wars) 2016, 11, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Kakoulidou, M.; Ingelman-Sundberg, H.; Johansson, E.; Cagigi, A.; Farouk, S.E.; Nilsson, A.; Johansen, K. Kinetics of antibody and memory B cell responses after MMR immunization in children and young adults. Vaccine 2013, 31, 711–717. [Google Scholar] [CrossRef]
- Kennedy, R.B.; Ovsyannikova, I.G.; Thomas, A.; Larrabee, B.R.; Rubin, S.; Poland, G.A. Differential durability of immune responses to measles and mumps following MMR vaccination. Vaccine 2019, 37, 1775–1784. [Google Scholar] [CrossRef]
| Characteristics | Number | Percentage |
|---|---|---|
| Total | 506 | 100 |
| Age, Y | ||
| Range | ||
| 17–18 | 407 | 80 |
| 19–20 | 97 | 19 |
| ≥21 | 2 | 0 |
| Sex | ||
| Female | 160 | 32 |
| Male | 346 | 68 |
| Vaccination | ||
| 1 | 56 | 11 |
| 2 | 399 | 79 |
| 3 | 21 | 4 |
| Unvaccinated | 30 | 6 |
| Medical history of measles | 22 | 4 |
| Item | Mean | Range | Number |
|---|---|---|---|
| Measles-specific IgG titer | 13.4 | 0.8–128 | 506 |
| 1st vaccination month | 23.6 | 11–263 | 476 |
| 2nd vaccination month | 152.2 | 16–265 | 420 |
| 3rd vaccination month | 156.0 | 53–238 | 21 |
| Elapsed months after last vaccination | 83.3 | 0–237 | 476 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sasaki, H.; Fukunaga, T.; Asano, A.; Suzuki, Y.; Nakanishi, Y.; Kondo, J.; Ishikawa, H.; Shibata, N. A Survey of Vaccine-Induced Measles IgG Antibody Titer to Verify Temporal Changes in Response to Measles Vaccination in Young Adults. Vaccines 2019, 7, 118. https://doi.org/10.3390/vaccines7030118
Sasaki H, Fukunaga T, Asano A, Suzuki Y, Nakanishi Y, Kondo J, Ishikawa H, Shibata N. A Survey of Vaccine-Induced Measles IgG Antibody Titer to Verify Temporal Changes in Response to Measles Vaccination in Young Adults. Vaccines. 2019; 7(3):118. https://doi.org/10.3390/vaccines7030118
Chicago/Turabian StyleSasaki, Hiraku, Tomoko Fukunaga, Ai Asano, Yoshio Suzuki, Yuko Nakanishi, Junzi Kondo, Hiroki Ishikawa, and Nobuto Shibata. 2019. "A Survey of Vaccine-Induced Measles IgG Antibody Titer to Verify Temporal Changes in Response to Measles Vaccination in Young Adults" Vaccines 7, no. 3: 118. https://doi.org/10.3390/vaccines7030118
APA StyleSasaki, H., Fukunaga, T., Asano, A., Suzuki, Y., Nakanishi, Y., Kondo, J., Ishikawa, H., & Shibata, N. (2019). A Survey of Vaccine-Induced Measles IgG Antibody Titer to Verify Temporal Changes in Response to Measles Vaccination in Young Adults. Vaccines, 7(3), 118. https://doi.org/10.3390/vaccines7030118







