The Cellular Immune Response to Rabies Vaccination: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria and Outcomes of Interest
2.3. Selection Process
2.4. Data Collection Process
2.5. Risk of Bias Assessment
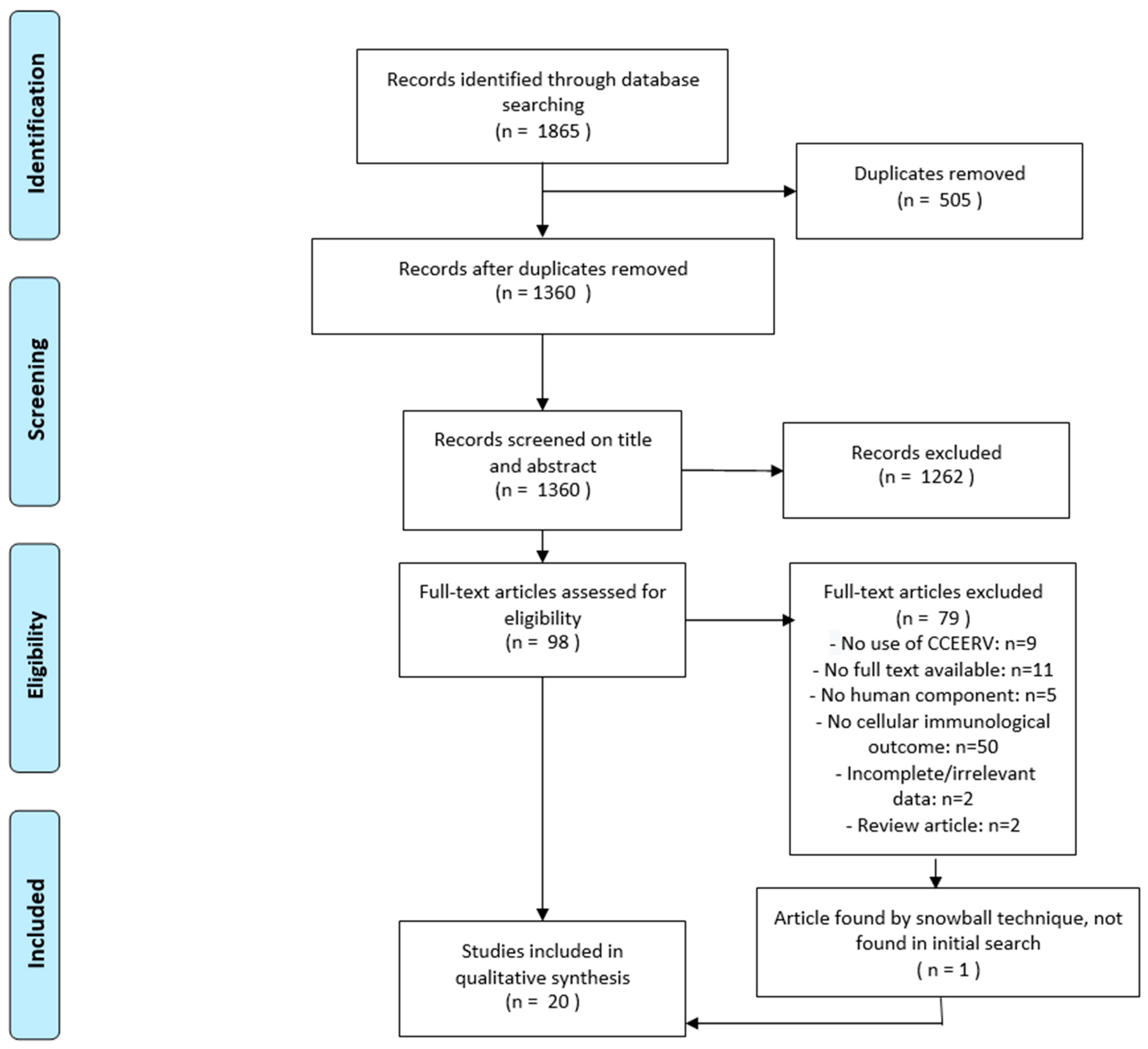
3. Results
3.1. Characteristics of Included Studies
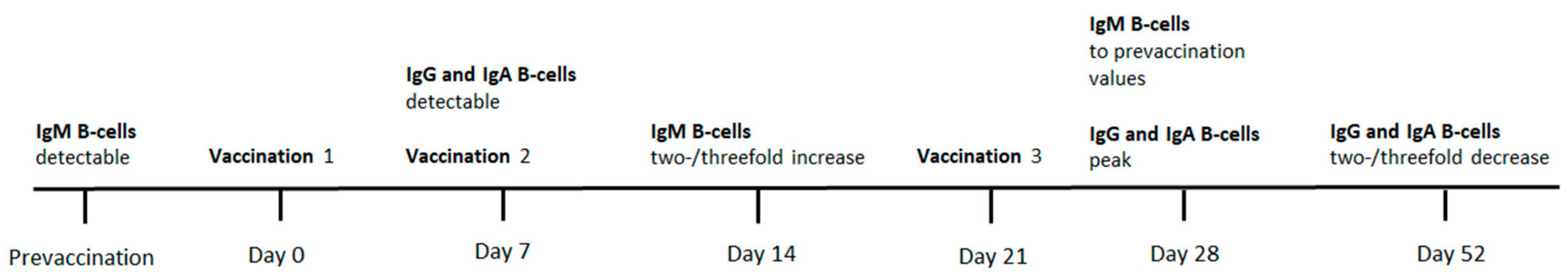
3.2. B-Cell Responses
3.3. T-Cell Responses
3.4. Intramuscular and Intradermal Vaccination
3.5. Cellular Immune Response in the Immunocompromised
4. Discussion
Supplementary Materials
Supplementary File 1Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. Embase
Appendix A.2. Web of Science
Appendix A.3. OR
Appendix A.4. COCHRANE Library
Appendix A.5. Academic Search Premier
References
- Fooks, A.R.; Banyard, A.C.; Horton, D.L.; Johnson, N.; McElhinney, L.M.; Jackson, A.C. Current status of rabies and prospects for elimination. Lancet 2014, 384, 1389–1399. [Google Scholar] [CrossRef]
- Hampson, K.; Coudeville, L.; Lembo, T.; Sambo, M.; Kieffer, A.; Attlan, M.; Barrat, J.; Blanton, J.D.; Briggs, D.J.; Cleaveland, S.; et al. Estimating the Global Burden of Endemic Canine Rabies. PLoS Negl. Trop. Dis. 2015, 9, e0003786. [Google Scholar] [CrossRef]
- WHO. Rabies Vaccines: WHO Position Paper. Available online: https://apps.who.int/iris/bitstream/handle/10665/272371/WER9316.pdf?ua=1 (accessed on 16 June 2019).
- Denis, M.; Knezevic, I.; Wilde, H.; Hemachudha, T.; Briggs, D.; Knopf, L. An overview of the immunogenicity and effectiveness of current human rabies vaccines administered by intradermal route. Vaccines 2018. [Google Scholar] [CrossRef] [PubMed]
- Langedijk, A.C.; De Pijper, C.A.; Spijker, R.; Holman, R.; Grobusch, M.P.; Stijnis, C. Rabies Antibody Response After Booster Immunization: A Systematic Review and Meta-analysis. Clin. Infect. Dis. 2018, 67, 1932–1947. [Google Scholar] [CrossRef] [PubMed]
- WHO. Rabies Working Group Report, SAGE Meeting of October 2017. Available online: http://www.who.int/immunization/sage/meetings/2017/october/1_Background_paper_WG_RABIES_final.pdf?ua=1 (accessed on 20 June 2019).
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- UytdeHaag, F.G.; Osterhaus, A.D.; Loggen, H.G.; Bakker, R.H.; A Van Asten, J.; Kreeftenberg, J.G.; Van Der Marel, P.; Van Steenis, B. Induction of antigen-specific antibody response in human peripheral blood lymphocytes in vitro by a dog kidney cell vaccine against rabies virus (DKCV). J. Immunol. 1983, 131, 1234–1239. [Google Scholar]
- Celis, E.; Wiktor, T.J.; Dietzschold, B.; Koprowski, H. Amplification of rabies virus-induced stimulation of human T-cell lines and clones by antigen-specific antibodies. J. Virol. 1985, 56, 426–433. [Google Scholar]
- Celis, E.; Miller, R.W.; Wiktor, T.J.; Dietzschold, B.; Koprowski, H. Isolation and characterization of human T cell lines and clones reactive to rabies virus: antigen specificity and production of interferon-gamma. J. Immunol. 1986, 136, 692–697. [Google Scholar]
- Celis, E.; Ou, D.; Dietzschold, B.; Koprowski, H. Recognition of rabies and rabies-related viruses by T cells derived from human vaccine recipients. J. Virol. 1988, 62, 3128–3134. [Google Scholar]
- Bunschoten, H.; Klapmuts, R.J.; Claassen, I.J.T.M.; Reyneveld, S.D.; Osterhaus, A.D.M.E.; UytdeHaag, F.G.C.M. Rabies Virus-specific Human T Cell Clones Provide Help for an in vitro Antibody Response against Neutralizing Antibody-inducing Determinants of the Viral Glycoprotein. J. Gen. Virol. 1989, 70, 1513–1521. [Google Scholar] [CrossRef] [PubMed]
- Ueki, Y. Clonal analysis of a human antibody response. Quantitation of precursors of antibody-producing cells and generation and characterization of monoclonal IgM, IgG, and IgA to rabies virus. J. Exp. Med. 1990, 171, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Herzog, M.; Fritzell, C.; Lafage, M.; Hirose, J.A.M.; Scott-Algara, D.; Lafon, M. T and B cell human responses to European bat lyssavirus after post-exposure rabies vaccination. Clin. Exp. Immunol. 1991, 85, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Herzog, M.; Lafage, M.; Montaño-Hirose, J.A.; Fritzell, C.; Scott-Algara, D.; Lafon, M. Nucleocapsid specific T and B cell responses in humans after rabies vaccination. Virus Res. 1992, 24, 77–89. [Google Scholar] [CrossRef]
- Van der Heijden, R.W.; Langedijk, J.P.; Groen, J.; UytdeHaag, F.G.; Meloen, R.H.; Osterhaus, A.D. Structural and functional studies on a unique linear neutralizing antigenic site (G5) of the rabies virus glycoprotein. J. Gen. Virol. 1993, 74, 1539–1545. [Google Scholar] [CrossRef] [PubMed]
- Thraenhart, O.; Kreuzfelder, E.; Hillebrandt, M.; Marcus, I.; Ramakrishnan, K.; Fu, Z.; Dietzschold, B. Long-Term Humoral and Cellular Immunity after Vaccination with Cell Culture Rabies Vaccines in Man. Clin. Immunol. Immunopathol. 1994, 71, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, G.; Passalacqua, D.J.; Bender, B.S.; Briggs, D.J.; Goodenow, M.M.; Sleasman, J.W. Human Lymphocyte Proliferation Responses following Primary Immunization with Rabies Vaccine as Neoantigen. Clin. Diagn. Lab. Immunol. 2001, 8, 880–883. [Google Scholar] [CrossRef]
- Brinkman, D.M.C.; Der Zijde, C.M.J.-V.; Dam, M.M.T.; Vossen, J.M.; Osterhaus, A.D.M.E.; Kroon, F.P.; Van Tol, M.J.D. Vaccination with rabies to study the humoral and cellular immune response to a T-cell dependent neoantigen in man. J. Clin. Immunol. 2003, 23, 528–538. [Google Scholar] [CrossRef]
- Gomez, I.; Marx, F.; Gould, E.; Grubeck-Loebenstein, B. T cells from elderly persons respond to neoantigenic stimulation with an unimpaired IL-2 production and an enhanced differentiation into effector cells. Exp. Gerontol. 2004, 39, 597–605. [Google Scholar] [CrossRef]
- Moore, S.M.; Wilkerson, M.J.; Davis, R.D.; Wyatt, C.R.; Briggs, D.J. Detection of Cellular Immunity to Rabies Antigens in Human Vaccinees. J. Clin. Immunol. 2006, 26, 533–545. [Google Scholar] [CrossRef]
- Brinkman, D.M.C.; Der Zijde, C.M.J.-V.; Dam, M.M.T.; Boekhorst, P.A.W.T.; Cate, R.T.; Wulffraat, N.M.; Hintzen, R.Q.; Vossen, J.M.; Van Tol, M.J.D. Resetting the Adaptive Immune System After Autologous Stem Cell Transplantation: Lessons from Responses to Vaccines. J. Clin. Immunol. 2007, 27, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Blanchard-Rohner, G.; Pulickal, A.S.; Der Zijde, C.M.J.-V.; Snape, M.D.; Pollard, A.J. Appearance of peripheral blood plasma cells and memory B cells in a primary and secondary immune response in humans. Blood 2009, 114, 4998–5002. [Google Scholar] [CrossRef] [PubMed]
- Sirikwin, S.; Likanonsakul, S.; Waradejwinyoo, S.; Pattamadilok, S.; Kumperasart, S.; Chaovavanich, A.; Manatsathit, S.; Malerczyk, C.; Wasi, C. Antibody response to an eight-site intradermal rabies vaccination in patients infected with Human Immunodeficiency Virus. Vaccines 2009, 27, 4350–4354. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, A.; Behrens, R.H.; Okell, L.; Fooks, A.R.; Riley, E.M. NK cells as effectors of acquired immune responses: Effector CD4+ T cell-dependent activation of NK cells following vaccination. J. Immunol. 2010, 185, 2808–2818. [Google Scholar] [CrossRef] [PubMed]
- Vejpongsa, P.; Leelasinjaroen, P.; Sodsai, P.; Hirankarn, N.; Tantawichien, T. Increasing CD4+CD25hiFoxP3+ regulatory T-cells in subjects after repeated booster doses of rabies vaccination. In Proceedings of the 21st ECCMID/27th ICC, Milan, Italy, 7–10 May 2011. P2215. [Google Scholar]
- Venkataswamy, M.M.; Madhusudana, S.N.; Sanyal, S.S.; Taj, S.; Belludi, A.Y.; Mani, R.S.; Hazra, N. Cellular immune response following pre-exposure and postexposure rabies vaccination by intradermal and intramuscular routes. Clin. Exp. Vaccine Res. 2015, 4, 68–74. [Google Scholar] [CrossRef] [PubMed]
| Authors | Year | Country | Population | N. | Follow-Up | Vaccine Type | Primary or Secondary A | ROA | Immunization Schedule | Challenging Antigen | Risk of Bias |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Uytdehaag et al. [9] | 1983 | The Netherlands | Healthy, naïve | N/R | 2 or 3 weeks after each vaccination. | DKCV | Secondary | IM | Day 0, 1mo, 6mo | DKCV | Low |
| N/R | 2 or 3 weeks after each vaccination. | DKCV | Secondary | IM | Day 0, 7, 21 | DKCV | |||||
| Celis et al. [10] | 1985 | USA | Immunized | 5 | Incubation. | N/R | Secondary | N/R | N/R | PM | Moderate |
| Celis et al. [11] | 1986 | USA | Immunized (high RVNA titer) | 5 | Maximum 6 mo after last vaccination. Incubation. | HDCV | Secondary | N/R | N/R | PMV | Moderate |
| Celis et al. [12] | 1988 | USA | Healthy | 5 | 7–10 days after last immunization. | HDCV | Secondary | N/R | PrEP (N/S) | PM, CVS, ERA, PV, North American bat (NAB; strain 6014) | Moderate |
| Bunschoten et al. [13] | 1989 | The Netherlands | Naïve | 7 | Incubation. | DKCV | Secondary | IM | Day 0, 7, 21 | PM-DKCV and PMV | Moderate |
| Ueki et al. [14] | 1990 | USA | Healthy, naïve (30–52 years) | 3 | Day -1, 7, 14, 28, 52, 163. | HDCV | Both | ID | Day 0, 7, 21, 142 | ERA | Moderate |
| 1 | Day -1, 7, 14, 28, 52, 163. | HDCV | Both | ID | Day 0, 7, 21 | ERA | |||||
| Herzog et al. [15] | 1991 | France | Healthy, post-exposure, naïve to the vaccine (19–48 years) | 22 | Day 7, 21, 28/35. | PMV | Both | N/R | 2× on day 0, 1× on 7, 21 | CVS, ERA | Moderate |
| Herzog et al. [16] | 1992 | France | Healthy, immunized | 18 | Day 21. | PMV | Secondary | N/R | 2× on day 0, 1x on 7, 21 | ERA, NC | Serious |
| Healthy, immunized | 18 | 1–36 mo after the last vaccination. | PMV | Secondary | N/R | Day 0, 7, 28 + booster each two years | ERA, NC | ||||
| Van der Heijden et al. [17] | 1993 | The Netherlands | Naïve | 25 | Before immunization and variable times. | DKCV | Secondary | N/R | 3 vaccinations | N/R | Serious |
| Thraenhart et al. [18] | 1994 | Germany | Immunized | 18 | After last immunization (2–14 years earlier). Incubation. Cell counts on day 0 and 7. | HDCV or PCECV | Secondary | N/R | N/R | PCECV, Flury LEP, GP, NP, RNP | Moderate |
| Naïve | 18 | N/A | N/A | Primary | N/A | N/A | PCECV, Flury LEP, GP, NP, RNP | ||||
| Ghaffari et al. [19] | 2001 | USA | Healthy, naïve (23–33 years) | 14 | Prior to vaccination, 4 weeks after last vaccination. | HDCV | Secondary | IM | Day 0, 7, 28 | N/R | Low |
| Brinkman et al. [20] | 2003 | The Netherlands | Healthy (19–49 years) | 18 | Day 0, 7, 14, 28, 365 days after 1st vaccination. Day 0, 7, 14, 28 after each booster. | HDCV | Both | IM | Day 0, 3 mo | PM-DKCV | Moderate |
| CID patients (4–13 years) | 5 | Day 0, 7, 14, 28, 365 days after 1st vaccination. Day 0, 7, 14, 28 after each booster. | HDCV | Both | IM | Day 0, 3 mo | PM-DKCV | ||||
| Gomez et al. [21] | 2004 | Austria | Healthy, naïve (<35 years) | 7 | Incubation. Cell counts on day 0 and 7. | N/A | Both | N/A | N/A | HDCV | Moderate |
| Healthy, naïve (>60 years) | 8 | Incubation. Cell counts on day 0 and 7. | N/A | Both | N/A | N/A | HDCV | ||||
| Moore et al. [22] | 2006 | USA | Healthy, naïve | 5 | N/R | N/A | Primary | N/A | N/A | CVS | Moderate |
| Healthy, immunized | 10 | After last immunization (5 mo to 19 years earlier). | HDCV or PCECV | Secondary | N/A | N/A | CVS | ||||
| Brinkman et al. [23] | 2007 | The Netherlands | JIA or SLE patients (4–15 years) undergoing ASCT | 6 | Day 0, 28 after each vaccination. | HDCV | Both | IM | Day of bone marrow harvest, 6 mo after ASCT | PM-DKCV | Moderate |
| MS patients (23–50 years) undergoing ASCT | 10 | Day 0, 28 after each vaccination. | HDCV | Both | IM | Day of bone marrow harvest, 6 mo after ASCT | PM-DKCV | ||||
| Healthy (19–49 years) | 18 | Day 0, 28 after each vaccination. | HDCV | Both | IM | Day 0, 3 mo | PM-DKCV | ||||
| Blanchard-Rohner et al. [24] | 2009 | UK | Healthy, naïve (18–50 years) | 10 | Before immunization, day 2, 4, 7, 10, 14, 28 after 1st and 3rd vaccination. | HDCV | Both | IM | Day 0, 28, 56 | N/R | Moderate |
| Healthy, immunized (18–50 years) | 10 | After a single booster. | HDCV | Secondary | IM | Day 0 | N/R | ||||
| Sirikwin et al. [25] | 2009 | Thailand | HIV1 patients, naïve (>15 years) | 27 | Day 0, 3, 7, 14, 30, 90, 180, 365. | PCECV | Both | 8-site ID | Day 0, 3, 7, 14, 30 | N/R | Moderate |
| Horowitz et al. [26] | 2010 | UK | Healthy, naïve (26–31 years) | 30 | Day 0, 21. | HDCV | Both | IM | Day 0, 7, 21 | Inactivated virus | Moderate |
| Vejpongsa et al. [27] (unpublished) | 2011 | Thailand | Immunized (20–55 years) | 41 | Day 0, 14. | PVRV | Secondary | 2-site ID | Day 0 | NR | Moderate |
| Venkataswamy et al. [28] | 2015 | India | Naïve (25–40 years) | 10 | N/A | N/A | Primary | N/A | N/A | HDCV | Low |
| Healthy, immunized (27–58 years) | 10 | Day 7 after the last vaccination. | PCECV | Secondary | ID | Day 0, 7, 28 | HDCV | ||||
| Healthy, immunized (27–58 years) | 20 | Day 7 after the last vaccination. | PCECV | Secondary | ID | Day 0, 7, 28, 180, 183 | HDCV | ||||
| Post-exposure (10–45 years) | 18 | Day 7 after the last vaccination. | PCECV | Secondary | ID | Day 0, 3, 7, 28 | HDCV | ||||
| Post-exposure (10–45 years) | 20 | Day 7 after the last vaccination. | PCECV | Secondary | IM | Day 0, 3, 7, 14, 28 | HDCV |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Overduin, L.A.; van Dongen, J.J.M.; Visser, L.G. The Cellular Immune Response to Rabies Vaccination: A Systematic Review. Vaccines 2019, 7, 110. https://doi.org/10.3390/vaccines7030110
Overduin LA, van Dongen JJM, Visser LG. The Cellular Immune Response to Rabies Vaccination: A Systematic Review. Vaccines. 2019; 7(3):110. https://doi.org/10.3390/vaccines7030110
Chicago/Turabian StyleOverduin, Lisanne A., Jacques J.M. van Dongen, and Leonardus G. Visser. 2019. "The Cellular Immune Response to Rabies Vaccination: A Systematic Review" Vaccines 7, no. 3: 110. https://doi.org/10.3390/vaccines7030110
APA StyleOverduin, L. A., van Dongen, J. J. M., & Visser, L. G. (2019). The Cellular Immune Response to Rabies Vaccination: A Systematic Review. Vaccines, 7(3), 110. https://doi.org/10.3390/vaccines7030110





