Bacterial Outer Membrane Vesicles (OMVs)-Based Dual Vaccine for Influenza A H1N1 Virus and MERS-CoV
Abstract
:1. Introduction
2. Materials and Methods
2.1. Viruses and Plasmids
2.2. Construction of Plasmids
2.3. Purification and Quantification of OMVs
2.4. Immunoblotting Analysis
2.5. Preparation of Inactivated Control Vaccines
2.6. Immunization of Mice
2.7. Hemagglutinin Inhibition (HAI)
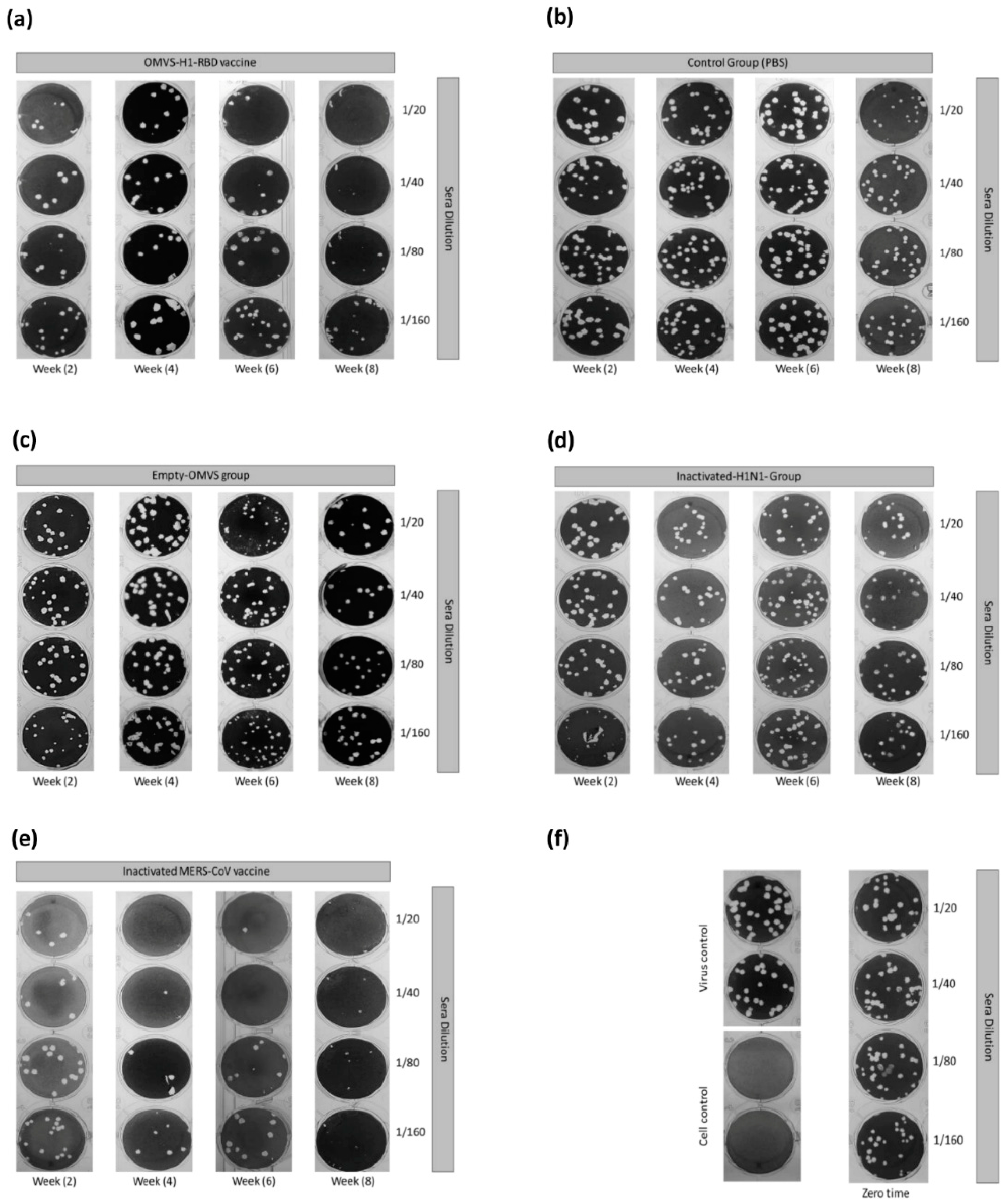
2.8. Plaque-Reduction Neutralization Test (PRNT)
2.9. Challenge Infection of Mice
2.10. Ethics Statement and Biosafety
3. Results
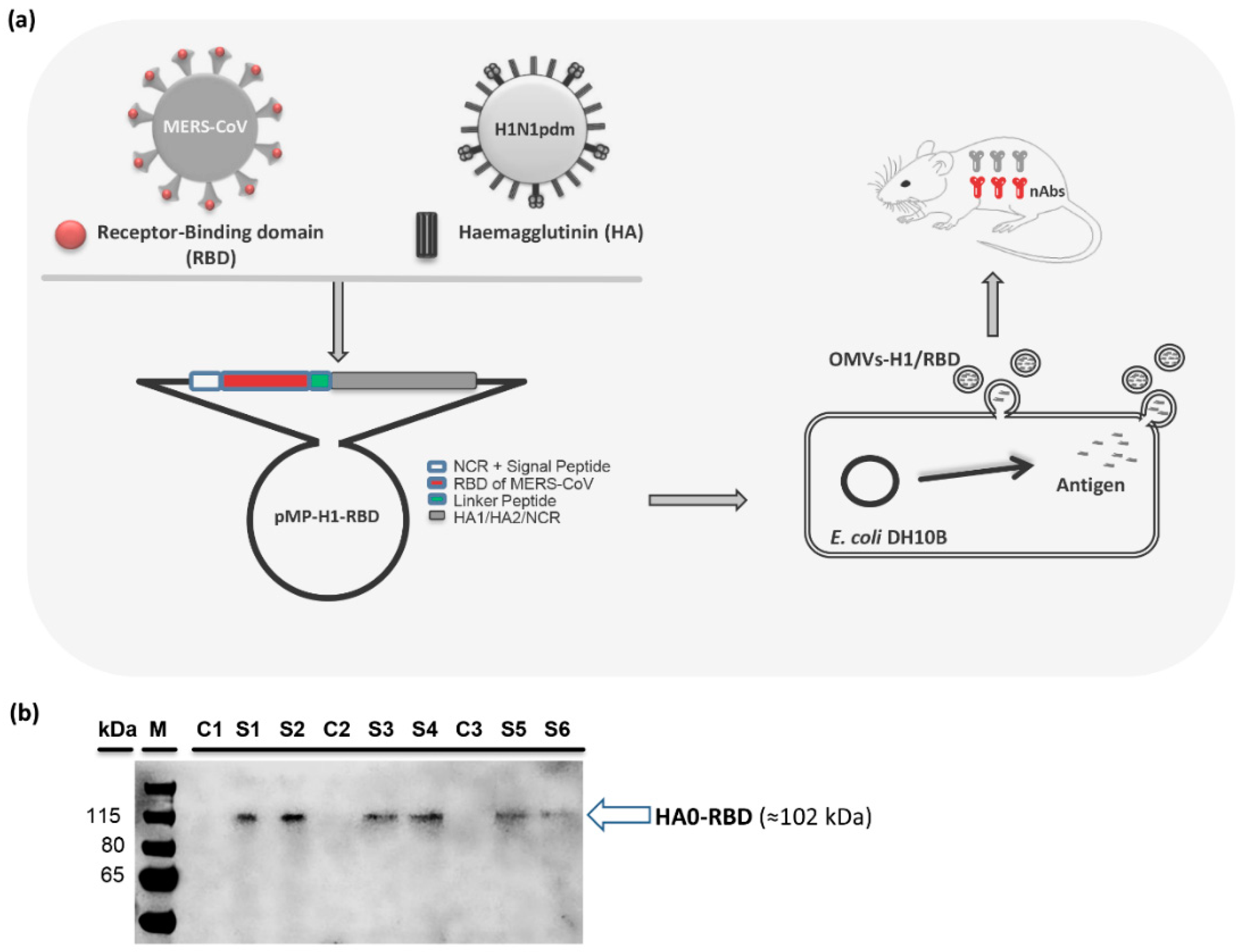
3.1. Design and Construction of Chimeric pMP-H1/RBD
3.2. Production and Characterization of OMVs with Chimeric HA/RBD Antigen (OMVs-H1/RBD)
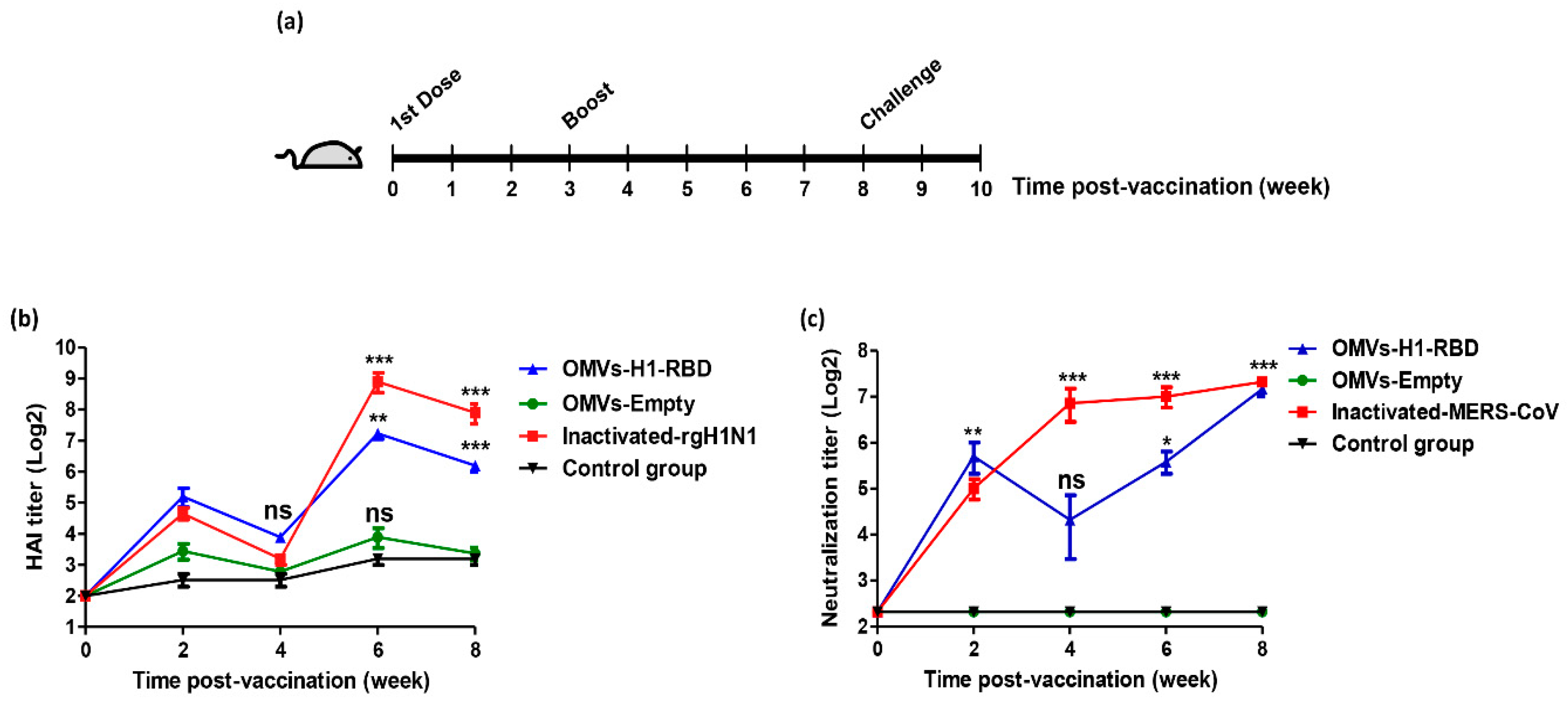
3.3. Immunization with OMVs-H1/RBD Elicits Specific IgG Titers
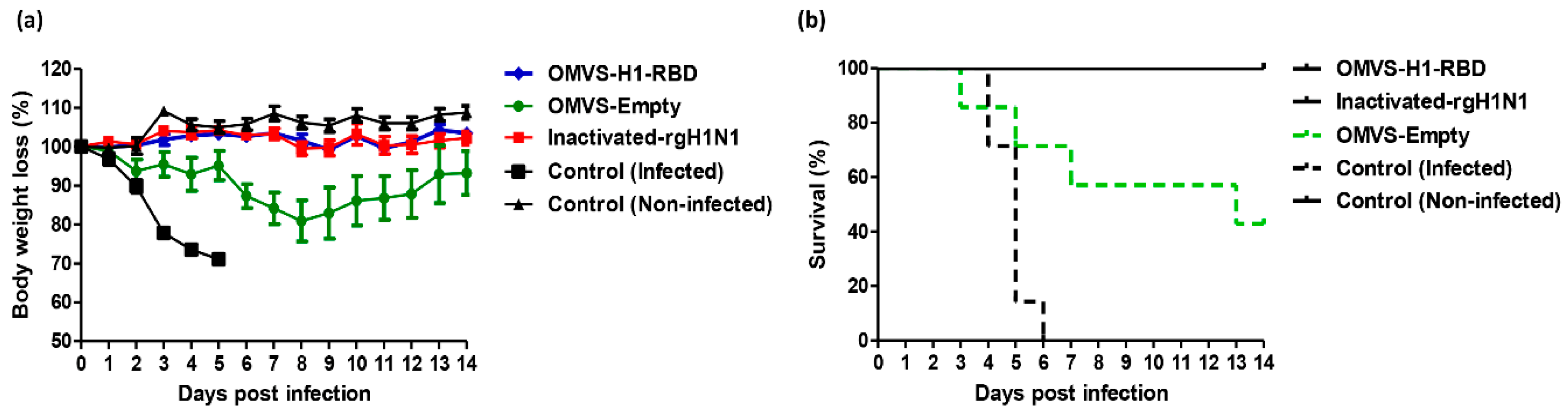
3.4. Mice Immunized with OMVs-H1/RBD Showed 100% Survival Upon Challenge with H1N1pdm09
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bhuyan, G.S.; Hossain, M.A.; Sarker, S.K.; Rahat, A.; Islam, M.T.; Haque, T.N.; Begum, N.; Qadri, S.K.; Muraduzzaman, A.K.; Islam, N.N.; et al. Bacterial and viral pathogen spectra of acute respiratory infections in under-5 children in hospital settings in Dhaka city. PLoS ONE 2017, 12, e0174488. [Google Scholar] [CrossRef] [PubMed]
- The top 10 Causes of Death. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 12 May 2019).
- Troeger, C.; Forouzanfar, M.; Rao, P.C.; Khalil, I.; Brown, A.; Swartz, S.; Fullman, N.; Mosser, J.; Thompson, R.L.; Reiner, R.C.; et al. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory tract infections in 195 countries: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Infect. Dis. 2017, 17, 1133–1161. [Google Scholar] [CrossRef] [Green Version]
- Burden of Disease. Available online: https://www.who.int/influenza/surveillance_monitoring/bod/en/ (accessed on 12 May 2019).
- Potter, C.W. A history of influenza. J. Appl. Microbiol. 2001, 91, 572–579. [Google Scholar] [CrossRef]
- Parry, J. H7N9 avian flu infects humans for the first time. BMJ 2013, 346, f2151. [Google Scholar] [CrossRef] [PubMed]
- Bahgat, M.M.; Kutkat, M.A.; Nasraa, M.H.; Mostafa, A.; Webby, R.; Bahgat, I.M.; Ali, M.A. Characterization of an avian influenza virus H5N1 Egyptian isolate. J. Virol. Methods. 2009, 159, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yuan, H.; Gao, R.; Zhang, J.; Wang, D.; Xiong, Y.; Fan, G.; Yang, F.; Li, X.; Zhou, J.; et al. Clinical and epidemiological characteristics of a fatal case of avian influenza A H10N8 virus infection: A descriptive study. Lancet 2014, 383, 714–721. [Google Scholar] [CrossRef]
- Shi, W.; Shi, Y.; Wu, Y.; Liu, D.; Gao, G.F. Origin and molecular characterization of the human-infecting H6N1 influenza virus in Taiwan. Protein Cell 2013, 4, 846–853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lessler, J.; Reich, N.G.; Cummings, D.A.; Nair, H.P.; Jordan, H.T.; Thompson, N. Outbreak of 2009 pandemic influenza A (H1N1) at a New York City school. N. Engl. J. Med. 2009, 361, 2628–2636. [Google Scholar] [CrossRef] [PubMed]
- Neumann, G.; Noda, T.; Kawaoka, Y. Emergence and pandemic potential of swine-origin H1N1 influenza virus. Nature 2009, 459, 931–939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Middle East Respiratory Syndrome Coronavirus (MERS-CoV). Available online: https://www.who.int/emergencies/mers-cov/en/ (accessed on 14 May 2019).
- Chafekar, A.; Fielding, B.C. MERS-CoV: Understanding the Latest Human Coronavirus Threat. Viruses 2018, 10, 93. [Google Scholar] [CrossRef]
- Mostafa, A.; Pleschka, S. Influenza H3N2 Vaccines: Recent Challenges. Trends Microbiol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Zost, S.J.; Parkhouse, K.; Gumina, M.E.; Kim, K.; Diaz Perez, S.; Wilson, P.C.; Treanor, J.J.; Sant, A.J.; Cobey, S.; Hensley, S.E. Contemporary H3N2 influenza viruses have a glycosylation site that alters binding of antibodies elicited by egg-adapted vaccine strains. Proc. Natl. Acad. Sci. USA 2017, 114, 12578–12583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, N.C.; Zost, S.J.; Thompson, A.J.; Oyen, D.; Nycholat, C.M.; McBride, R.; Paulson, J.C.; Hensley, S.E.; Wilson, I.A. A structural explanation for the low effectiveness of the seasonal influenza H3N2 vaccine. PLoS Pathogens 2017, 13, e1006682. [Google Scholar] [CrossRef] [PubMed]
- Gerritzen, M.J.H.; Martens, D.E.; Wijffels, R.H.; van der Pol, L.; Stork, M. Bioengineering bacterial outer membrane vesicles as vaccine platform. Biotechnol. Adv. 2017, 35, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Anand, D.; Chaudhuri, A. Bacterial outer membrane vesicles: New insights and applications. Mol. Membr. Biol. 2016, 33, 125–137. [Google Scholar] [CrossRef] [Green Version]
- Zollinger, W.D.; Poolman, J.T.; Maiden, M.C. Meningococcal serogroup B vaccines: Will they live up to expectations? Expert. Rev. Vaccines 2011, 10, 559–561. [Google Scholar] [CrossRef] [PubMed]
- Fantappie, L.; de Santis, M.; Chiarot, E.; Carboni, F.; Bensi, G.; Jousson, O.; Margarit, I.; Grandi, G. Antibody-mediated immunity induced by engineered Escherichia coli OMVs carrying heterologous antigens in their lumen. J. Extracell Vesicles 2014, 3. [Google Scholar] [CrossRef]
- Mostafa, A.; Kanrai, P.; Ziebuhr, J.; Pleschka, S. Improved dual promotor-driven reverse genetics system for influenza viruses. J. Virol. Methods 2013, 193, 603–610. [Google Scholar] [CrossRef]
- Bommakanti, G.; Citron, M.P.; Hepler, R.W.; Callahan, C.; Heidecker, G.J.; Najar, T.A.; Lu, X.; Joyce, J.G.; Shiver, J.W.; Casimiro, D.R.; et al. Design of an HA2-based Escherichia coli expressed influenza immunogen that protects mice from pathogenic challenge. Proc. Natl. Acad. Sci. USA 2010, 107, 13701–13706. [Google Scholar] [CrossRef]
- Alshukairi, A.N.; Zheng, J.; Zhao, J.; Nehdi, A.; Baharoon, S.A.; Layqah, L.; Bokhari, A.; Al Johani, S.M.; Samman, N.; Boudjelal, M.; et al. High Prevalence of MERS-CoV Infection in Camel Workers in Saudi Arabia. MBio 2018, 9. [Google Scholar] [CrossRef]
- Ali, M.; El-Shesheny, R.; Kandeil, A.; Shehata, M.; Elsokary, B.; Gomaa, M.; Hassan, N.; El Sayed, A.; El-Taweel, A.; Sobhy, H.; et al. Cross-sectional surveillance of Middle East respiratory syndrome coronavirus (MERS-CoV) in dromedary camels and other mammals in Egypt, August 2015 to January 2016. Euro. Surveill. 2017, 22. [Google Scholar] [CrossRef] [PubMed]
- Raj, V.S.; Mou, H.; Smits, S.L.; Dekkers, D.H.; Muller, M.A.; Dijkman, R.; Muth, D.; Demmers, J.A.; Zaki, A.; Fouchier, R.A.; et al. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature 2013, 495, 251–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Zhou, P.; Wang, P.; Li, Y.; Jiang, L.; Jia, W.; Wang, H.; Fan, A.; Wang, D.; Shi, X.; et al. Structural Definition of a Unique Neutralization Epitope on the Receptor-Binding Domain of MERS-CoV Spike Glycoprotein. Cell Rep. 2018, 24, 441–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Zheng, X.; Gai, W.; Wong, G.; Wang, H.; Jin, H.; Feng, N.; Zhao, Y.; Zhang, W.; Li, N.; et al. Novel chimeric virus-like particles vaccine displaying MERS-CoV receptor-binding domain induce specific humoral and cellular immune response in mice. Antiviral Res. 2017, 140, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tai, W.; Yang, J.; Zhao, G.; Sun, S.; Tseng, C.K.; Jiang, S.; Zhou, Y.; Du, L.; Gao, J. Receptor-binding domain of MERS-CoV with optimal immunogen dosage and immunization interval protects human transgenic mice from MERS-CoV infection. Hum. Vaccin. Immunother. 2017, 13, 1615–1624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, L.; Kou, Z.; Ma, C.; Tao, X.; Wang, L.; Zhao, G.; Chen, Y.; Yu, F.; Tseng, C.T.; Zhou, Y.; et al. A truncated receptor-binding domain of MERS-CoV spike protein potently inhibits MERS-CoV infection and induces strong neutralizing antibody responses: implication for developing therapeutics and vaccines. PloS ONE 2013, 8, e81587. [Google Scholar] [CrossRef]
- Zhao, G.; He, L.; Sun, S.; Qiu, H.; Tai, W.; Chen, J.; Li, J.; Chen, Y.; Guo, Y.; Wang, Y.; et al. A Novel Nanobody Targeting Middle East Respiratory Syndrome Coronavirus (MERS-CoV) Receptor-Binding Domain Has Potent Cross-Neutralizing Activity and Protective Efficacy against MERS-CoV. J. Virol. 2018. [Google Scholar] [CrossRef]
- Shehata, M.M.; Gomaa, M.R.; Ali, M.A.; Kayali, G. Middle East respiratory syndrome coronavirus: A comprehensive review. Front. Med. 2016, 10, 120–136. [Google Scholar] [CrossRef]
- Mostafa, A.; Kanrai, P.; Ziebuhr, J.; Pleschka, S. The PB1 segment of an influenza A virus H1N1 2009pdm isolate enhances the replication efficiency of specific influenza vaccine strains in cell culture and embryonated eggs. J. Gen. Virol. 2016, 97, 620–631. [Google Scholar] [CrossRef]
- Khanna, M.; Sharma, S.; Kumar, B.; Rajput, R. Protective immunity based on the conserved hemagglutinin stalk domain and its prospects for universal influenza vaccine development. Biomed. Res. Int. 2014. [Google Scholar] [CrossRef]
- Lynch, M. Evolution of the mutation rate. Trends Genet. 2010, 26, 345–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pritsch, M.; Ben-Khaled, N.; Chaloupka, M.; Kobold, S.; Berens-Riha, N.; Peter, A.; Liegl, G.; Schubert, S.; Hoelscher, M.; Loscher, T.; et al. Comparison of Intranasal Outer Membrane Vesicles with Cholera Toxin and Injected MF59C.1 as Adjuvants for Malaria Transmission Blocking Antigens AnAPN1 and Pfs48/45. J. Immunol. Res. 2016, 2016, 3576028. [Google Scholar] [CrossRef] [PubMed]
- Adriani, R.; Mousavi Gargari, S.L.; Nazarian, S.; Sarvary, S.; Noroozi, N. Immunogenicity of Vibrio cholerae outer membrane vesicles secreted at various environmental conditions. Vaccine 2018, 36, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.I.; Kim, M.; Jeon, J.; Han, J.K.; Kim, K.S. Overexpression of MicA induces production of OmpC-enriched outer membrane vesicles that protect against Salmonella challenge. Biochem. Biophys. Res. Commun. 2017, 490, 991–996. [Google Scholar] [CrossRef] [PubMed]
- Roy, N.; Barman, S.; Ghosh, A.; Pal, A.; Chakraborty, K.; Das, S.S.; Saha, D.R.; Yamasaki, S.; Koley, H. Immunogenicity and protective efficacy of Vibrio cholerae outer membrane vesicles in rabbit model. FEMS Immunol. Med. Microbiol. 2010, 60, 18–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Oliveira Santos, F.A.; Lincopan, N.; De Gaspari, E. Evaluation of intranasal and subcutaneous route of immunization in neonatal mice using DODAB-BF as adjuvant with outer membrane vesicles of Neisseria meningitis B. Immunobiology 2018. [Google Scholar] [CrossRef] [PubMed]
- Persson, G.; Pors, S.E.; Thofner, I.C.N.; Bojesen, A.M. Vaccination with outer membrane vesicles and the fimbrial protein FlfA offers improved protection against lesions following challenge with Gallibacterium anatis. Vet. Microbiol. 2018, 217, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, F.; Zou, J.; Wu, W.; Jing, H.; Gou, Q.; Li, H.; Gu, J.; Zou, Q.; Zhang, J. Immunization with Pseudomonas aeruginosa outer membrane vesicles stimulates protective immunity in mice. Vaccine 2018, 36, 1047–1054. [Google Scholar] [CrossRef]
- van der Pol, L.; Stork, M.; van der Ley, P. Outer membrane vesicles as platform vaccine technology. Biotechnol. J. 2015, 10, 1689–1706. [Google Scholar] [CrossRef]
- Wang, S.; Huang, W.; Li, K.; Yao, Y.; Yang, X.; Bai, H.; Sun, W.; Liu, C.; Ma, Y. Engineered outer membrane vesicle is potent to elicit HPV16E7-specific cellular immunity in a mouse model of TC-1 graft tumor. Int. J. Nanomed. 2017, 12, 6813–6825. [Google Scholar] [CrossRef]
- Bae, E.H.; Seo, S.H.; Kim, C.U.; Jang, M.S.; Song, M.S.; Lee, T.Y.; Jeong, Y.J.; Lee, M.S.; Park, J.H.; Lee, P.; et al. Bacterial Outer Membrane Vesicles Provide Broad-Spectrum Protection against Influenza Virus Infection via Recruitment and Activation of Macrophages. J. Innate Immun. 2019. [Google Scholar] [CrossRef] [PubMed]
- Raetz, C.R.; Whitfield, C. Lipopolysaccharide endotoxins. Annu. Rev. Biochem. 2002, 71, 635–700. [Google Scholar] [CrossRef] [PubMed]
- van der Ley, P.; van den Dobbelsteen, G. Next-generation outer membrane vesicle vaccines against neisseria meningitidis based on nontoxic lps mutants. Hum. Vaccines 2011, 7, 886–890. [Google Scholar] [CrossRef] [PubMed]
- Mamat, U.; Wilke, K.; Bramhill, D.; Schromm, A.B.; Lindner, B.; Kohl, T.A.; Corchero, J.L.; Villaverde, A.; Schaffer, L.; Head, S.R.; et al. Detoxifying escherichia coli for endotoxin-free production of recombinant proteins. Microbial. Cell Factories 2015, 14, 57. [Google Scholar] [CrossRef] [PubMed]
- Mamat, U.; Woodard, R.W.; Wilke, K.; Souvignier, C.; Mead, D.; Steinmetz, E.; Terry, K.; Kovacich, C.; Zegers, A.; Knox, C. Endotoxin-free protein production—ClearColi™ technology (Application Note). Nature Methods 2013, 10, 916. [Google Scholar] [CrossRef]
- Bottero, D.; Zurita, M.E.; Gaillard, M.E.; Carriquiriborde, F.; Martin Aispuro, P.; Elizagaray, M.; Bartel, E.; Castuma, C.; Hozbor, D. Outer-membrane-vesicle-associated o antigen, a crucial component for protecting against bordetella parapertussis infection. Front. Immunology 2018, 9, 2501. [Google Scholar] [CrossRef] [PubMed]
- Rappazzo, C.G.; Watkins, H.C.; Guarino, C.M.; Chau, A.; Lopez, J.L.; DeLisa, M.P.; Leifer, C.A.; Whittaker, G.R.; Putnam, D. Recombinant m2e outer membrane vesicle vaccines protect against lethal influenza a challenge in balb/c mice. Vaccine 2016, 34, 1252–1258. [Google Scholar] [CrossRef] [PubMed]
- Watkins, H.C.; Rappazzo, C.G.; Higgins, J.S.; Sun, X.; Brock, N.; Chau, A.; Misra, A.; Cannizzo, J.P.B.; King, M.R.; Maines, T.R.; et al. Safe Recombinant Outer Membrane Vesicles that Display M2e Elicit Heterologous Influenza Protection. Mol. Ther. 2017, 25, 989–1002. [Google Scholar] [CrossRef] [Green Version]
| PCR Fragment | Primer Name | Primer Sequence (5’ to 3’) | Amplicon Description | Size (bp) | Ref. |
|---|---|---|---|---|---|
| Fragment (1) | F1-NcoI-PHW | CTATTACCATGGTGATGCGGTTTTGGCAGT | Part of pMPccdB, and 5’-NCR/SP of HA from H1N1pdm | 419 | * |
| R1-Bm-H1Gi-PHW | ATATCGTCTCGCTTCTGCATTTGCGGTTGCAAATG | ||||
| Fragment (2) | F2-Bm-RBD | TATTCGTCTCAGAAGCAAAACCTTCTGGCTC | RBD/peptide linker | 741 | * |
| R2-RBD-Linker | TCCGGCGCTACCGGCGCTACCATATTCCACGCAATTGCCTA | ||||
| R2-Bm-RBD- linker | ATATCGTCTCGTGTCTCCGGCGCTACCGGCGCTACC | ||||
| Fragment (3) | F3-Bm-HA1 | TATTCGTCTCAGACACATTATGTATAGGTTA | Coding sequence of HA and 3’-NCR from H1N1pdm | 1694 | * |
| Bm-NS-890R | ATATCGTCTCGTATTAGTAGAAACAAGGGTGTTTT | Raetz et al. 2002 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shehata, M.M.; Mostafa, A.; Teubner, L.; Mahmoud, S.H.; Kandeil, A.; Elshesheny, R.; Boubak, T.A.; Frantz, R.; Pietra, L.L.; Pleschka, S.; et al. Bacterial Outer Membrane Vesicles (OMVs)-Based Dual Vaccine for Influenza A H1N1 Virus and MERS-CoV. Vaccines 2019, 7, 46. https://doi.org/10.3390/vaccines7020046
Shehata MM, Mostafa A, Teubner L, Mahmoud SH, Kandeil A, Elshesheny R, Boubak TA, Frantz R, Pietra LL, Pleschka S, et al. Bacterial Outer Membrane Vesicles (OMVs)-Based Dual Vaccine for Influenza A H1N1 Virus and MERS-CoV. Vaccines. 2019; 7(2):46. https://doi.org/10.3390/vaccines7020046
Chicago/Turabian StyleShehata, Mahmoud M., Ahmed Mostafa, Lisa Teubner, Sara H. Mahmoud, Ahmed Kandeil, Rabeh Elshesheny, Thamer A. Boubak, Renate Frantz, Luigi La Pietra, Stephan Pleschka, and et al. 2019. "Bacterial Outer Membrane Vesicles (OMVs)-Based Dual Vaccine for Influenza A H1N1 Virus and MERS-CoV" Vaccines 7, no. 2: 46. https://doi.org/10.3390/vaccines7020046










