Computational Approaches and Challenges to Developing Universal Influenza Vaccines
Abstract
1. Introduction
2. Current Approach for Influenza Vaccine Design
2.1. Selection of Circulating Influenza Viruses for Seasonal Vaccine Design
2.2. Universal Influenza Vaccine Design
3. Computational Design of Universal Influenza Vaccines
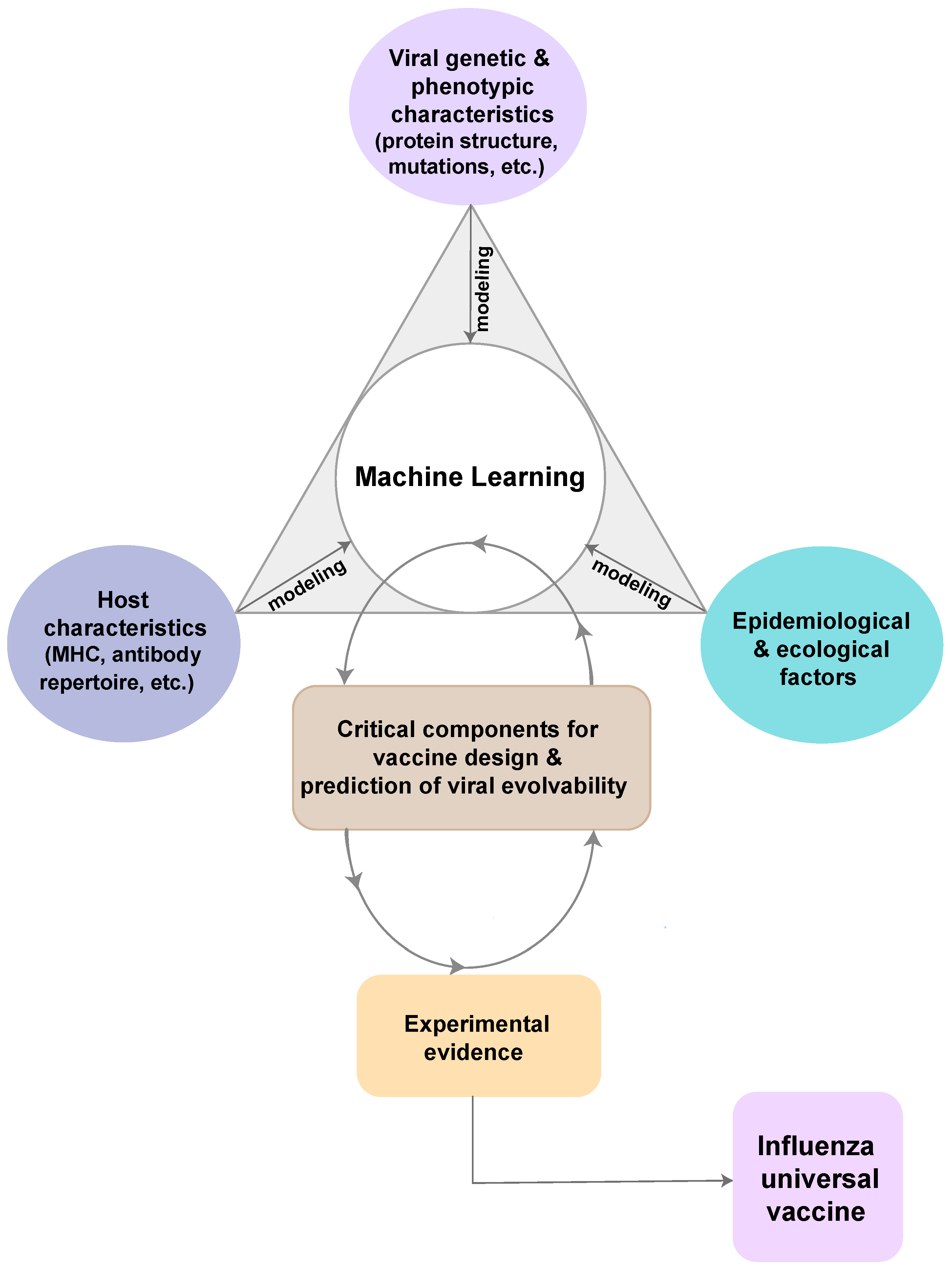
3.1. The Rationale of Computational Design Approaches
3.2. The Host
3.2.1. Immunoinformatics to Immunomics
3.2.2. Advanced Universal Influenza Vaccines in Clinical Development
3.2.3. Computational Approaches that Incorporate Host Immunological Factors
3.3. The Pathogen
3.3.1. Model-free Consensus-Based Optimized Approach
3.3.2. Phylogenetic Model-Based Approaches to Ancestral Sequence Reconstruction
3.4. The Environment
Pathogen Evolvability
4. Resources and Efforts Needed for Computational Vaccine Design
4.1. Data Collection and Sampling Efforts
4.2. Integration of Experimental Evidence and Model Development
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Erbelding, E.J.; Post, D.J.; Stemmy, E.J.; Roberts, P.C.; Augustine, A.D.; Ferguson, S.; Paules, C.I.; Graham, B.S.; Fauci, A.S. A Universal Influenza Vaccine: The Strategic Plan for the National Institute of Allergy and Infectious Diseases. J. Infect. Dis. 2018, 218, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Rappuoli, R.; Aderem, A. A 2020 vision for vaccines against HIV, tuberculosis and malaria. Nature 2011, 473, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Medina, R.A.; Stertz, S.; Manicassamy, B.; Zimmermann, P.; Sun, X.; Albrecht, R.A.; Uusi-Kerttula, H.; Zagordi, O.; Belshe, R.B.; Frey, S.E.; et al. Glycosylations in the Globular Head of the Hemagglutinin Protein Modulate the Virulence and Antigenic Properties of the H1N1 Influenza Viruses. Sci. Transl. Med. 2013, 5. [Google Scholar] [CrossRef] [PubMed]
- Ekiert, D.C.; Friesen, R.H.; Bhabha, G.; Kwaks, T.; Jongeneelen, M.; Yu, W.; Ophorst, C.; Cox, F.; Korse, H.J.; Brandenburg, B.; et al. A Highly Conserved Neutralizing Epitope on Group 2 Influenza A Viruses. Science 2011, 333, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Palatnik-de-Sousa, C.B.; Soares, I.S.; Rosa, D.S. Epitope Discovery and Synthetic Vaccine Design. Front. Immunol. 2018, 9, 826. [Google Scholar] [CrossRef] [PubMed]
- Flower, D.R.; Macdonald, I.K.; Ramakrishnan, K.; Davies, M.N.; Doytchinova, I.A. Computer aided selection of candidate vaccine antigens. Immunome Res. 2010, 6, S1. [Google Scholar] [CrossRef] [PubMed]
- Margine, I.; Hai, R.; Albrecht, R.A.; Obermoser, G.; Harrod, A.C.; Banchereau, J.; Palucka, K.; García-Sastre, A.; Palese, P.; Treanor, J.J.; et al. H3N2 Influenza Virus Infection Induces Broadly Reactive Hemagglutinin Stalk Antibodies in Humans and Mice. J. Virol. 2013, 87, 4728–4737. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Selecting Viruses for the Seasonal Influenza Vaccine | CDC. 2018. Available online: https://www.cdc.gov/flu/about/season/vaccine-selection.htm (accessed on 17 February 2019).
- Centers for Disease Control and Prevention. Antigenic Characterization | CDC. 2017. Available online: https://www.cdc.gov/flu/professionals/laboratory/antigenic.htm (accessed on 17 February 2019).
- Morris, D.H.; Gostic, K.M.; Pompei, S.; Bedford, T.; Łuksza, M.; Neher, R.A.; Grenfell, B.T.; Lässig, M.; McCauley, J.W. Predictive Modeling of Influenza Shows the Promise of Applied Evolutionary Biology. Trends Microbiol. 2018, 26, 102–118. [Google Scholar] [CrossRef]
- Wong, S.-S.; Webby, R.J. Traditional and new influenza vaccines. Clin. Microbiol. Rev. 2013, 26, 476–492. [Google Scholar] [CrossRef]
- Anderson, C.S.; Ortega, S.; Chaves, F.A.; Clark, A.M.; Yang, H.; Topham, D.J.; DeDiego, M.L. Natural and directed antigenic drift of the H1 influenza virus hemagglutinin stalk domain. Sci. Rep. 2017, 7, 14614. [Google Scholar] [CrossRef]
- Berlanda Scorza, F.; Tsvetnitsky, V.; Donnelly, J.J. Universal influenza vaccines: Shifting to better vaccines. Vaccine 2016, 34, 2926–2933. [Google Scholar] [CrossRef] [PubMed]
- Skowronski, D.M.; Janjua, N.Z.; De Serres, G.; Sabaiduc, S.; Eshaghi, A.; Dickinson, J.A.; Fonseca, K.; Winter, A.L.; Gubbay, J.B.; Krajden, M.; et al. Low 2012–13 Influenza Vaccine Effectiveness Associated with Mutation in the Egg-Adapted H3N2 Vaccine Strain Not Antigenic Drift in Circulating Viruses. PLoS ONE 2014, 9, e92153. [Google Scholar] [CrossRef] [PubMed]
- Zost, S.J.; Parkhouse, K.; Gumina, M.E.; Kim, K.; Diaz Perez, S.; Wilson, P.C.; Treanor, J.J.; Sant, A.J.; Cobey, S.; Hensley, S.E. Contemporary H3N2 influenza viruses have a glycosylation site that alters binding of antibodies elicited by egg-adapted vaccine strains. Proc. Natl. Acad. Sci. USA 2017, 114, 12578–12583. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.C.; Zost, S.J.; Thompson, A.J.; Oyen, D.; Nycholat, C.M.; McBride, R.; Paulson, J.C.; Hensley, S.E.; Wilson, I.A. A structural explanation for the low effectiveness of the seasonal influenza H3N2 vaccine. PLoS Pathog. 2017, 13, e1006682. [Google Scholar] [CrossRef] [PubMed]
- Paules, C.I.; Sullivan, S.G.; Subbarao, K.; Fauci, A.S. Chasing Seasonal Influenza—The Need for a Universal Influenza Vaccine. N. Engl. J. Med. 2018, 378, 7–9. [Google Scholar] [CrossRef]
- CDC. Seasonal Influenza Vaccine Effectiveness, 2004–2018. 2018. Available online: https://www.cdc.gov/flu/professionals/vaccination/effectiveness-studies.htm (accessed on 19 February 2019).
- Klingen, T.R.; Reimering, S.; Guzmán, C.A.; McHardy, A.C. In Silico Vaccine Strain Prediction for Human Influenza Viruses. Trends Microbiol. 2018, 26, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Webster, R.G.; Bean, W.J.; Gorman, O.T.; Chambers, T.M.; Kawaoka, Y. Evolution and ecology of influenza A viruses. Microbiol. Rev. 1992, 56, 152–179. [Google Scholar]
- Guan, Y.; Vijaykrishna, D.; Bahl, J.; Zhu, H.; Wang, J.; Smith, G.J.D. The emergence of pandemic influenza viruses. Protein Cell 2010, 1, 9–13. [Google Scholar] [CrossRef]
- Smith, G.J.D.; Bahl, J.; Vijaykrishna, D.; Zhang, J.; Poon, L.L.; Chen, H.; Webster, R.G.; Peiris, J.S.; Guan, Y. Dating the emergence of pandemic influenza viruses. Proc. Natl. Acad. Sci. USA 2009, 106, 11709–11712. [Google Scholar] [CrossRef]
- Neumann, G.; Noda, T.; Kawaoka, Y. Emergence and pandemic potential of swine-origin H1N1 influenza virus. Nature 2009, 459, 931–939. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, L.; Compans, R.W.; Wang, B.-Z. Universal Influenza Vaccines, a Dream to Be Realized Soon. Viruses 2014, 6, 1974. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.H.; Seong, B.L. Options and obstacles for designing a universal influenza vaccine. Viruses 2014, 6, 3159–3180. [Google Scholar] [CrossRef] [PubMed]
- Kirchenbaum, G.A.; Ross, T.M. Eliciting broadly protective antibody responses against influenza. Curr. Opin. Immunol. 2014, 28, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Sautto, G.A.; Kirchenbaum, G.A.; Ross, T.M. Towards a universal influenza vaccine: Different approaches for one goal. Virol. J. 2018, 15, 17. [Google Scholar] [CrossRef] [PubMed]
- Carter, D.M.; Darby, C.A.; Lefoley, B.C.; Crevar, C.J.; Alefantis, T.; Oomen, R.; Anderson, S.F.; Strugnell, T.; Cortés-Garcia, G.; Vogel, T.U. Design and Characterization of a Computationally Optimized Broadly Reactive Hemagglutinin Vaccine for H1N1 Influenza Viruses. J. Virol. 2016, 90, 4720–4734. [Google Scholar] [CrossRef] [PubMed]
- Job, E.R.; Ysenbaert, T.; Smet, A.; Christopoulou, I.; Strugnell, T.; Oloo, E.O.; Oomen, R.P.; Kleanthous, H.; Vogel, T.U.; Saelens, X. Broadened immunity against influenza by vaccination with computationally designed influenza virus N1 neuraminidase constructs. NPJ Vaccines 2018, 3, 55. [Google Scholar] [CrossRef]
- He, L.; Zhu, J. Computational tools for epitope vaccine design and evaluation. Curr. Opin. Virol. 2015, 11, 103–112. [Google Scholar] [CrossRef]
- Hurwitz, J.L. Respiratory syncytial virus vaccine development. Expert Rev. Vaccines 2011, 10, 1415–1433. [Google Scholar] [CrossRef]
- Chabas, H.; Lion, S.; Nicot, A.; Meaden, S.; van Houte, S.; Moineau, S.; Wahl, L.M.; Westra, E.R.; Gandon, S. Evolutionary emergence of infectious diseases in heterogeneous host populations. PLoS Biol. 2018, 16, e2006738. [Google Scholar] [CrossRef]
- Long, B.C.; Goldberg, T.L.; Swenson, S.L.; Erickson, G.; Scherba, G. Adaptation and Limitations of Established Hemagglutination Inhibition Assays for the Detection of Porcine Anti—Swine Influenza Virus H1N2 Antibodies. J. Vet. Diagnostic Investig. 2004, 16, 264–270. [Google Scholar] [CrossRef]
- Giles, B.M.; Bissel, S.J.; Dealmeida, D.R.; Wiley, C.A.; Ross, T.M. Antibody breadth and protective efficacy are increased by vaccination with computationally optimized hemagglutinin but not with polyvalent hemagglutinin-based H5N1 virus-like particle vaccines. Clin. Vaccine Immunol. 2012, 19, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.; McCauley, J. GISAID: Global initiative on sharing all influenza data––From vision to reality. Eurosurveillance 2017, 22, 30494. [Google Scholar] [CrossRef] [PubMed]
- NCBI Influenza virus database. Available online: https://www.ncbi.nlm.nih.gov/genomes/FLU/Database/nph-select.cgi?go=database (accessed on 28 April 2019).
- WHO. FluID—A global influenza epidemiological data sharing platform. WHO. 2017. Available online: https://www.who.int/influenza/surveillance_monitoring/fluid/en/ (accessed on 5 March 2019).
- Liljeroos, L.; Malito, E.; Ferlenghi, I.; Bottomley, M.J. Structural and Computational Biology in the Design of Immunogenic Vaccine Antigens. J. Immunol. Res. 2015, 2015, 156241. [Google Scholar] [CrossRef] [PubMed]
- Galvani, A.P. Epidemiology meets evolutionary ecology. Trends Ecol. Evol. 2003, 18, 132–139. [Google Scholar] [CrossRef]
- Nabel, G.J.; Fauci, A.S. Induction of unnatural immunity: Prospects for a broadly protective universal influenza vaccine. Nat. Med. 2010, 16, 1389–1391. [Google Scholar] [CrossRef] [PubMed]
- Giles, B.M.; Ross, T.M. A computationally optimized broadly reactive antigen (COBRA) based H5N1 VLP vaccine elicits broadly reactive antibodies in mice and ferrets. Vaccine 2011, 29, 3043–3054. [Google Scholar] [CrossRef] [PubMed]
- Crevar, C.J.; Carter, D.M.; Lee, K.Y.J.; Ross, T.M. Cocktail of H5N1 COBRA HA vaccines elicit protective antibodies against H5N1 viruses from multiple clades. Hum. Vaccin. Immunother. 2015, 11, 572–583. [Google Scholar] [CrossRef]
- Giles, B.M.; Crevar, C.J.; Carter, D.M.; Bissel, S.J.; Schultz-Cherry, S.; Wiley, C.A.; Ross, T.M. A Computationally Optimized Hemagglutinin Virus-Like Particle Vaccine Elicits Broadly Reactive Antibodies that Protect Nonhuman Primates from H5N1 Infection. J. Infect. Dis. 2012, 205, 1562–1570. [Google Scholar] [CrossRef]
- Ducatez, M.F.; Bahl, J.; Griffin, Y.; Stigger-Rosser, E.; Franks, J.; Barman, S.; Vijaykrishna, D.; Webb, A.; Guan, Y.; Webster, R.G. Feasibility of reconstructed ancestral H5N1 influenza viruses for cross-clade protective vaccine development. Proc. Natl. Acad. Sci. USA 2011, 108, 349–354. [Google Scholar] [CrossRef]
- Wong, T.M.; Allen, J.D.; Bebin-Blackwell, A.G.; Carter, D.M.; Alefantis, T.; DiNapoli, J.; Kleanthous, H.; Ross, T.M. Computationally Optimized Broadly Reactive Hemagglutinin Elicits Hemagglutination Inhibition Antibodies against a Panel of H3N2 Influenza Virus Cocirculating Variants. J. Virol. 2017, 91, e01581-17. [Google Scholar] [CrossRef]
- Baum, D.A.; Smith, S.D. Tree Thinking: An. Introduction to Phylogenetic Biology; Roberts and Co.: Greenwood Village, CO, USA, 2012. [Google Scholar]
- Lemey, P.; Rambaut, A.; Drummond, A.J.; Suchard, M.A. Bayesian phylogeography finds its roots. PLoS Comput. Biol. 2009, 5, e1000520. [Google Scholar] [CrossRef] [PubMed]
- King, B.; Lee, M.S.Y. Ancestral State Reconstruction, Rate Heterogeneity, and the Evolution of Reptile Viviparity. Syst. Biol. 2015, 64, 532–544. [Google Scholar] [CrossRef] [PubMed]
- Brusic, V. From immunoinformatics to immunomics. J. Bioinform. Comput. Biol. 2003, 1, 179–181. [Google Scholar] [CrossRef] [PubMed]
- Sette, A.; Fleri, W.; Peters, B.; Sathiamurthy, M.; Bui, H.-H.; Wilson, S. A roadmap for the immunomics of category A-C pathogens. Immunity 2005, 22, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Brusic, V.; Petrovsky, N. Immunoinformatics and its relevance to understanding human immune disease. Expert Rev. Clin. Immunol. 2005, 1, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Rappuoli, R. Reverse vaccinology. Curr. Opin. Microbiol. 2000, 3, 445–450. [Google Scholar] [CrossRef]
- Sette, A.; Rappuoli, R. Reverse Vaccinology: Developing Vaccines in the Era of Genomics. Immunity 2010, 33, 530–541. [Google Scholar] [CrossRef] [PubMed]
- De Groot, A.S. Immunomics: Discovering new targets for vaccines and therapeutics. Drug Discov. Today 2006, 11, 203–209. [Google Scholar] [CrossRef]
- Potocnakova, L.; Bhide, M.; Pulzova, L.B. An Introduction to B-Cell Epitope Mapping and In Silico Epitope Prediction. J. Immunol. Res. 2016, 2016, 1–11. [Google Scholar] [CrossRef]
- Patronov, A.; Doytchinova, I. T-cell epitope vaccine design by immunoinformatics. Open Biol. 2013, 3, 120139. [Google Scholar] [CrossRef]
- Greenbaum, J.A.; Andersen, P.H.; Blythe, M.; Bui, H.H.; Cachau, R.E.; Crowe, J.; Davies, M.; Kolaskar, A.S.; Lund, O.; Morrison, S.; et al. Towards a consensus on datasets and evaluation metrics for developing B-cell epitope prediction tools. J. Mol. Recognit. 2007, 20, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Trincado, J.L.; Gomez-Perosanz, M.; Reche, P.A. Fundamentals and Methods for T- and B-Cell Epitope Prediction. J. Immunol. Res. 2017, 2017, 1–14. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Rappuoli, R.; De Groot, A.S.; Chen, R.T. Emerging Vaccine Informatics. J. Biomed. Biotechnol. 2010, 2010, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Tomar, N.; De, R.K. Immunoinformatics: An integrated scenario. Immunology 2010, 131, 153–168. [Google Scholar] [CrossRef] [PubMed]
- Backert, L.L.; Kohlbacher, O. Immunoinformatics and epitope prediction in the age of genomic medicine. Genome Med. 2015, 7, 119. [Google Scholar] [CrossRef]
- Hegde, N.R.; Gauthami, S.; Sampath Kumar, H.M.; Bayry, J. The use of databases, data mining and immunoinformatics in vaccinology: Where are we? Expert Opin. Drug Discov. 2018, 13, 117–130. [Google Scholar] [CrossRef]
- Luo, H.; Ye, H.; Ng, H.W.; Shi, L.; Tong, W.; Mendrick, D.L.; Hong, H. Machine Learning Methods for Predicting HLA-Peptide Binding Activity. Bioinform. Biol. Insights 2015, 9. [Google Scholar] [CrossRef]
- Bui, H.-H.; Sidney, J.; Dinh, K.; Southwood, S.; Newman, M.J.; Sette, A. Predicting population coverage of T-cell epitope-based diagnostics and vaccines. BMC Bioinformatics 2006, 7, 153. [Google Scholar] [CrossRef]
- Molero-Abraham, M.; Lafuente, E.M.; Flower, D.R.; Reche, P.A. Selection of conserved epitopes from hepatitis C virus for pan-populational stimulation of T-cell responses. Clin. Dev. Immunol. 2013, 2013, 601943. [Google Scholar] [CrossRef]
- McMichael, A.J.; Gotch, F.M.; Noble, G.R.; Beare, P.A.S. Cytotoxic T-Cell Immunity to Influenza. N. Engl. J. Med. 1983, 309, 13–17. [Google Scholar] [CrossRef]
- McKinstry, K.K.; Strutt, T.M.; Swain, S.L. Hallmarks of CD4 T cell immunity against influenza. J. Intern. Med. 2011, 269, 507–518. [Google Scholar] [CrossRef] [PubMed]
- La Gruta, N.L.; Turner, S.J. T cell mediated immunity to influenza: Mechanisms of viral control. Trends Immunol. 2014, 35, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Effros, R.B.; Doherty, P.C.; Gerhard, W.; Bennink, J. Generation of both cross-reactive and virus-specific T-cell populations after immunization with serologically distinct influenza A viruses. J. Exp. Med. 1977, 145, 557–568. [Google Scholar] [CrossRef] [PubMed]
- Kreijtz, J.H.; Bodewes, R.; van Amerongen, G.; Kuiken, T.; Fouchier, R.A.; Osterhaus, A.D.; Rimmelzwaan, G.F. Primary influenza A virus infection induces cross-protective immunity against a lethal infection with a heterosubtypic virus strain in mice. Vaccine 2007, 25, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Zweerink, H.J.; Courtneidge, S.A.; Skehel, J.J.; Crumpton, M.J.; Askonas, B.A. Cytotoxic T cells kill influenza virus infected cells but do not distinguish between serologically distinct type A viruses. Nature 1977, 267, 354–356. [Google Scholar] [CrossRef] [PubMed]
- Duvvuri, V.R.; Moghadas, S.M.; Guo, H.; Duvvuri, B.; Heffernan, J.M.; Fisman, D.N.; Wu, G.E.; Wu, J. Original Article: Highly conserved cross-reactive CD4+ T-cell HA-epitopes of seasonal and the 2009 pandemic influenza viruses. Influenza Other Respi. Viruses 2010, 4, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Duvvuri, V.R.; Duvvuri, B.; Jamnik, V.; Gubbay, J.B.; Wu, J.; Wu, G.E. T cell memory to evolutionarily conserved and shared hemagglutinin epitopes of H1N1 viruses: A pilot scale study. BMC Infect. Dis. 2013, 13, 204. [Google Scholar] [CrossRef] [PubMed]
- De Groot, A.S.; Ardito, M.; McClaine, E.M.; Moise, L.; Martin, W.D. Immunoinformatic comparison of T-cell epitopes contained in novel swine-origin influenza A (H1N1) virus with epitopes in 2008–2009 conventional influenza vaccine. Vaccine 2009, 27, 5740–5747. [Google Scholar] [CrossRef]
- Ge, X.; Tan, V.; Bollyky, P.L.; Standifer, N.E.; James, E.A.; Kwok, W.W. Assessment of Seasonal Influenza A Virus-Specific CD4 T-Cell Responses to 2009 Pandemic H1N1 Swine-Origin Influenza A Virus. J. Virol. 2010, 84, 3312–3319. [Google Scholar] [CrossRef]
- Greenbaum, J.A.; Kotturi, M.F.; Kim, Y.; Oseroff, C.; Vaughan, K.; Salimi, N.; Vita, R.; Ponomarenko, J.; Scheuermann, R.H.; Sette, A.; et al. Pre-existing immunity against swine-origin H1N1 influenza viruses in the general human population. Proc. Natl. Acad. Sci. USA 2009, 106, 20365–20370. [Google Scholar] [CrossRef]
- Weinfurter, J.T.; Brunner, K.; Capuano, S.V., 3rd; Li, C.; Broman, K.W.; Kawaoka, Y.; Friedrich, T.C. Cross-Reactive T Cells Are Involved in Rapid Clearance of 2009 Pandemic H1N1 Influenza Virus in Nonhuman Primates. PLoS Pathog. 2011, 7, e1002381. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, T.M.; Li, C.K.; Chui, C.S.; Huang, A.K.; Perkins, M.; Liebner, J.C.; Lambkin-Williams, R.; Gilbert, A.; Oxford, J.; Nicholas, B.; et al. Preexisting influenza-specific CD4+ T cells correlate with disease protection against influenza challenge in humans. Nat. Med. 2012, 18, 74–280. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov Identifier:NCT01265914 A Study to Evaluate the Safety, Tolerability and Immunogenicity of a Universal Influenza A Vaccine. 2010. Available online: https://clinicaltrials.gov/ct2/show/NCT01265914 (accessed on 20 February 2019).
- Francis, J.N.; Bunce, C.J.; Horlock, C.; Watson, J.M.; Warrington, S.J.; Georges, B.; Brown, C.B. A novel peptide-based pan-influenza A vaccine: A double blind, randomised clinical trial of immunogenicity and safety. Vaccine 2015, 33, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Pleguezuelos, O.; Robinson, S.; Stoloff, G.A.; Caparrós-Wanderley, W. Synthetic Influenza vaccine (FLU-v) stimulates cell mediated immunity in a double-blind, randomised, placebo-controlled Phase I trial. Vaccine 2012, 30, 4655–4660. [Google Scholar] [CrossRef] [PubMed]
- Pleguezuelos, O.; Robinson, S.; Fernández, A.; Stoloff, G.A.; Mann, A.; Gilbert, A.; Balaratnam, G.; Wilkinson, T.; Lambkin-Williams, R.; Oxford, J.; et al. A Synthetic Influenza Virus Vaccine Induces a Cellular Immune Response That Correlates with Reduction in Symptomatology and Virus Shedding in a Randomized Phase Ib Live-Virus Challenge in Humans. Clin. Vaccine Immunol. 2015, 22, 828–835. [Google Scholar] [CrossRef] [PubMed]
- van Doorn, E.; Pleguezuelos, O.; Liu, H.; Fernandez, A.; Bannister, R.; Stoloff, G.; Oftung, F.; Norley, S.; Huckriede, A.; Frijlink, H.W.; et al. Evaluation of the immunogenicity and safety of different doses and formulations of a broad spectrum influenza vaccine (FLU-v) developed by SEEK: Study protocol for a single-center, randomized, double-blind and placebo-controlled clinical phase IIb trial. BMC Infect. Dis. 2017, 17, 241. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov Identifier: NCT03450915. A Pivotal Trial to Assess the Safety and Clinical Efficacy of the M-001 as a Standalone Universal Flu Vaccine. Available online: https://clinicaltrials.gov/ct2/show/NCT03450915?term=epitope&cond=Influenza&rank=6 (accessed on 20 February 2019).
- Gottlieb, T.; Ben-Yedidia, T. Epitope-based approaches to a universal influenza vaccine. J. Autoimmun. 2014, 54, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Atsmon, J.; Caraco, Y.; Ziv-Sefer, S.; Shaikevich, D.; Abramov, E.; Volokhov, I.; Bruzil, S.; Haima, K.Y.; Gottlieb, T.; Ben-Yedidia, T. Priming by a novel universal influenza vaccine (Multimeric-001)—A gateway for improving immune response in the elderly population. Vaccine 2014, 32, 5816–5823. [Google Scholar] [CrossRef]
- Georgiou, G.; Ippolito, G.C.; Beausang, J.; Busse, C.E.; Wardemann, H.; Quake, S.R. The promise and challenge of high-throughput sequencing of the antibody repertoire. Nat. Biotechnol. 2014, 32, 158–168. [Google Scholar] [CrossRef]
- Six, A.; Bellier, B.; Thomas-Vaslin, V.; Klatzmann, D. Systems biology in vaccine design. Microb. Biotechnol. 2012, 5, 295–304. [Google Scholar] [CrossRef]
- Yermanos, A.D.; Dounas, A.K.; Stadler, T.; Oxenius, A.; Reddy, S.T. Tracing Antibody Repertoire Evolution by Systems Phylogeny. Front. Immunol. 2018, 9, 2149. [Google Scholar] [CrossRef] [PubMed]
- Koff, W.C.; Burton, D.R.; Johnson, P.R.; Walker, B.D.; King, C.R.; Nabel, G.J.; Ahmed, R.; Bhan, M.K.; Plotkin, S.A. Accelerating Next-Generation Vaccine Development for Global Disease Prevention. Science 2013, 340, 1232910. [Google Scholar] [CrossRef] [PubMed]
- Koff, W.C.; Gust, I.D.; Plotkin, S.A. Toward a Human Vaccines Project. Nat. Immunol. 2014, 15, 589–592. [Google Scholar] [CrossRef] [PubMed]
- Sok, D.; Laserson, U.; Laserson, J.; Liu, Y.; Vigneault, F.; Julien, J.P.; Briney, B.; Ramos, A.; Saye, K.F.; Le, K.; et al. The Effects of Somatic Hypermutation on Neutralization and Binding in the PGT121 Family of Broadly Neutralizing HIV Antibodies. PLoS Pathog. 2013, 9, e1003754. [Google Scholar] [CrossRef]
- Wu, X.; Zhou, T.; Zhu, J.; Zhang, B.; Georgiev, I.; Wang, C.; Chen, X.; Longo, N.S.; Louder, M.; McKee, K.; et al. Focused Evolution of HIV-1 Neutralizing Antibodies Revealed by Structures and Deep Sequencing. Science 2011, 333, 1593–1602. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-G.; Johnson, T.A.; Su, J.Q.; Qiao, M.; Guo, G.X.; Stedtfeld, R.D.; Hashsham, S.A.; Tiedje, J.M. Diverse and abundant antibiotic resistance genes in Chinese swine farms. Proc. Natl. Acad. Sci. USA 2013, 110, 3435–3440. [Google Scholar] [CrossRef] [PubMed]
- María, R.R.; Arturo, C.J.; Alicia, J.A.; Paulina, M.G.; Gerardo, A.O. The Impact of Bioinformatics on Vaccine Design and Development. Vaccines 2017. [Google Scholar] [CrossRef]
- Van Regenmortel, M.H.V. Structure-Based Reverse Vaccinology Failed in the Case of HIV Because it Disregarded Accepted Immunological Theory. Int. J. Mol. Sci. 2016, 17, 9. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, C.; Song, W. Rational derivation, extension, and cyclization of self-inhibitory peptides to target TGF-β/BMP signaling in ONFH. Amino Acids 2017, 49, 283–290. [Google Scholar] [CrossRef]
- Kmiecik, S.; Gront, D.; Kolinski, M.; Wieteska, L.; Dawid, A.E.; Kolinski, A. Coarse-Grained Protein Models and Their Applications. Chem. Rev. 2016, 116, 7898–7936. [Google Scholar] [CrossRef]
- Laddy, D.J.; Yan, J.; Corbitt, N.; Kobasa, D.; Kobinger, G.P.; Weiner, D.B. Immunogenicity of novel consensus-based DNA vaccines against avian influenza. Vaccine 2007, 25, 2984–2989. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-Y.; Yeh, Y.C.; Yang, Y.C.; Chou, C.; Liu, M.T.; Wu, H.S.; Chan, J.T.; Hsiao, P.W. Mammalian Expression of Virus-Like Particles for Advanced Mimicry of Authentic Influenza Virus. PLoS ONE 2010, 5, e9784. [Google Scholar] [CrossRef] [PubMed]
- Bright, R.A.; Carter, D.M.; Crevar, C.J.; Toapanta, F.R.; Steckbeck, J.D.; Cole, K.S.; Kumar, N.M.; Pushko, P.; Smith, G.; Tumpey, T.M.; et al. Cross-Clade Protective Immune Responses to Influenza Viruses with H5N1 HA and NA Elicited by an Influenza Virus-Like Particle. PLoS ONE 2008, 3, e1501. [Google Scholar] [CrossRef] [PubMed]
- Ben-Dor, A.; Lancia, G.; Ravi, R.; Perone, J. Banishing Bias from Consensus Sequences; Springer: Berlin/Heidelberg, Germany, 1997; pp. 247–261. [Google Scholar]
- Thornton, J.W. Resurrecting ancient genes: Experimental analysis of extinct molecules. Nat. Rev. Genet. 2004, 5, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.A.; Hart, K.M.; Harms, M.J.; Marqusee, S. Evolutionary trend toward kinetic stability in the folding trajectory of RNases H. Proc. Natl. Acad. Sci. USA 2016, 113, 13045–13050. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Nielsen, R.; Goldman, N.; Pedersen, A.M. Codon-substitution models for heterogeneous selection pressure at amino acid sites. Genetics 2000, 155, 431–449. [Google Scholar] [PubMed]
- Huelsenbeck, J.P.; Ronquist, F.; Nielsen, R.; Bollback, J.P. Bayesian inference of phylogeny and its impact on evolutionary biology. Scienc 2001, 294, 2310–2314. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Pei, J.; Grishin, N.V. Reconstruction of ancestral protein sequences and its applications. BMC E Biol. 2004, 4, 33. [Google Scholar]
- Baele, G.; Suchard, M.A.; Rambaut, A.; Lemey, P. Emerging Concepts of Data Integration in Pathogen Phylodynamics. Syst. Biol. 2017, 66, e47–e65. [Google Scholar] [CrossRef]
- Kirkpatrick, E.; Qiu, X.; Wilson, P.C.; Bahl, J.; Krammer, F. The influenza virus hemagglutinin head evolves faster than the stalk domain. Sci. Rep. 2018, 8, 10432. [Google Scholar] [CrossRef]
- Qiu, X.; Bahl, J. Structurally informed evolutionary models improve phylogenetic reconstruction for emerging, seasonal, and pandemic influenza viruses. bioRxiv 2017. [Google Scholar] [CrossRef]
- Kleinman, C.L.; Rodrigue, N.; Lartillot, N.; Philippe, H. Statistical Potentials for Improved Structurally Constrained Evolutionary Models. Mol. Biol. Evol. 2010, 27, 1546–1560. [Google Scholar] [CrossRef] [PubMed]
- Bloom, J.D. An Experimentally Informed Evolutionary Model Improves Phylogenetic Fit to Divergent Lactamase Homologs. Mol. Biol. Evol. 2014, 31, 2753–2769. [Google Scholar] [CrossRef] [PubMed]
- Booker, T.R.; Keightley, P.D. Understanding the factors that shape patterns of nucleotide diversity in the house mouse genome. Mol. Biol. Evol. 2018, 35, 2971–2988. [Google Scholar] [CrossRef] [PubMed]
- Bloom, J.D. An Experimentally Determined Evolutionary Model Dramatically Improves Phylogenetic Fit. Mol. Biol. Evol. 2014, 31, 1956–1978. [Google Scholar] [CrossRef]
- Fowler, D.M.; Araya, C.L.; Fleishman, S.J.; Kellogg, E.H.; Stephany, J.J.; Baker, D.; Fields, S. High-resolution mapping of protein sequence-function relationships. Nat. Methods 2010, 7, 741–746. [Google Scholar] [CrossRef]
- Araya, C.L.; Fowler, D.M. Deep mutational scanning: Assessing protein function on a massive scale. Trends Biotechnol. 2011, 29, 435–442. [Google Scholar] [CrossRef]
- Traxlmayr, M.W.; Hasenhindl, C.; Hackl, M.; Stadlmayr, G.; Rybka, J.D.; Borth, N.; Grillari, J.; Rüker, F.; Obinger, C. Construction of a Stability Landscape of the CH3 Domain of Human IgG1 by Combining Directed Evolution with High Throughput Sequencing. J. Mol. Biol. 2012, 423, 397–412. [Google Scholar] [CrossRef]
- Melamed, D.; Young, D.L.; Gamble, C.E.; Miller, C.R.; Fields, S. Deep mutational scanning of an RRM domain of the Saccharomyces cerevisiae poly(A)-binding protein. RNA 2013, 19, 1537–1551. [Google Scholar] [CrossRef]
- Roscoe, B.P.; Thayer, K.M.; Zeldovich, K.B.; Fushman, D.; Bolon, D.N.A. Analyses of the Effects of All Ubiquitin Point Mutants on Yeast Growth Rate. J. Mol. Biol. 2013, 425, 1363–1377. [Google Scholar] [CrossRef]
- Firnberg, E.; Labonte, J.W.; Gray, J.J.; Ostermeier, M. A comprehensive, high-resolution map of a gene’s fitness landscape. Mol. Biol. Evol. 2014, 31, 1581–1592. [Google Scholar] [CrossRef] [PubMed]
- Hanson-Smith, V.; Kolaczkowski, B.; Thornton, J.W. Robustness of ancestral sequence reconstruction to phylogenetic uncertainty. Mol. Biol. Evol. 2010, 27, 1988–1999. [Google Scholar] [CrossRef] [PubMed]
- Holmes, E.C. What can we predict about viral evolution and emergence? Curr. Opin. Virol. 2013, 3, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Kirschner, M.; Gerhart, J. Evolvability. Proc. Natl. Acad. Sci. USA 1998, 95, 8420–8427. [Google Scholar] [CrossRef] [PubMed]
- Volz, E.M.; Koelle, K.; Bedford, T. Viral Phylodynamics. PLoS Comput. Biol. 2013, 9, e1002947. [Google Scholar] [CrossRef]
- Grenfell, B.T.; Pybus, O.G.; Gog, J.R.; Wood, J.L.; Daly, J.M.; Mumford, J.A.; Holmes, E.C. Unifying the Epidemiological and Evolutionary Dynamics of Pathogens. Science 2004, 303, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Visher, E.; Whitefield, S.E.; McCrone, J.T.; Fitzsimmons, W.; Lauring, A.S. The Mutational Robustness of Influenza A Virus. PLOS Pathog. 2016, 12, e1005856. [Google Scholar] [CrossRef]
- Thyagarajan, B.; Bloom, J.D. The inherent mutational tolerance and antigenic evolvability of influenza hemagglutinin. Elife 2014, 3. [Google Scholar] [CrossRef]
- Bloom, J.D.; Gong, L.I.; Baltimore, D. Permissive Secondary Mutations Enable the Evolution of Influenza Oseltamivir Resistance. Science 2010, 328, 1272–1275. [Google Scholar] [CrossRef] [PubMed]
- Olson, S.H.; Parmley, J.; Soos, C.; Gilbert, M.; Latorre-Margalef, N.; Hall, J.S.; Hansbro, P.M.; Leighton, F.; Munster, V.; Joly, D. Sampling Strategies and Biodiversity of Influenza A Subtypes in Wild Birds. PLoS ONE 2014, 9, e90826. [Google Scholar] [CrossRef]
- Klingen, T.R.; Reimering, S.; Loers, J.; Mooren, K.; Klawonn, F.; Krey, T.; Gabriel, G.; McHardy, A.C. Sweep Dynamics (SD) plots: Computational identification of selective sweeps to monitor the adaptation of influenza A viruses. Sci. Rep. 2018, 8, 373. [Google Scholar] [CrossRef] [PubMed]
- Ampofo, W.K.; Azziz-Baumgartner, E.; Bashir, U.; Cox, N.J.; Fasce, R.; Giovanni, M.; Grohmann, G.; Huang, S.; Katz, J.; Mironenko, A.; et al. Strengthening the influenza vaccine virus selection and development process. Vaccine 2015, 33, 4368–4382. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine (US) Forum on Microbial Threats. The Domestic and International Impacts of the 2009-H1N1 Influenza A Pandemic: Global Challenges, Global Solutions: Workshop Summary; National Academies Press (US): Washington, DC, USA, 2010. [Google Scholar]
- Hoye, B.J.; Munster, V.J.; Nishiura, H.; Klaassen, M.; Fouchier, R.A. Surveillance of wild birds for avian influenza virus. Emerg. Infect. Dis. 2010, 16, 1827–1834. [Google Scholar] [CrossRef] [PubMed]
- Squires, R.B.; Noronha, J.; Hunt, V.; García-Sastre, A.; Macken, C.; Baumgarth, N.; Suarez, D.; Pickett, B.E.; Zhang, Y.; Larsen, C.N. Influenza research database: An integrated bioinformatics resource for influenza research and surveillance. Influenza Other Respi. Viruses 2012, 6, 404–416. [Google Scholar] [CrossRef] [PubMed]
- Gunnarsson, G.; Latorre-Margalef, N.; Hobson, K.A.; Van Wilgenburg, S.L.; Elmberg, J.; Olsen, B.; Fouchier, R.A.M.; Waldenström, J. Disease dynamics and bird migration––Linking mallards Anas platyrhynchos and subtype diversity of the influenza A virus in time and space. PLoS ONE 2012, 7, e35679. [Google Scholar] [CrossRef] [PubMed]
- Elbe, S.; Buckland-Merrett, G. Data, disease and diplomacy: GISAID’s innovative contribution to global health. Glob. Challenges 2017, 1, 33–46. [Google Scholar] [CrossRef]
- Baele, G.; Lemey, P.; Bedford, T.; Rambaut, A.; Suchard, M.A.; Alekseyenko, A.V. Improving the accuracy of demographic and molecular clock model comparison while accomodating phylogenetic uncertainty. Mol. Biol. Evol. 2012, 29, 2157–2167. [Google Scholar] [CrossRef]
- Dudas, G.; Carvalho, L.M.; Rambaut, A.; Bedford, T. MERS-CoV spillover at the camel-human interface. Elife 2018, 7. [Google Scholar] [CrossRef]
- Mu, N.F.; Rasmussen, D.A.; Stadler, T. The Structured Coalescent and Its Approximations. Mol. Biol. Evol. 2017, 34, 2970–2981. [Google Scholar]
- Duchene, S.; Bouckaert, R.; Duchene, D.A.; Stadler, T.; Drummond, A.J. Phylodynamic Model Adequacy Using Posterior Predictive Simulations. Syst. Biol. 2019, 68, 358–364. [Google Scholar] [CrossRef]
- Samuel, A.L. Some Studies in Machine Learning Using the Game of Checkers. IBM J. Res. Dev. 1959, 3, 210–229. [Google Scholar] [CrossRef]
- Bunker, R.P.; Thabtah, F. A machine learning framework for sport result prediction. Appl. Comput. Informatics 2019, 15, 27–33. [Google Scholar] [CrossRef]
- Fritz, B.A.; Chen, Y.; Murray-Torres, T.M.; Gregory, S.; Ben Abdallah, A.; Kronzer, A.; McKinnon, S.L.; Budelier, T.; Helsten, D.L.; Wildes, T.S.; et al. Using machine learning techniques to develop forecasting algorithms for postoperative complications: Protocol for a retrospective study. BMJ Open 2018, 8, e020124. [Google Scholar] [CrossRef] [PubMed]
- Zitnik, M.; Nguyen, F.; Wang, B.; Leskovec, J.; Goldenberg, A.; Hoffman, M.M. Machine learning for integrating data in biology and medicine: Principles, practice, and opportunities. Inf. Fusion 2019, 50, 71–91. [Google Scholar] [CrossRef] [PubMed]
- Salama, M.A.; Hassanien, A.E.; Mostafa, A. The prediction of virus mutation using neural networks and rough set techniques. EURASIP J. Bioinform. Syst. Biol. 2016, 2016, 10. [Google Scholar] [CrossRef] [PubMed]
- Shim, H. Feature Learning of Virus Genome Evolution With the Nucleotide Skip-Gram Neural Network. Evol. Bioinforma 2019, 15. [Google Scholar] [CrossRef] [PubMed]

| Approach | Conceptual Design | Evidence-Level | Advantages | Disadvantages | Examples |
|---|---|---|---|---|---|
| Consensus-based optimized approach | Figure 2A | Pre-clinical | (1) Efficiently generate a potentially full profile of conserved immunogenicity in viral genome; (2) Induce broad HA inhibition antibody titers that are cross-reactive with diverse strains within the same subtype; (3) Neutralize the receptor binding sites to prevent influenza disease with a clear path towards clinical proof of correlation for protective efficacy in humans | (1) Biased viral samples may not generate consensus sequences that represent full profile of conserved immunogenicity; (2) Large efforts on surveillance data required | Pre-clinical tests on H1, H3 and H5 HA [28,41,42,43,45] |
| Ancestral sequence reconstruction | Figure 2B | Pre-clinical | (1) Induce broad cross-reactive protection within highly diverse influenza subtype (2) Account for sampling bias and the variability of substitution rates among sites; (3) Potentially avoid the detrimental effects of antigenic drift with ancestral sequences; (4) Incorporate protein functional and structural domains | (1) More sophisticated and advanced models to incorporate protein domains are still under development; (2) Experimental data on protein function is needed | Pre-clinical tests on ancestral sequence of H5N1 HA and NA [44] |
| Immunomics | Figure 2C | Pre-clinical & Clinical | (1) Account for the heterogeneity of the major histocompatibility complex (MHC) in host; (2) Protections and viral clearance from T-cell response has been distinctively tested | (1) Indirect estimation on epitope affinity to MHC; (2) To keep conformational epitopes to be function when designed into vaccine can be challenge | FP-01.1 Flu-v Multimeric-001 See Table 2 for details |
| Vaccine | Company | Projects | Clinical Phase | Clinical Trial Registration# | Reference | ||
|---|---|---|---|---|---|---|---|
| I | II | III | |||||
| FP-01.1 | Immune Targeting Systems Ltd., London, UK. | FP-01.1 | completed | completed | NCT01265914, NCT01677676, NCT02071329 | Francis 2015 [80] | |
| FP-01.1-Adjuvant | completed | NCT01677676 | unpublished | ||||
| FP-01.1 + seasonal TIV + FP-01.1-Adjuvant | completed | NCT01701752 | unpublished | ||||
| Flu-v | PepTcell Limited | Flu-v | completed | NCT01226758, NCT01181336 | Pleguezuelos 2015 [82] | ||
| adjuvanted Flu-v | completed | NCT03180801, NCT02962908 | van Doorn 2017 [83] | ||||
| Multimeric-001 (M-001) | BiondVax Pharmaceuticals Ltd | M-001 | completed | completed | NCT01146119, NCT01010737 | Atsmon 2014 [86] | |
| M-001 (prime) + seasonal TIV vaccine (boost) | completed | completed | NCT03058692, NCT01419925, NCT02293317 | Atsmon 2014 [86] | |||
| M-001 (prime) + H5N1 vaccine (boost) | completed | completed | NCT02691130 | unpublished | |||
| M-001 as standalone vaccine | ongoing | NCT03450915 | unpublished | ||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, X.; Duvvuri, V.R.; Bahl, J. Computational Approaches and Challenges to Developing Universal Influenza Vaccines. Vaccines 2019, 7, 45. https://doi.org/10.3390/vaccines7020045
Qiu X, Duvvuri VR, Bahl J. Computational Approaches and Challenges to Developing Universal Influenza Vaccines. Vaccines. 2019; 7(2):45. https://doi.org/10.3390/vaccines7020045
Chicago/Turabian StyleQiu, Xueting, Venkata R. Duvvuri, and Justin Bahl. 2019. "Computational Approaches and Challenges to Developing Universal Influenza Vaccines" Vaccines 7, no. 2: 45. https://doi.org/10.3390/vaccines7020045
APA StyleQiu, X., Duvvuri, V. R., & Bahl, J. (2019). Computational Approaches and Challenges to Developing Universal Influenza Vaccines. Vaccines, 7(2), 45. https://doi.org/10.3390/vaccines7020045





