Safety and Immunogenicity of the Heterosubtypic Influenza A Vaccine MVA-NP+M1 Manufactured on the AGE1.CR.pIX Avian Cell Line
Abstract
:1. Introduction
2. Materials and Methods
2.1. MVA-NP+M1 (AGE1.CR.pIX) Vaccine
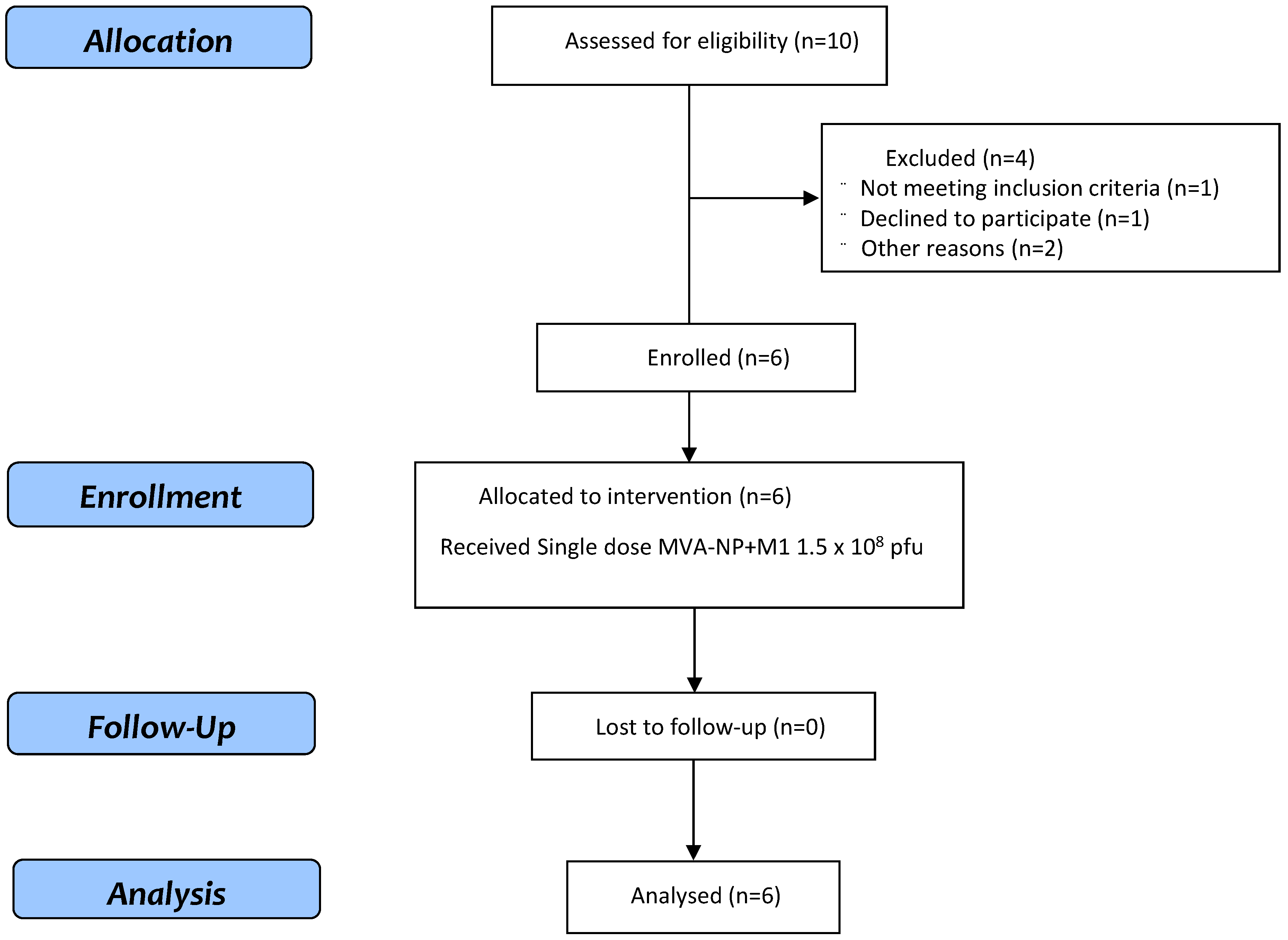
2.2. Study Design and Participants
2.3. Study Procedures
2.4. Endpoints
2.5. Ex-Vivo IFN-γ ELISpot
2.6. Data Presentation and Statistical Analysis
3. Results
3.1. Study Population
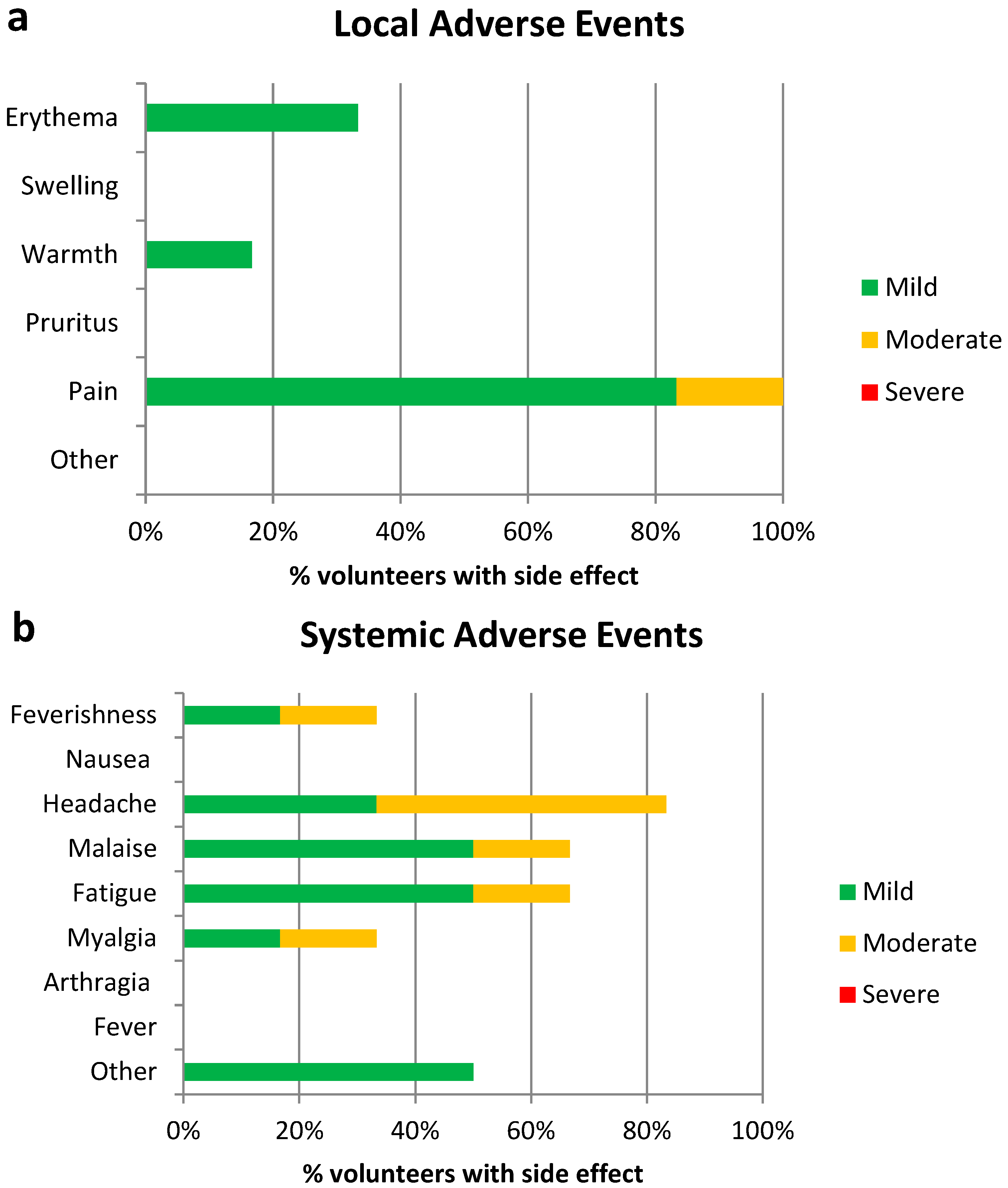
3.2. Vaccine Safety
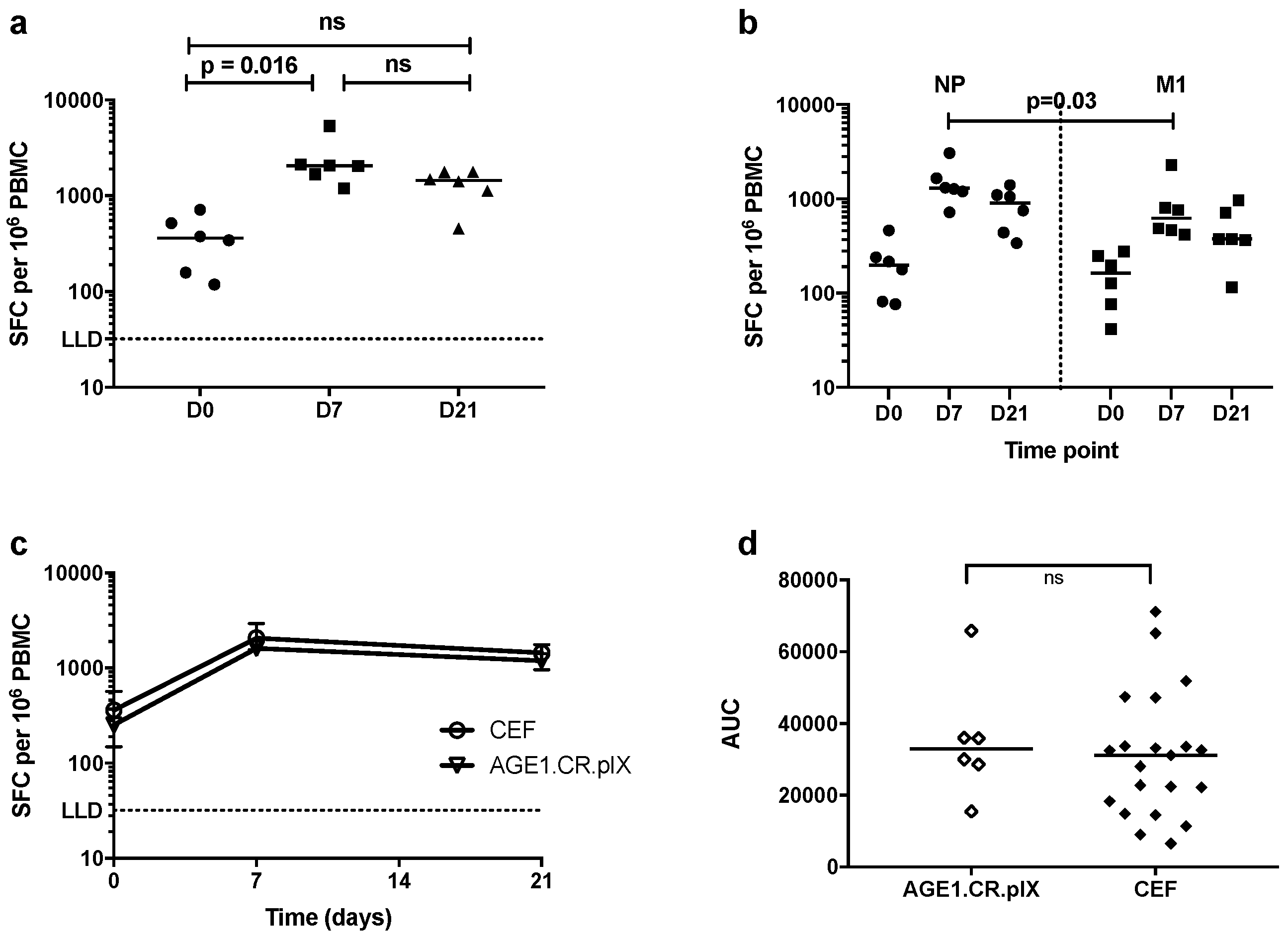
3.3. T-Cell Responses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Iuliano, A.D.; Roguski, K.M.; Chang, H.H.; Muscatello, D.J.; Palekar, R.; Tempia, S.; Cohen, C.; Gran, J.M.; Schanzer, D.; Cowling, B.J.; et al. Estimates of global seasonal influenza-associated respiratory mortality: A modelling study. Lancet 2018, 391, 1285–1300. [Google Scholar] [CrossRef]
- Putri, W.; Muscatello, D.J.; Stockwell, M.S.; Newall, A.T. Economic burden of seasonal influenza in the United States. Vaccine 2018, 36, 3960–3966. [Google Scholar] [CrossRef]
- Bardenheier, B.H.; Strikas, R.; Kempe, A.; Stokley, S.; Ellis, J. Influenza vaccine supply, 2005–2006: Did we come up short? BMC Health Serv Res. 2007, 7, 66. [Google Scholar] [CrossRef]
- Belongia, E.A.; Simpson, M.D.; King, J.P.; Sundaram, M.E.; Kelley, N.S.; Osterholm, M.T.; McLean, H.Q. Variable influenza vaccine effectiveness by subtype: A systematic review and meta-analysis of test-negative design studies. Lancet Infect. Dis. 2016, 16, 942–951. [Google Scholar] [CrossRef]
- Wu, N.C.; Zost, S.J.; Thompson, A.J.; Oyen, D.; Nycholat, C.M.; McBride, R.; Paulson, J.C.; Hensley, S.E.; Wilson, I.A. A structural explanation for the low effectiveness of the seasonal influenza H3N2 vaccine. PLoS Pathog. 2017, 13, e1006682. [Google Scholar] [CrossRef]
- Demicheli, V.; Jefferson, T.; Di Pietrantonj, C.; Ferroni, E.; Thorning, S.; Thomas, R.E.; Rivetti, A. Vaccines for preventing influenza in the elderly. Cochrane Database Syst. Rev. 2018, 2, Cd004876. [Google Scholar] [CrossRef]
- Haq, K.; McElhaney, J.E. Immunosenescence: Influenza vaccination and the elderly. Curr. Opin. Immunol. 2014, 29, 38–42. [Google Scholar] [CrossRef]
- Jefferson, T.; Rivetti, D.; Rivetti, A.; Rudin, M.; Di Pietrantonj, C.; Demicheli, V. Efficacy and effectiveness of influenza vaccines in elderly people: A systematic review. Lancet 2005, 366, 1165–1174. [Google Scholar] [CrossRef]
- Lillie, P.J.; Berthoud, T.K.; Powell, T.J.; Lambe, T.; Mullarkey, C.; Spencer, A.J.; Hamill, M.; Peng, Y.; Blais, M.E.; Duncan, C.J.; et al. Preliminary assessment of the efficacy of a T-cell-based influenza vaccine, MVA-NP+M1, in humans. Clin. Infect. Dis. 2012, 55, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Coughlan, L.; Sridhar, S.; Payne, R.; Edmans, M.; Milicic, A.; Venkatraman, N.; Lugonja, B.; Clifton, L.; Qi, C.; Folegatti, P.M.; et al. Heterologous Two-Dose Vaccination with Simian Adenovirus and Poxvirus Vectors Elicits Long-Lasting Cellular Immunity to Influenza Virus A in Healthy Adults. EBioMedicine 2018, 29, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Antrobus, R.D.; Berthoud, T.K.; Mullarkey, C.E.; Hoschler, K.; Coughlan, L.; Zambon, M.; Hill, A.V.; Gilbert, S.C. Coadministration of seasonal influenza vaccine and MVA-NP+M1 simultaneously achieves potent humoral and cell-mediated responses. Mol. Ther. 2014, 22, 233–238. [Google Scholar] [CrossRef]
- Antrobus, R.D.; Lillie, P.J.; Berthoud, T.K.; Spencer, A.J.; McLaren, J.E.; Ladell, K.; Lambe, T.; Milicic, A.; Price, D.A.; Hill, A.V.; et al. A T cell-inducing influenza vaccine for the elderly: Safety and immunogenicity of MVA-NP+M1 in adults aged over 50 years. PLoS ONE 2012, 7, e48322. [Google Scholar] [CrossRef]
- Berthoud, T.K.; Hamill, M.; Lillie, P.J.; Hwenda, L.; Collins, K.A.; Ewer, K.J.; Milicic, A.; Poyntz, H.C.; Lambe, T.; Fletcher, H.A.; et al. Potent CD8+ T-cell immunogenicity in humans of a novel heterosubtypic influenza A vaccine, MVA-NP+M1. Clin. Infect. Dis. 2011, 52, 1–7. [Google Scholar] [CrossRef]
- Jordan, I.; Vos, A.; Beilfuss, S.; Neubert, A.; Breul, S.; Sandig, V. An avian cell line designed for production of highly attenuated viruses. Vaccine 2009, 27, 748–756. [Google Scholar] [CrossRef]
- Weissmahr, R.N.; Schüpbach, J.; Böni, J. Reverse transcriptase activity in chicken embryo fibroblast culture supernatants is associated with particles containing endogenous avian retrovirus EAV-0 RNA. J. Virol. 1997, 71, 3005–3012. [Google Scholar] [PubMed]
- Shoyab, M.; Baluda, M.A. Homology between avian oncornavirus RNAs and DNA from several avian species. J. Virol. 1975, 16, 1492–1502. [Google Scholar] [PubMed]
- Lohr, V.; Rath, A.; Genzel, Y.; Jordan, I.; Sandig, V.; Reichl, U. New avian suspension cell lines provide production of influenza virus and MVA in serum-free media: Studies on growth, metabolism and virus propagation. Vaccine 2009, 27, 4975–4982. [Google Scholar] [CrossRef] [PubMed]
- DiazGranados, C.A.; Dunning, A.J.; Kimmel, M.; Kirby, D.; Treanor, J.; Collins, A.; Pollak, R.; Christoff, J.; Earl, J.; Landolfi, V.; et al. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N. Engl. J. Med. 2014, 371, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Van Buynder, P.G.; Konrad, S.; Van Buynder, J.L.; Brodkin, E.; Krajden, M.; Ramler, G.; Bigham, M. The comparative effectiveness of adjuvanted and unadjuvanted trivalent inactivated influenza vaccine (TIV) in the elderly. Vaccine 2013, 31, 6122–6128. [Google Scholar] [CrossRef]
- Mannino, S.; Villa, M.; Apolone, G.; Weiss, N.S.; Groth, N.; Aquino, I.; Boldori, L.; Caramaschi, F.; Gattinoni, A.; Malchiodi, G.; et al. Effectiveness of adjuvanted influenza vaccination in elderly subjects in northern Italy. Am. J. Epidemiol. 2012, 176, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Dunkle, L.M.; Izikson, R.; Patriarca, P.; Goldenthal, K.L.; Muse, D.; Callahan, J.; Cox, M.M.J.; Team, P.S.C.S. Efficacy of Recombinant Influenza Vaccine in Adults 50 Years of Age or Older. N. Engl. J. Med. 2017, 376, 2427–2436. [Google Scholar] [CrossRef]
- Rajao, D.S.; Perez, D.R. Universal Vaccines and Vaccine Platforms to Protect against Influenza Viruses in Humans and Agriculture. Front. Microbiol. 2018, 9, 123. [Google Scholar] [CrossRef]
- Kumar, A.; Meldgaard, T.S.; Bertholet, S. Novel Platforms for the Development of a Universal Influenza Vaccine. Front. Immunol. 2018, 9, 600. [Google Scholar] [CrossRef]
- Clemens, E.B.; van de Sandt, C.; Wong, S.S.; Wakim, L.M.; Valkenburg, S.A. Harnessing the Power of T Cells: The Promising Hope for a Universal Influenza Vaccine. Vaccines 2018, 6, 18. [Google Scholar] [CrossRef]
- Hayward, A.C.; Wang, L.; Goonetilleke, N.; Fragaszy, E.B.; Bermingham, A.; Copas, A.; Dukes, O.; Millett, E.R.; Nazareth, I.; Nguyen-Van-Tam, J.S.; et al. Natural T Cell-mediated Protection against Seasonal and Pandemic Influenza. Results of the Flu Watch Cohort Study. Am. J. Respir. Crit. Care Med. 2015, 191, 1422–1431. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, S.; Begom, S.; Bermingham, A.; Hoschler, K.; Adamson, W.; Carman, W.; Bean, T.; Barclay, W.; Deeks, J.J.; Lalvani, A. Cellular immune correlates of protection against symptomatic pandemic influenza. Nat. Med. 2013, 19, 1305–1312. [Google Scholar] [CrossRef]
- Clinicaltrials.gov. NCT03300362. Improved Novel VaccIne CombinaTion InflUenza Study (INVICTUS). Available online: https://clinicaltrials.gov/ct2/show/NCT03300362 (accessed on 4 September 2019).
- Cottingham, M.G.; Carroll, M.W. Recombinant MVA vaccines: Dispelling the myths. Vaccine 2013, 31, 4247–4251. [Google Scholar] [CrossRef]
- Ramírez, J.C.; Gherardi, M.M.; Rodríguez, D.; Esteban, M. Attenuated Modified Vaccinia Virus Ankara Can Be Used as an Immunizing Agent under Conditions of Preexisting Immunity to the Vector. J. Virol. 2000, 74, 7651. [Google Scholar] [CrossRef] [PubMed]
- Harrop, R.; Connolly, N.; Redchenko, I.; Valle, J.; Saunders, M.; Ryan, M.G.; Myers, K.A.; Drury, N.; Kingsman, S.M.; Hawkins, R.E.; et al. Vaccination of Colorectal Cancer Patients with Modified Vaccinia Ankara Delivering the Tumor Antigen 5T4 (TroVax) Induces Immune Responses which Correlate with Disease Control: A Phase I/II Trial. Clin. Cancer Res. 2006, 12, 3416. [Google Scholar] [CrossRef] [PubMed]
| Variable | Number | Percentage |
|---|---|---|
| Age | ||
| Median | 32.5 | |
| Interquartile range | 24.25–48.5 | |
| Sex | ||
| Male | 2 | 33.33 |
| Female | 4 | 66.67 |
| Ethnicity | ||
| White | 5 | 83.33 |
| Mixed (White and Black) | 1 | 16.67 |
| Adverse Event | Proportion of Moderate/Severe AEs–AGE1.CR.pIX (n = 6) | Proportion of Moderate/Severe AEs–CEF (n = 24) | Difference between Proportions | 95% CI | p Value * |
|---|---|---|---|---|---|
| Pain at injection site | 0.1667 | 0.5417 | 0.375 | 0.1228 to 0.8881 | 0.1755 |
| Feverishness | 0.1667 | 0.125 | 0.04167 | −0.2208 to 0.5192 | >0.9999 |
| Arthralgia | 0 | 0.0769 | 0.07692 | −0.1128 to 0.5643 | |
| Myalgia | 0.1667 | 0.2917 | 0.125 | −0.1465 to 0.6192 | |
| Headache | 0.5 | 0.25 | 0.25 | −0.1726 to 0.6382 | 0.3287 |
| Fatigue | 0.1667 | 0.1667 | 0 | −0.2669 to 0.4817 | >0.9999 |
| Nausea | 0 | 0.125 | 0.1250 | −0.08518 to 0.6169 | |
| Malaise | 0.1667 | 0.0833 | 0.08333 | −0.1726 to 0.5569 | 0.5015 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Folegatti, P.M.; Bellamy, D.; Flaxman, A.; Mair, C.; Ellis, C.; L. Ramon, R.; Ramos Lopez, F.; Mitton, C.; Baker, M.; Poulton, I.; et al. Safety and Immunogenicity of the Heterosubtypic Influenza A Vaccine MVA-NP+M1 Manufactured on the AGE1.CR.pIX Avian Cell Line. Vaccines 2019, 7, 33. https://doi.org/10.3390/vaccines7010033
Folegatti PM, Bellamy D, Flaxman A, Mair C, Ellis C, L. Ramon R, Ramos Lopez F, Mitton C, Baker M, Poulton I, et al. Safety and Immunogenicity of the Heterosubtypic Influenza A Vaccine MVA-NP+M1 Manufactured on the AGE1.CR.pIX Avian Cell Line. Vaccines. 2019; 7(1):33. https://doi.org/10.3390/vaccines7010033
Chicago/Turabian StyleFolegatti, Pedro M., Duncan Bellamy, Amy Flaxman, Catherine Mair, Chris Ellis, Raquel L. Ramon, Fernando Ramos Lopez, Celia Mitton, Megan Baker, Ian Poulton, and et al. 2019. "Safety and Immunogenicity of the Heterosubtypic Influenza A Vaccine MVA-NP+M1 Manufactured on the AGE1.CR.pIX Avian Cell Line" Vaccines 7, no. 1: 33. https://doi.org/10.3390/vaccines7010033
APA StyleFolegatti, P. M., Bellamy, D., Flaxman, A., Mair, C., Ellis, C., L. Ramon, R., Ramos Lopez, F., Mitton, C., Baker, M., Poulton, I., Lawrie, A., Roberts, R., Minassian, A., Ewer, K. J., Evans, T. G., Hill, A. V. S., & Gilbert, S. C. (2019). Safety and Immunogenicity of the Heterosubtypic Influenza A Vaccine MVA-NP+M1 Manufactured on the AGE1.CR.pIX Avian Cell Line. Vaccines, 7(1), 33. https://doi.org/10.3390/vaccines7010033





