Attenuation and Stability of CHIKV-NoLS, a Live-Attenuated Chikungunya Virus Vaccine Candidate
Abstract
1. Introduction
2. Materials and Methods
2.1. Viruses and Cells
2.2. Mice
2.3. Histology
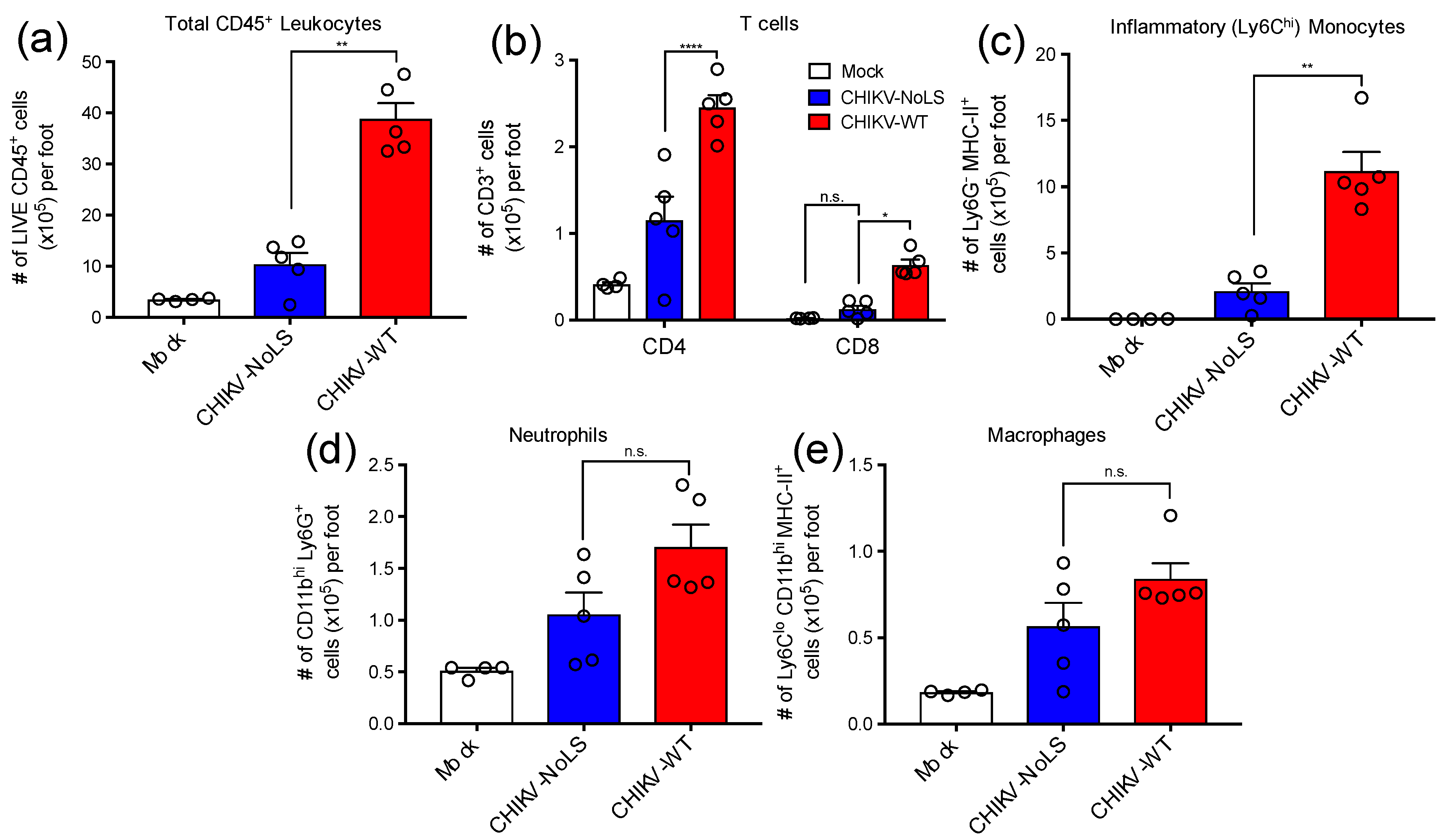
2.4. Enumeration of Foot Cellular Infiltrates by Flow Cytometry
2.5. Extended in Vitro Passage
2.6. Thermostability Assays
2.7. Statistical Analysis
3. Results
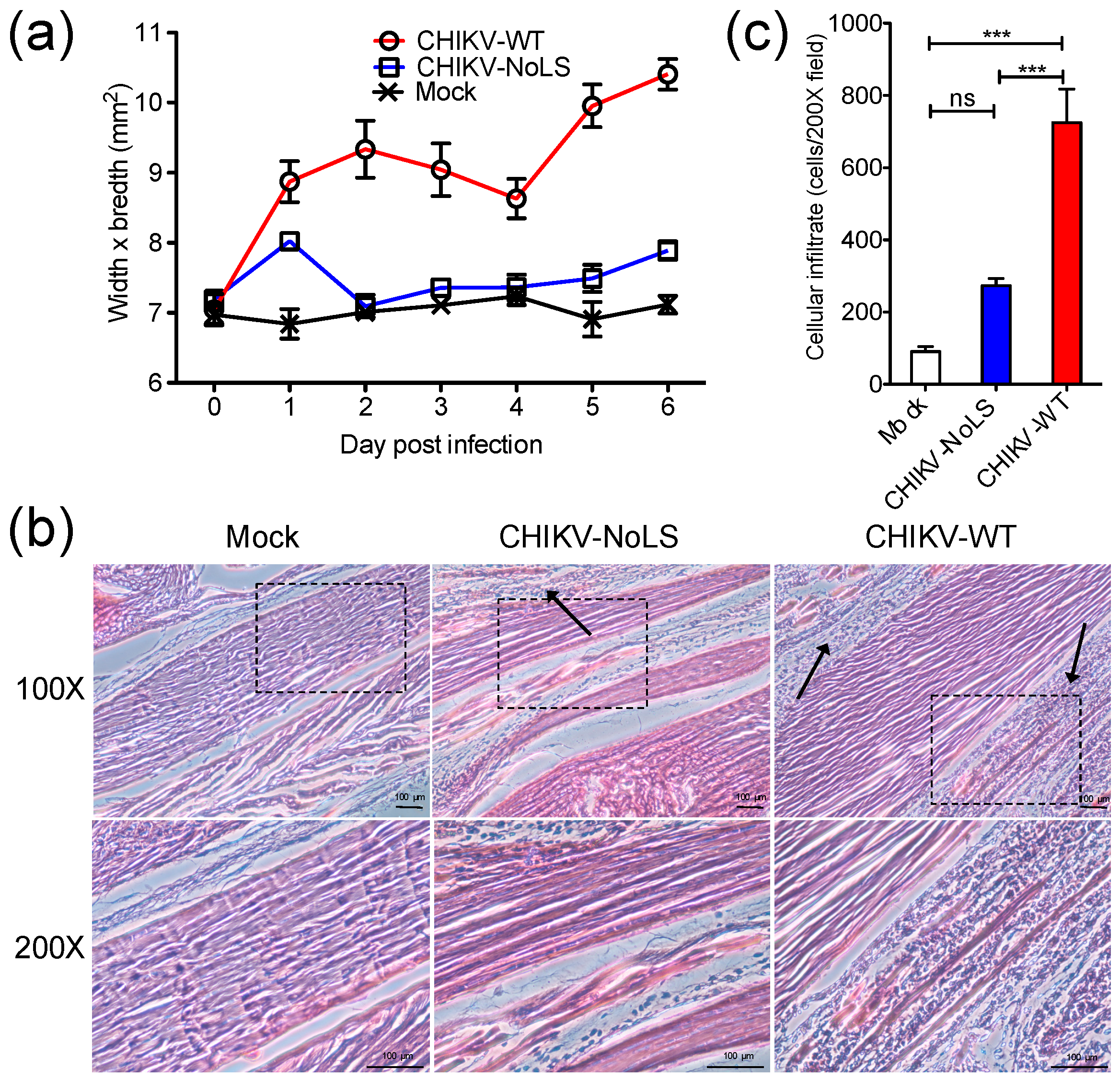
3.1. Immunopathology Associated with CHIKV-NoLS Inoculation
3.2. Stability of CHIKV-NoLS Attenuation Following Extended in Vitro Passage
3.3. Examining the Thermotability of CHIKV-NoLS
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Conflicts of Interest
References
- Silva, L.A.; Dermody, T.S. Chikungunya virus: Epidemiology, replication, disease mechanisms, and prospective intervention strategies. J. Clin. Invest. 2017, 127, 737–749. [Google Scholar] [CrossRef] [PubMed]
- Powers, A.M. How chikungunya virus virology affects its epidemiology and transmission: Implications for influencing public health. J. Infect. Dis. 2016, 214, 4494. [Google Scholar] [CrossRef] [PubMed]
- Queyriaux, B.; Simon, F.; Grandadam, M.; Michel, R.; Tolou, H.; Boutin, J.P. Clinical burden of chikungunya virus infection. Lancet Infect. Dis. 2008, 8, 2–3. [Google Scholar] [CrossRef]
- Sissoko, D.; Malvy, D.; Ezzedine, K.; Renault, P.; Moscetti, F.; Ledrans, M.; Pierre, V. Post-epidemic chikungunya disease on reunion island: Course of rheumatic manifestations and associated factors over a 15-month period. PLoS Negl. Trop. Dis. 2009, 3, e389. [Google Scholar] [CrossRef] [PubMed]
- Lemant, J.; Boisson, V.; Winer, A.; Thibault, L.; Andre, H.; Tixier, F.; Lemercier, M.; Antok, E.; Cresta, M.P.; Grivard, P.; et al. Serious acute chikungunya virus infection requiring intensive care during the reunion island outbreak in 2005-2006. Crit. Care Med. 2008, 36, 2536–2541. [Google Scholar] [CrossRef] [PubMed]
- Weaver, S.C. Arrival of chikungunya virus in the new world: Prospects for spread and impact on public health. PLoS Negl. Trop. Dis. 2014, 8. [Google Scholar] [CrossRef]
- Gerardin, P.; Guernier, V.; Perrau, J.; Fianu, A.; Le Roux, K.; Grivard, P.; Michault, A.; De Lamballerie, X.; Flahault, A.; Favier, F. Estimating chikungunya prevalence in la reunion island outbreak by serosurveys: Two methods for two critical times of the epidemic. BMC Infect. Dis. 2008, 8. [Google Scholar] [CrossRef]
- Arankalle, V.A.; Shrivastava, S.; Cherian, S.; Gunjikar, R.S.; Walimbe, A.M.; Jadhav, S.M.; Sudeep, A.B.; Mishra, A.C. Genetic divergence of chikungunya viruses in india (1963-2006) with special reference to the 2005-2006 explosive epidemic. J. Gen. Virol. 2007, 88, 1967–1976. [Google Scholar] [CrossRef]
- Win, M.K.; Chow, A.; Dimatatac, F.; Go, C.J.; Leo, Y.S. Chikungunya fever in singapore: Acute clinical and laboratory features, and factors associated with persistent arthralgia. J. Clin. Virol. 2010, 49, 111–114. [Google Scholar] [CrossRef]
- Leparc-Goffart, I.; Nougairede, A.; Cassadou, S.; Prat, C.; de Lamballerie, X. Chikungunya in the americas. Lancet 2014, 383, 514. [Google Scholar] [CrossRef]
- Soumahoro, M.K.; Boelle, P.Y.; Gauzere, B.A.; Atsou, K.; Pelat, C.; Lambert, B.; La Ruche, G.; Gastellu-Etchegorry, M.; Renault, P.; Sarazin, M.; et al. The chikungunya epidemic on la reunion island in 2005-2006: A cost-of-illness study. PLoS Negl. Trop. Dis. 2011, 5. [Google Scholar] [CrossRef] [PubMed]
- Cardona-Ospina, J.A.; Villamil-Gomez, W.E.; Jimenez-Canizales, C.E.; Castaneda-Hernandez, D.M.; Rodriguez-Morales, A.J. Estimating the burden of disease and the economic cost attributable to chikungunya, colombia, 2014. Trans. R Soc. Trop. Med. Hyg. 2015, 109, 793–802. [Google Scholar] [CrossRef]
- Fredericks, A.C.; Fernandez-Sesma, A. The burden of dengue and chikungunya worldwide: Implications for the southern united states and california. Ann. Glob. Health 2014, 80, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Tsetsarkin, K.A.; Vanlandingham, D.L.; McGee, C.E.; Higgs, S. A single mutation in chikungunya virus affects vector specificity and epidemic potential. PLoS Pathog. 2007, 3, e201. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.; Liu, X.; Zaid, A.; Goh, L.Y.H.; Hobson-Peters, J.; Hall, R.A.; Merits, A.; Mahalingam, S. Mutation of the n-terminal region of chikungunya virus capsid protein: Implications for vaccine design. Mbio 2017, 8, e01970-16. [Google Scholar] [CrossRef]
- Teo, T.H.; Lum, F.M.; Claser, C.; Lulla, V.; Lulla, A.; Merits, A.; Renia, L.; Ng, L.F. A pathogenic role for CD4+ t cells during chikungunya virus infection in mice. J. Immunol. 2013, 190, 259–269. [Google Scholar] [CrossRef]
- Hallengard, D.; Lum, F.M.; Kummerer, B.M.; Lulla, A.; Lulla, V.; Garcia-Arriaza, J.; Fazakerley, J.K.; Roques, P.; Le Grand, R.; Merits, A.; et al. Prime-boost immunization strategies against chikungunya virus. J. Virol. 2014, 88, 13333–13343. [Google Scholar] [CrossRef]
- Lum, F.M.; Couderc, T.; Chia, B.S.; Ong, R.Y.; Her, Z.; Chow, A.; Leo, Y.S.; Kam, Y.W.; Renia, L.; Lecuit, M.; et al. Antibody-mediated enhancement aggravates chikungunya virus infection and disease severity. Sci. Rep. 2018, 8, 1860. [Google Scholar] [CrossRef]
- Edelman, R.; Tacket, C.O.; Wasserman, S.S.; Bodison, S.A.; Perry, J.G.; Mangiafico, J.A. Phase ii safety and immunogenicity study of live chikungunya virus vaccine tsi-gsd-218. Am. J. Trop. Med. Hyg. 2000, 62, 681–685. [Google Scholar] [CrossRef]
- Gardner, J.; Anraku, I.; Le, T.T.; Larcher, T.; Major, L.; Roques, P.; Schroder, W.A.; Higgs, S.; Suhrbier, A. Chikungunya virus arthritis in adult wild-type mice. J. Virol. 2010, 84, 8021–8032. [Google Scholar] [CrossRef]
- Hansen, L.J.J.; Daoussi, R.; Vervaet, C.; Remon, J.P.; De Beer, T.R.M. Freeze-drying of live virus vaccines: A review. Vaccine 2015, 33, 5507–5519. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Owen, K.E.; Choi, H.K.; Lee, H.; Lu, G.G.; Wengler, G.; Brown, D.T.; Rossmann, M.G.; Kuhn, R.J. Identification of a protein binding site on the surface of the alphavirus nucleocapsid and its implication in virus assembly. Structure 1996, 4, 531–541. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abeyratne, E.; Freitas, J.R.; Zaid, A.; Mahalingam, S.; Taylor, A. Attenuation and Stability of CHIKV-NoLS, a Live-Attenuated Chikungunya Virus Vaccine Candidate. Vaccines 2019, 7, 2. https://doi.org/10.3390/vaccines7010002
Abeyratne E, Freitas JR, Zaid A, Mahalingam S, Taylor A. Attenuation and Stability of CHIKV-NoLS, a Live-Attenuated Chikungunya Virus Vaccine Candidate. Vaccines. 2019; 7(1):2. https://doi.org/10.3390/vaccines7010002
Chicago/Turabian StyleAbeyratne, Eranga, Joseph R. Freitas, Ali Zaid, Suresh Mahalingam, and Adam Taylor. 2019. "Attenuation and Stability of CHIKV-NoLS, a Live-Attenuated Chikungunya Virus Vaccine Candidate" Vaccines 7, no. 1: 2. https://doi.org/10.3390/vaccines7010002
APA StyleAbeyratne, E., Freitas, J. R., Zaid, A., Mahalingam, S., & Taylor, A. (2019). Attenuation and Stability of CHIKV-NoLS, a Live-Attenuated Chikungunya Virus Vaccine Candidate. Vaccines, 7(1), 2. https://doi.org/10.3390/vaccines7010002





