The Modified Vaccination Technique
Abstract
:1. Introduction
2. Modified Vaccination Technique (MVT)
3. Antibody Information Transfer (AIT)
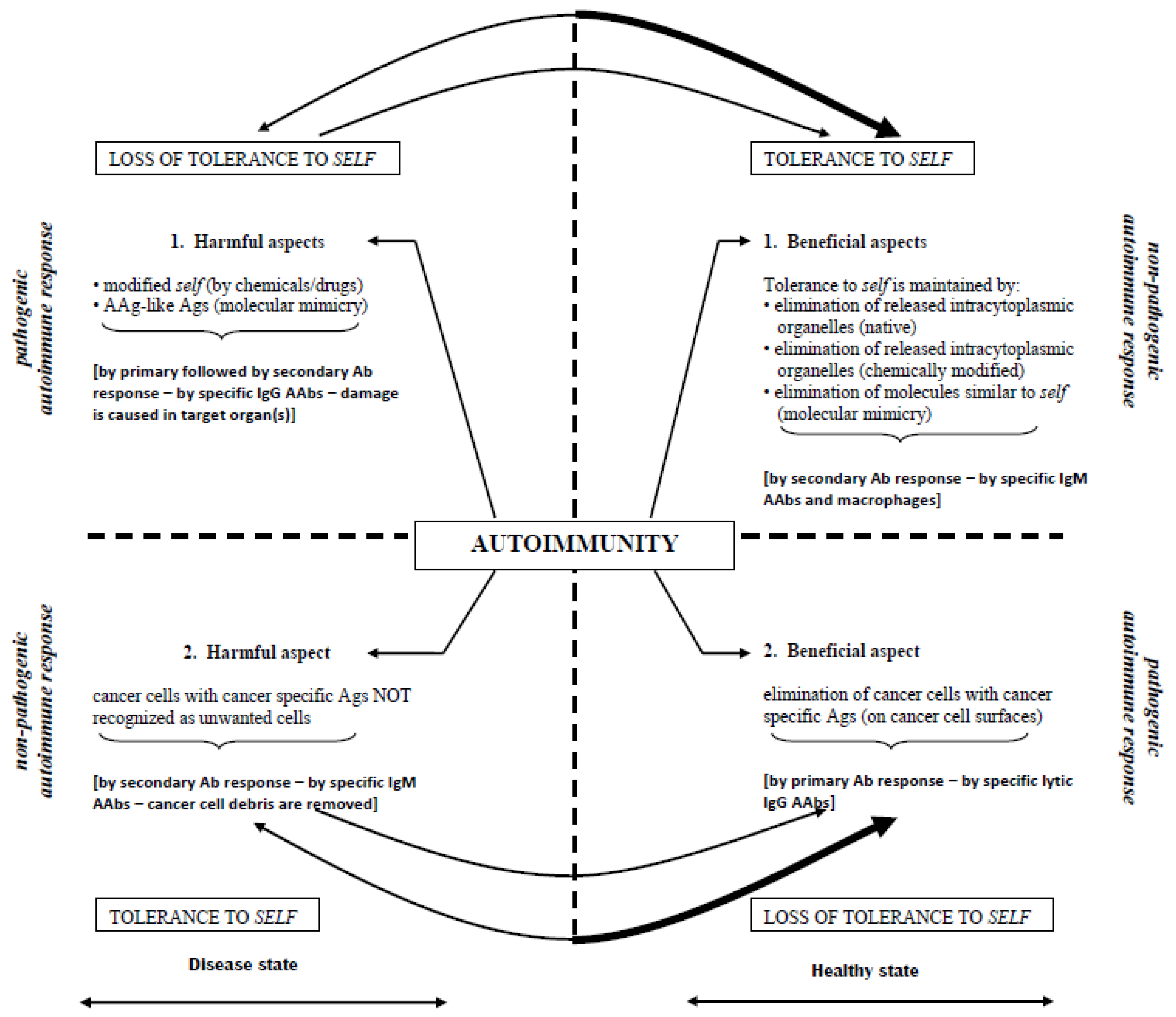
- Down-regulation of pathogenic IgG aab responses in autoimmune diseases, thereby preventing/terminating immune events that attempt to destroy self targets (Figure 2);
- Up-regulation of pathogenic immune responses against cancer-specific ags to lyse cells (irrespective of their locations (metastatic spread)) (Figure 3); and
- Initiation of immune responses against agents causing acute/chronic infections (e.g., malaria, Ebola, cholera, influenza, etc.).
4. Therapeutic Immunization Program
5. Preventative and Therapeutic Possibilities in Acute and Chronic Immunological Disorders
5.1. Example of Vaccination against A Hyper-Acute Infection by the MVT
- Ebola virus ag (inactivated but immunogenic), laboratory-produced/tested;
- Human anti-Ebola virus ag poly-specific IgG abs, obtained from sera of recovered patients;
- To employ MVT, produce ICs comprised of human Ebola ag × human anti-Ebola neutralizing IgG abs.
- The same components as above and the same MVT to induce, by repeated injections, high ab titres of human anti-Ebola neutralizing IgG abs. If the modified vaccine is given in time, patients should survive.
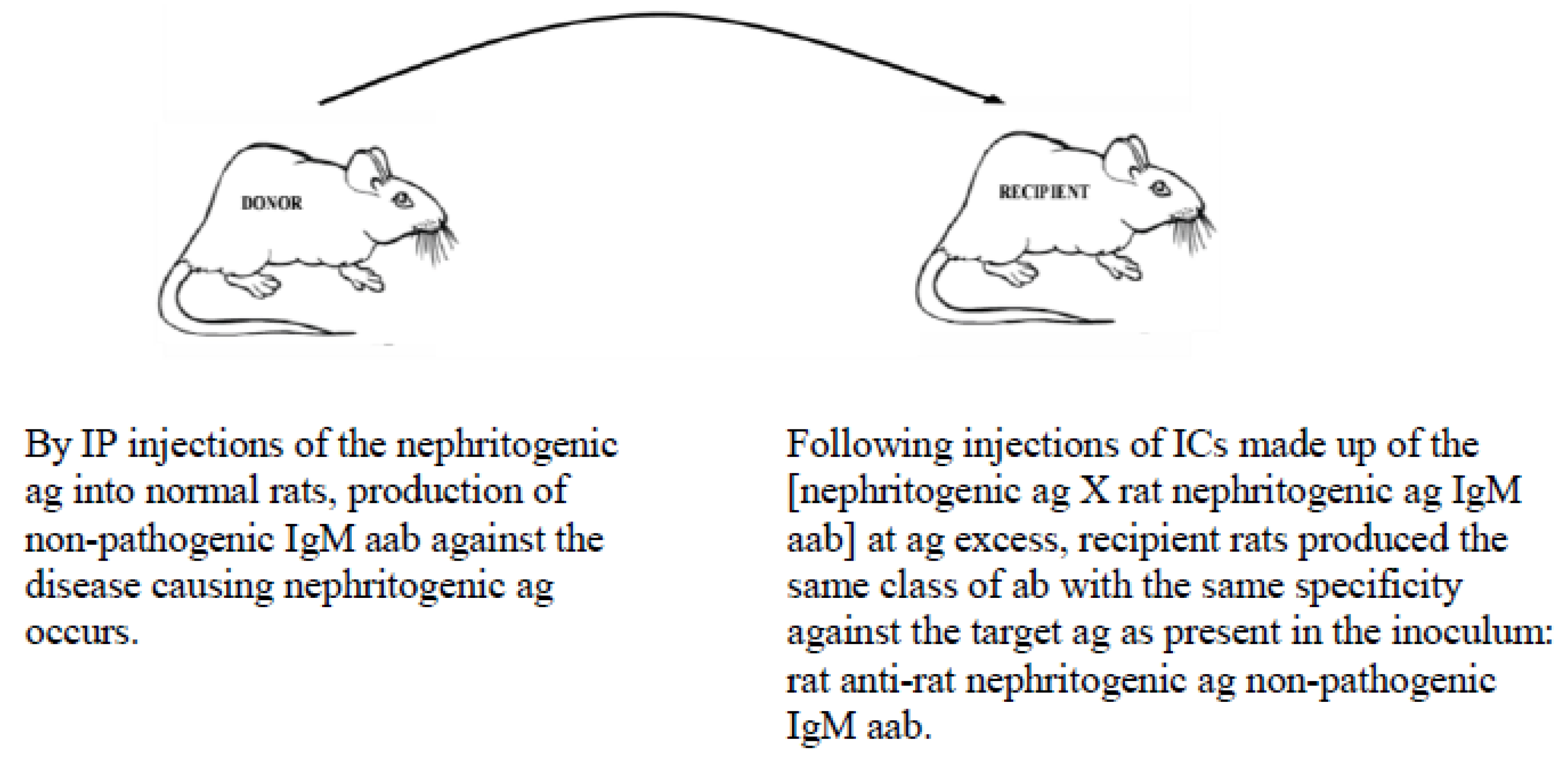
5.2. Example of Vaccination against an Autoimmune Disease-Causing Ag Using the MVT
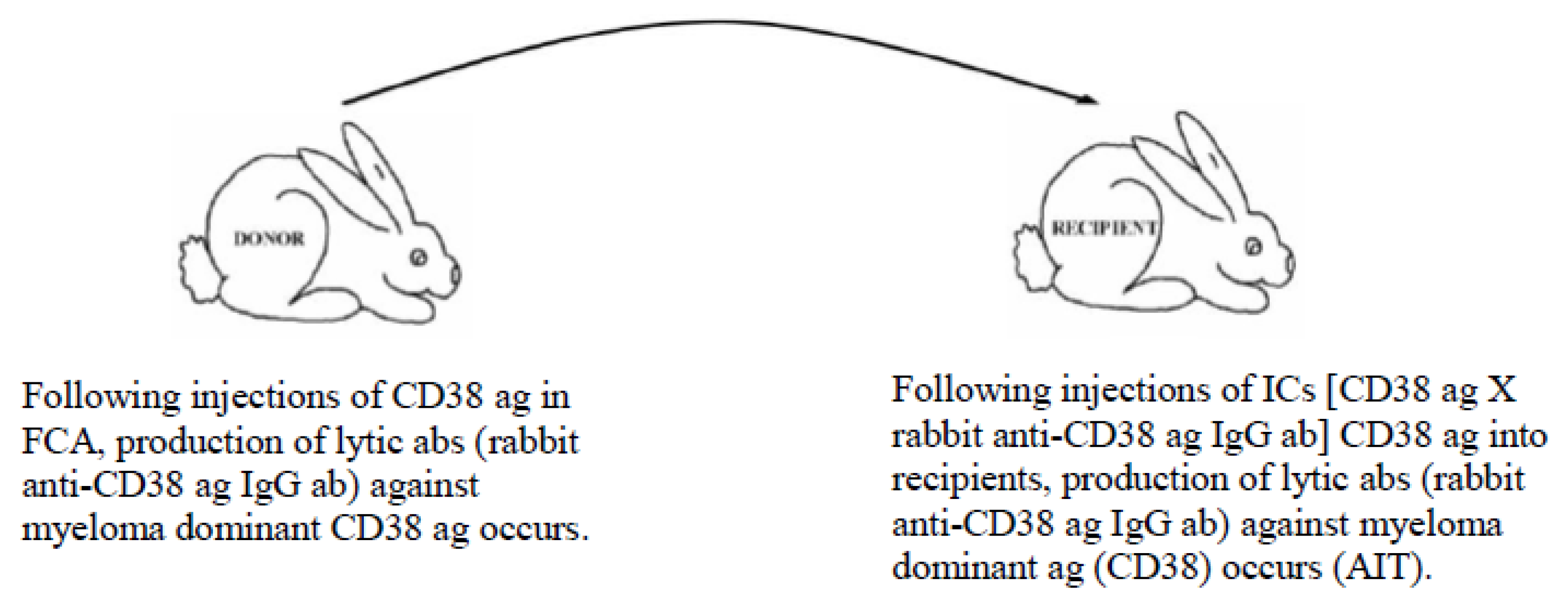
5.3. Example of Vaccination against Cancer-Causing Ags Utilizing the MVT
6. Conclusions
- It is both prophylactic and therapeutic with equal effectiveness (no other vaccination program offers this);
- It can be used against exogenous and endogenous ags (e.g., with the elimination of modified self ag from the system that could otherwise induce an autoimmune disease, etc.);
- It can downregulate/terminate pathogenic immune responses in certain autoimmune diseases by non-pathogenic poly-specific IgM aabs targeting native and modified disease-causing ags;
- It can upregulate pathogenic immune responses against target ags (i.e., cancer-associated and cancer-specific ags) by inducing production of lytic poly-specific IgG aabs;
- When specific ICs are prepared for immunization, only small amounts (micro-gram doses) of ag and abs against the ag are required to evoke the desired immune responses;
- The ICs do not require adjuvants for adequate responses in recipients, although acceptable adjuvants included in the IC will enhance immune response, such as that initiated by the elimination of modified self ags that are causing autoimmune diseases, and enhance ab response to target cancer-specific ags to lyse cancer cells;
- It can be produced cost-effectively and should considerably reduce health care expenses while allowing highly effective treatments to be initiated with minimal delay.
Author Contributions
Funding
Conflicts of Interest
References
- Berinstein, N.L. Enhancing cancer vaccines with immunomodulators. Vaccine 2007, 25 (Suppl. 2), B72–B88. [Google Scholar] [CrossRef]
- Cope, A.P.; Feldmann, M. Emerging approaches for the therapy of autoimmune and chronic inflammatory disease. Curr. Opin. Immunol. 2004, 16, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Feldmann, M.; Steinman, L. Design of effective immunotherapy for human autoimmunity. Nature 2005, 435, 612–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lollini, P.L.; Forni, G. Antitumor vaccines: Is it possible to prevent a tumor? Cancer Immunol. Immunother. 2002, 51, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Milstein, C.; Waldmann, H. Optimism after much pessimism: What next? Curr. Opin. Immunol. 1999, 11, 589–591. [Google Scholar] [CrossRef]
- Petrovsky, N.; Aguilar, J.C. Vaccine adjuvants: Current state and future trends. Immunol. Cell Biol. 2004, 82, 488–496. [Google Scholar] [CrossRef] [Green Version]
- Brent, L.; Cohen, I.R.; Doherty, P.C.; Feldmann, M.; Matzinger, P.; Holtgate, S.T.; Lachmann, P.; Mitchison, N.A.; Nossal, G.; Rose, N.R.; et al. Crystal-ball gazing: The future of immunological research viewed from the cutting edge. Clin. Exp. Immunol. 2007, 147, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bruley-Rosset, M.; Mouthon, L.; Chanseaud, Y.; Dhainaut, F.; Lirochon, J.; Bourel, D. Polyreactive autoantibodies purified from human intravenous immunoglobulins prevent the development of experimental autoimmune diseases. Lab. Investig. 2003, 83, 1013–1023. [Google Scholar] [CrossRef]
- Coiffier, B. Rituximab in the treatment of diffuse large B-cell lymphomas. Semin. Oncol. 2002, 29, 30–35. [Google Scholar] [CrossRef]
- Czuczman, M.S.; Fallon, A.; Mohr, A.; Stewart, C.; Bernstein, Z.P.; McCarthy, P.; Skipper, M.; Brown, K.; Miller, K.; Wentling, D.; et al. Rituximab in combination with CHOP or fludarabine in low-grade lymphoma. Semin. Oncol. 2002, 29, 36–40. [Google Scholar] [CrossRef]
- Dupont, B. Introduction: Current concepts in immunity to human cancer and therapeutic antitumor vaccines. Immunol. Rev. 2002, 188, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Ephrem, A.; Chamat, S.; Miquel, C.; Fisson, S.; Mouthon, L.; Caligiuri, G.; Delignat, S.; Elluru, S.; Bayry, J.; Lacroix-Desmazes, S.; et al. Expansion of CD4+CD25+ regulatory T cells by intravenous immunoglobulin: A critical factor in controlling experimental autoimmune encephalomyelitis. Blood 2007, 111, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Fox, R.J.; Ransohoff, R.M. New directions in MS therapeutics: Vehicles of hope. Trends Immunol. 2004, 25, 632–636. [Google Scholar] [CrossRef] [PubMed]
- Ichim, C.V. Revisiting immunosurveillance and immunostimulation: Implications for cancer immunotherapy. J. Transl. Med. 2005, 3, 8. [Google Scholar] [CrossRef]
- Romond, E.H.; Perez, E.A.; Bryant, J.; Suman, V.J.; Geyer, C.E., Jr.; Davidson, N.E.; Tan-Chiu, E.; Martino, S.; Paik, S.; Kaufman, P.A.; et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N. Engl. J. Med. 2005, 353, 1673–1684. [Google Scholar] [CrossRef] [PubMed]
- Sewell, W.A.; Jolles, S. Immunomodulatory action of intravenous immunoglobulin. Immunology 2002, 107, 387–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shoenfeld, Y.; Katz, U. IVIg therapy in autoimmunity and related disorders: Our experience with a large cohort of patients. Autoimmunity 2005, 38, 123–137. [Google Scholar] [CrossRef]
- Slamon, D.J.; Leyland-Jones, B.; Shak, S.; Fuchs, H.; Paton, V.; Bajamonde, A.; Fleming, T.; Eiermann, W.; Wolter, J.; Pegram, M.; et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N. Engl. J. Med. 2001, 344, 783–792. [Google Scholar] [CrossRef]
- Aruffo, A.; Hollenbaugh, D. Therapeutic intervention with inhibitors of co-stimulatory pathways in autoimmune disease. Curr. Opin. Immunol. 2001, 13, 683–686. [Google Scholar] [CrossRef]
- Biesecker, G.; Noble, B.; Andres, G.A.; Koffler, D. Immunopathogenesis of Heymann’s nephritis. Clin. Immunol. Immunopathol. 1984, 33, 333–338. [Google Scholar] [CrossRef]
- Cattran, D.C. Effect of ciclosporin on active Heymann nephritis. Nephron 1988, 48, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, T. Membranous nephropathy. Insights from Heymann nephritis. Am. J. Pathol. 1994, 144, 651–658. [Google Scholar] [PubMed]
- Hasegawa, Y.; Kaneoka, H.; Tanaka, T.; Ogahara, S.; Matsumae, T.; Noda, R.; Yoshitake, K.; Murata, T.; Naito, S. Suppression of experimental membranous glomerulonephritis in rats by an anti-MHC class II antibody. Nephron 2001, 88, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Heymann, W.; Hackel, D.B.; Harwood, S.; Wilson, S.G.; Hunter, J.L.P. Production of the nephritic syndrome in rat by Freund’s adjuvant and rat kidney suspension. Proc. Soc. Exp. Biol. Med. 1959, 100, 660–664. [Google Scholar] [CrossRef] [PubMed]
- Kerjaschki, D.; Farquhar, M.G. The pathogenic antigen of Heymann nephritis is a membrane glycoprotein of the renal proximal tubule brush border. Proc. Natl. Acad. Sci. USA 1982, 79, 5557–5581. [Google Scholar] [CrossRef] [PubMed]
- Kerjaschki, D. Molecular development of immune deposits and proteinuria in Heymann nephritis. Clin. Investig. 1993, 71, 817–821. [Google Scholar] [CrossRef]
- Kerjaschki, D.; Neale, T.J. Molecular mechanisms of glomerular injury in rat experimental membranous nephropathy (Heymann nephritis). J. Am. Soc. Nephrol. 1996, 7, 2518–2526. [Google Scholar] [PubMed]
- Kerjaschki, D. Megalin/GP330 and pathogenetic concepts of membranous glomerulopathy (MGN). Kidney Blood Press Res. 2000, 23, 163–166. [Google Scholar]
- Kupor, L.R.; Lowance, D.C.; McPhaul, J.J., Jr. Single and multiple drug therapy in autologous immune complex nephritis in rats. J. Lab. Clin. Med. 1976, 87, 27–36. [Google Scholar]
- Makker, S.P.; Moorthy, B. In situ immune complex formation in isolated perfused kidney using homologous antibody. Lab. Investig. 1981, 44, 1–5. [Google Scholar]
- Makker, S.P.; Makker, D.M. A simple technique for detecting the antigen of Heymann nephritis in glomeruli by immunofluorescence. Clin. Exp. Immunol. 1986, 64, 615–622. [Google Scholar] [PubMed]
- Matsukawa, W.; Hara, S.; Yoshida, F.; Suzuki, N.; Fukatsu, A.; Yuzawa, Y.; Sakamoto, N.; Matsuo, S. Effects of a new immunosuppressive agent, FK506, in rats with active Heymann nephritis. J. Lab. Clin. Med. 1992, 119, 116–123. [Google Scholar] [PubMed]
- Nangaku, M.; Pippin, J.; Richardson, C.A.; Schulze, M.; Young, B.A.; Alpers, C.E.; Gordon, K.L.; Johnson, R.J.; Couser, W.G. Beneficial effects of systemic immunoglobulin in experimental membranous nephropathy. Kidney Int. 1996, 50, 2054–2062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Penny, M.J.; Boyd, R.A.; Hall, B.M. Permanent CD8(+) T cell depletion prevents proteinuria in active Heymann nephritis. J. Exp. Med. 1998, 188, 1775–1784. [Google Scholar] [CrossRef] [PubMed]
- Schiller, B.; He, C.; Salant, D.J.; Lim, A.; Alexander, J.J.; Quigg, R.J. Inhibition of complement regulation is key to the pathogenesis of active Heymann nephritis. J. Exp. Med. 1998, 188, 1353–1358. [Google Scholar] [CrossRef]
- Singh, A.K.; Kasinath, B.S. Metabolic fate of monovalent and multivalent antibodies of Heymann nephritis following formation of surface immune complexes on glomerular epithelial cells. Clin. Exp. Immunol. 1993, 94, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Spicer, S.T.; Ha, H.; Boyd, R.A.; He, X.Y.; Carter, N.; Tran, G.; Penny, M.J.; Hodgkinson, S.J.; Hall, B.M. Il-4 therapy prevents the development of proteinuria in active Heymann nephritis by inhibition of Tc1 cells. J. Immunol. 2001, 167, 3725–3733. [Google Scholar] [CrossRef]
- Yokoyama, H.; Goshima, S.; Wada, T.; Takaeda, M.; Furuichi, K.; Kobayashi, K.I.; Kida, H. The short- and long-term outcomes of membranous nephropathy treated with intravenous immune globulin therapy. Kanazawa Study Group for Renal Diseases and Hypertension. Nephrol. Dial. Transplant. 1999, 14, 2379–2386. [Google Scholar] [CrossRef]
- Barabas, A.Z.; Cole, C.D.; Barabas, A.D.; Lafreniere, R. Down-regulation of pathogenic autoantibody response in a slowly progressive Heymann nephritis kidney disease model. Int. J. Exp. Pathol. 2004, 85, 321–334. [Google Scholar] [CrossRef]
- Barabas, A.Z.; Lafreniere, R. Antigen-specific down-regulation of immunopathological events in an experimental autoimmune kidney disease. Autoimmun. Rev. 2005, 4, 565–570. [Google Scholar] [CrossRef]
- Barabas, A.Z.; Cole, C.D.; Barabas, A.D.; Barabas, A.N.; Lafreniere, R. Reduced incidence of slowly progressive Heymann nephritis in rats immunized with a modified vaccination technique. Clin. Dev. Immunol. 2006, 13, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Barabas, A.Z.; Cole, C.D.; Barabas, A.D.; Lafreniere, R. Preventative and therapeutic vaccination to combat an experimental autoimmune kidney disease. Biol. Targets Therapy 2007, 1, 59–68. [Google Scholar]
- Barabas, A.Z.; Cole, C.D.; Barabas, A.D.; Bahlis, N.J.; Lafreniere, R. A vaccination technique to combat presently untreatable chronic ailments. BioProcess. J. 2007, 6, 12–18. [Google Scholar] [CrossRef]
- Barabas, A.Z.; Cole, C.D.; Barabas, A.D.; Lafreniere, R. A modified vaccination technique for the prevention and treatment of an experimental autoimmune kidney disease. Ann. N. Y. Acad. Sci. 2007, 1110, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Barabas, A.Z.; Cole, C.D.; Barabas, A.D.; Bahlis, N.J.; Lafreniere, R. New vaccination technology for endogenous antigen-derived ailments. IDrugs 2008, 11, 111–115. [Google Scholar] [PubMed]
- Barabas, A.Z.; Weir, D.M.; Cole, C.D.; Barabas, A.D.; Bahlis, N.J.; Graeff, R.M.; Lafreniere, R. Preventing and treating chronic disorders using the modified vaccination technique. Front. Biosci. 2009, 14, 3892–3898. [Google Scholar] [CrossRef]
- Barabas, A.Z.; Cole, C.D.; Graeff, R.M.; Morcol, T.; Lafreniere, R. A novel modified vaccination technique produces IgG antibodies that cause complement-mediated lysis of multiple myeloma cells carrying CD38 antigen. Hum. Antibodies 2017, 24, 45–51. [Google Scholar] [CrossRef]
- Barabas, A.Z.; Cole, C.D.; Lafreniere, R.; Weir, D.M. A new vaccination method for exogenous and endogenous antigen initiated disorders. In Vaccinations: Procedures, Types and Controversy; Bezio, A.I., Campbell, B.E., Eds.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2012; pp. 75–98. [Google Scholar]
- Albert, L.J.; Inman, R.D. Molecular mimicry and autoimmunity. N. Engl. J. Med. 1999, 341, 2068–2074. [Google Scholar] [CrossRef]
- Ban, Y.; Tomer, Y. Susceptibility genes in thyroid autoimmunity. Clin. Dev. Immunol. 2005, 12, 47–58. [Google Scholar] [CrossRef]
- Barabas, A.Z.; Cole, C.D.; Barabas, A.D.; Lafreniere, R. Production of Heymann nephritis by a chemically modified renal antigen. Int. J. Exp. Pathol. 2004, 85, 277–285. [Google Scholar] [CrossRef]
- Conti, F.; Rezai, S.; Valesini, G. Vaccination and autoimmune rheumatic diseases. Autoimmun. Rev. 2008, 8, 124–128. [Google Scholar] [CrossRef]
- Davidson, A.; Diamond, B. Autoimmune diseases. N. Engl. J. Med. 2001, 345, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Ebringer, A.; Thorpe, C.; Pirt, J.; Wilson, C.; Cunningham, P.; Ettelaie, C. Bovine spongiform encephalopathy: Is it an autoimmune disease due to bacteria showing molecular mimicry with brain antigens? Environ. Health Perspect. 1997, 105, 1172–1174. [Google Scholar] [CrossRef]
- Ebringer, A. Molecular mimicry as the basis of a new theory of autoimmunity. In Frontiers in Autoimmunity: Fundamental Aspects and Clinical Perspectives; Zouali, M., Ed.; IOS Press: Amsterdam, The Netherlands, 2003; Volume 354, pp. 79–99. [Google Scholar]
- Harel, M.; Shoenfeld, Y. Predicting and preventing autoimmunity, myth or reality? Ann. N. Y. Acad. Sci. 2006, 1069, 322–345. [Google Scholar] [CrossRef] [PubMed]
- Hess, E.V. Are there environmental forms of systemic autoimmune diseases? Environ. Health Perspect. 1999, 107 (Suppl. 5), 709–711. [Google Scholar] [CrossRef]
- Molina, V.; Shoenfeld, Y. Infection, vaccines and other environmental triggers of autoimmunity. Autoimmunity 2005, 38, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Morahan, G.; Morel, L. Genetics of autoimmune diseases in humans and in animal models. Curr. Opin. Immunol. 2002, 14, 803–811. [Google Scholar] [CrossRef]
- Orbach, H.; Shoenfeld, Y. Vaccination infection and autoimmunity: Myth and reality VIAMR 2005-10-26-28, Beau-Rivage Palace Hotel, Lausanne, Switzerland. Autoimmun. Rev. 2007, 6, 261–266. [Google Scholar] [CrossRef]
- Pramatarov, K.D. Drug-induced lupus erythematosus. Clin. Dermatol. 1998, 16, 367–377. [Google Scholar] [CrossRef]
- Price, E.J.; Venables, P.J. Drug-induced lupus. Drug Saf. 1995, 12, 283–290. [Google Scholar] [CrossRef]
- Ramsey-Goldman, R.; Franz, T.; Solano, F.X.; Medsger, T.A., Jr. Hydralazine induced lupus and Sweet’s syndrome. Report and review of the literature. J. Rheumatol. 1990, 17, 682–684. [Google Scholar] [PubMed]
- Rao, T.; Richardson, B. Environmentally induced autoimmune diseases: Potential mechanisms. Environ. Health Perspect. 1999, 107 (Suppl. 5), 737–742. [Google Scholar] [PubMed]
- Shoenfeld, Y.; Zandman-Goddard, G.; Stojanovich, L.; Cutolo, M.; Amital, H.; Levy, Y.; Abu-Shakra, M.; Barzilai, O.; Berkun, Y.; Blank, M.; et al. The mosaic of autoimmunity: Hormonal and environmental factors involved in autoimmune diseases—2008. Isr. Med. Assoc. J. 2008, 10, 8–12. [Google Scholar] [PubMed]
- Haddad, E.E.; Whitfill, C.E.; Avakian, A.P.; Ricks, C.A.; Andrews, P.D.; Thoma, J.A.; Wakenell, P.S. Efficacy of a novel infectious bursal disease virus immune complex vaccine in broiler chickens. Avian Dis. 1997, 41, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Nie, X.; Basu, S.; Cerny, J. Immunization with immune complex alters the repertoire of antigen-reactive B cells in the germinal centers. Eur. J. Immunol. 1997, 27, 3517–3525. [Google Scholar] [CrossRef] [PubMed]
- Stoner, R.D.; Terres, G. Enhanced antitoxin responses in irradiated mice elicited by complexes of tetanus toxoid and specific antibody. J. Immunol. 1963, 91, 761–770. [Google Scholar] [PubMed]
- Stoner, R.D.; Terres, G.; Hess, M.W. Early and enhanced antioxin responses elicited with complexes of tetanus toxoid and specific mouse and human antibodies. J. Infect. Dis. 1975, 131, 230–238. [Google Scholar] [CrossRef]
- Whitfill, C.E.; Haddad, E.E.; Ricks, C.A.; Skeeles, J.K.; Newberry, L.A.; Beasley, J.N.; Andrews, P.D.; Thoma, J.A.; Wakenell, P.S. Determination of optimum formulation of a novel infectious bursal disease virus (IBDV) vaccine constructed by mixing bursal disease antibody with IBDV. Avian Dis. 1995, 39, 687–699. [Google Scholar] [CrossRef]
- Barabas, A.Z.; Cole, C.D.; Barabas, A.D.; Lafreniere, R. Downregulation of a pathogenic autoantibody response by IgM autoantibodies directed against the nephritogenic antigen in slowly progressive Heymann nephritis. Pathol. Int. 2006, 56, 181–190. [Google Scholar] [CrossRef]
- Barabas, A.Z.; Cole, C.D.; Barabas, A.D.; Barabas, A.N.; Lafreniere, R. Effect of rat kidney fraction 3 (rKF3) antigen and specific IgM antibody against rKF3 on the progression of slowly progressive Heymann nephritis. Pathol. Int. 2006, 56, 516–529. [Google Scholar] [CrossRef]
- Barabas, A.Z.; Cole, C.D.; Lafreniere, R.; Weir, D.M. Regaining tolerance to a self-antigen by the modified vaccination technique. Clin. Rev. Allergy Immunol. 2013, 45, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Lerner, A.; Jeremias, P.; Matthias, T. The world incidence and prevalence of autoimmune diseases is increasing. Int. J. Celiac Dis. 2015, 3, 151–155. [Google Scholar] [CrossRef]
- Fairweather, D.; Frisancho-Kiss, S.; Rose, N.R. Sex differences in autoimmune disease from a pathological perspective. Am. J. Pathol. 2008, 173, 600–609. [Google Scholar] [CrossRef]
- Turtle, C.J.; Hanafi, L.A.; Berger, C.; Gooley, T.A.; Cherian, S.; Hudecek, M.; Sommermeyer, D.; Melville, K.; Pender, B.; Budiarto, T.M.; et al. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J. Clin. Investig. 2016, 126, 2123–2138. [Google Scholar] [CrossRef]
- Shoenfeld, Y.; Gershwin, M.E. Preface. The autoimmunologist and the congresses of autoimmunity. Ann. N. Y. Acad. Sci. 2009, 1173, 1–3. [Google Scholar] [CrossRef]
- Toubi, E.; Shoenfeld, Y. Protective autoimmunity in cancer (review). Oncol. Rep. 2007, 17, 245–251. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barabas, A.; Cole, C.; Kovacs, Z.; Kovacs, E.; Lafreniere, R. The Modified Vaccination Technique. Vaccines 2019, 7, 1. https://doi.org/10.3390/vaccines7010001
Barabas A, Cole C, Kovacs Z, Kovacs E, Lafreniere R. The Modified Vaccination Technique. Vaccines. 2019; 7(1):1. https://doi.org/10.3390/vaccines7010001
Chicago/Turabian StyleBarabas, Arpad, Chad Cole, Zoltan Kovacs, Erno Kovacs, and Rene Lafreniere. 2019. "The Modified Vaccination Technique" Vaccines 7, no. 1: 1. https://doi.org/10.3390/vaccines7010001




