Frequency of Adverse Events Following Q Fever Immunisation in Young Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Q-VAX® and Q-VAX® Skin Test
2.3. Pre-Vaccination Testing
2.4. Data Collection and Survey Design
2.5. Statistical Analysis
3. Results
3.1. Demographics
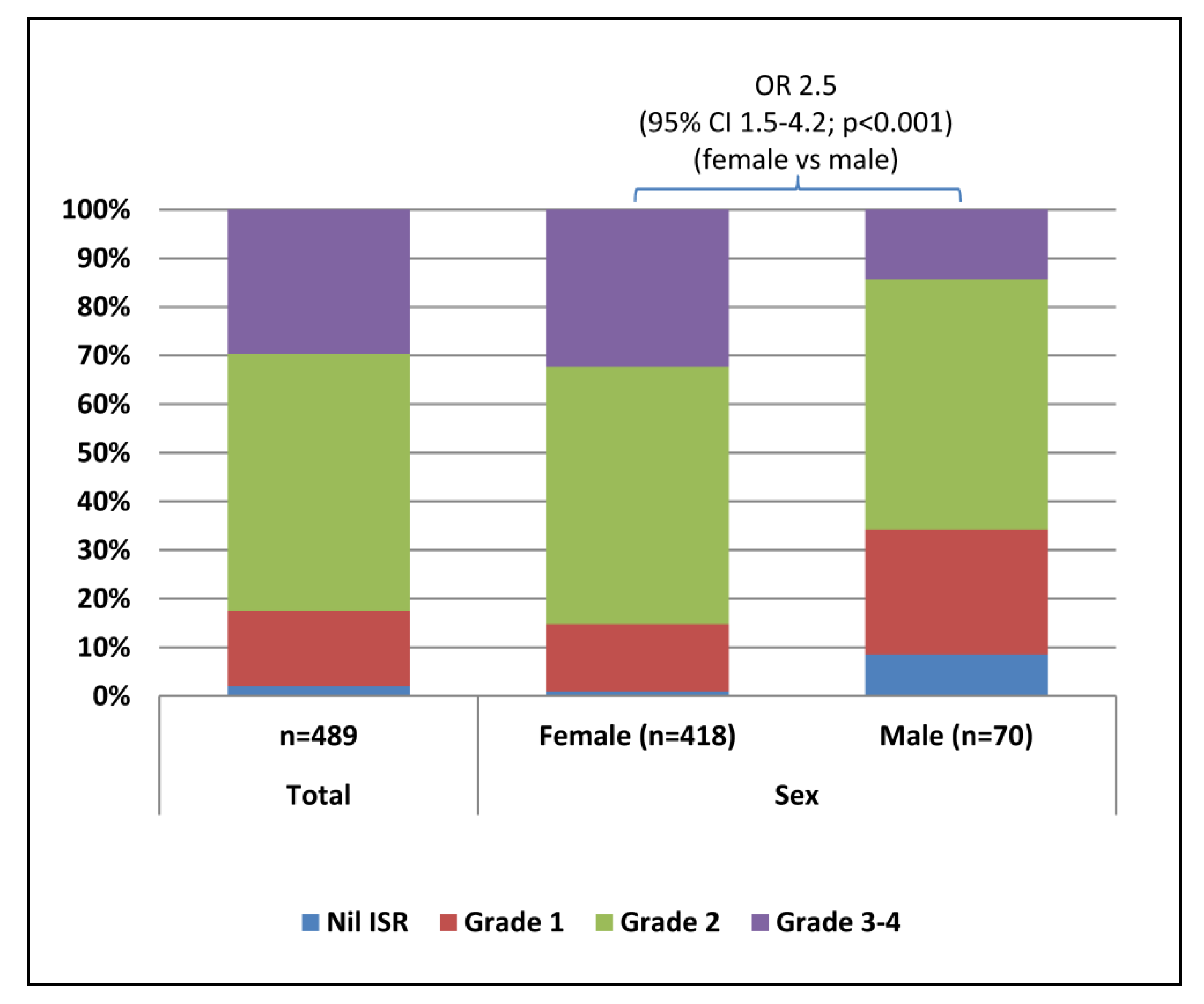
3.2. Injection Site Reactions
3.3. Systemic Adverse Events
3.4. Medical Attention Following Vaccination
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Angelakis, E.; Raoult, D. Emergence of Q Fever. Iran. J. Public Health 2011, 40, 1–18. [Google Scholar] [PubMed]
- Million, M.; Raoult, D. Recent advances in the study of Q fever epidemiology, diagnosis and management. J. Infect. 2015, 71, S2–S9. [Google Scholar] [CrossRef] [PubMed]
- Angelakis, E.; Raoult, D. Q Fever. Vet. Microbiol. 2010, 140, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Guatteo, R.; Beaudeau, F.; Berri, M.; Rodolakis, A.; Joly, A.; Seegers, H. Shedding routes of Coxiella burnetii in dairy cows: Implications for detection and control. Vet. Res. 2006, 37, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Raoult, D.; Marrie, T.J.; Mege, J.L. Natural history and pathophysiology of Q fever. Lancet Infect. Dis. 2005, 5, 219–226. [Google Scholar] [CrossRef]
- Hogerwerf, L.; Borlee, F.; Still, K.; Heederik, D.; van Rotterdam, B.; de Bruin, A.; Nielen, M.; Wouters, I.M. Detection of Coxiella burnetii DNA in inhalable airborne dust samples from goat farms after mandatory culling. Appl. Environ. Microbiol. 2012, 78, 5410–5412. [Google Scholar] [CrossRef] [PubMed]
- Kersh, G.J.; Fitzpatrick, K.A.; Self, J.S.; Priestley, R.A.; Kelly, A.J.; Lash, R.R.; Marsden-Haug, N.; Nett, R.J.; Bjork, A.; Massung, R.F.; et al. Presence and persistence of Coxiella burnetii in the environments of goat farms associated with a Q fever outbreak. Appl. Environ. Microbiol. 2013, 79, 1697–1703. [Google Scholar] [CrossRef]
- Tozer, S.J.; Lambert, S.B.; Strong, C.L.; Field, H.E.; Sloots, T.P.; Nissen, M.D. Potential Animal and Environmental Sources of Q Fever Infection for Humans in Queensland. Zoonoses Public Health. 2014, 61, 105–112. [Google Scholar] [CrossRef]
- Kosatsky, T. Household outbreak of Q fever pneumonia related to parturient cat. Lancet Infect. Dis. 1984, 324, 1447–1449. [Google Scholar] [CrossRef]
- Komiya, T.; Sadamasu, K.; Toriniwa, H.; Kato, K.; Arashima, Y.; Fukushi, H.; Hirai, K.; Arakawa, Y. Epidemiological survey on the route of Coxiella burnetii infection in an animal hospital. J. Infect. Chemother. 2003, 9, 151–155. [Google Scholar] [CrossRef]
- Gibbons, G.C.; White, P.J. Q Fever in a Veterinary Hospital; An unusual Epidemiology. Presented at the ASID Zoonoses 2014 Conference, Brisbane, Australia, 25–26 July 2014. poster 216. [Google Scholar]
- Shapiro, A.J.; Bosward, K.L.; Heller, J.; Norris, J.M. Seroprevalence of Coxiella burnetii in domesticated and feral cats in eastern Australia. Vet. Microbiol. 2015, 177, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Raoult, D.; Fenollar, F.; Stein, A. Q fever during pregnancy: Diagnosis, treatment, and follow-up. Arch. Intern. Med. 2002, 162, 701–704. [Google Scholar] [CrossRef] [PubMed]
- Langley, J.M.; Marrie, T.J.; LeBlanc, J.C.; Almudevar, A.; Raoult, D. Coxiella burnetii seropositivity in parturient women is associated with adverse pregnancy outcomes. Am. J. Obstet. Gynecol. 2003, 189, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Denman, J.; Woods, M. Acute Q fever in pregnancy: Report and literature review. Intern. Med. J. 2009, 39, 479–481. [Google Scholar] [CrossRef] [PubMed]
- Sukocheva, O.A.; Marmion, B.P.; Storm, P.A.; Lockhart, M.; Turra, M.; Graves, S. Long-term persistence after acute Q fever of non-infective Coxiella burnetii cell components, including antigens. Q. J. Med. 2010, 103, 847–863. [Google Scholar] [CrossRef] [PubMed]
- Wildman, M.J.; Smith, E.G.; Groves, J.; Beattie, J.M.; Caul, E.O.; Ayres, J.G. Chronic fatigue following infection by Coxiella burnetii (Q fever): Ten-year follow-up of the 1989 UK outbreak cohort. Q. J. Med. 2002, 95, 527–538. [Google Scholar] [CrossRef]
- Morroy, G.; Keijmel, S.P.; Delsing, C.E.; Bleijenberg, G.; Langendam, M.; Timen, A.; Bleeker-Rovers, C.P. Fatigue following Acute Q-Fever: A Systematic Literature Review. PLoS ONE 2016, 11, e0155884. [Google Scholar] [CrossRef]
- Gefenaite, G.; Munster, J.M.; van Houdt, R.; Hak, E. Effectiveness of the Q fever vaccine: A meta-analysis. Vaccine 2011, 29, 395–398. [Google Scholar] [CrossRef]
- Marmion, B.P. Q fever: The long journey to control by vaccination. Med. J. Aust. 2007, 186, 164–166. [Google Scholar]
- Bond, K.A.; Franklin, L.J.; Sutton, B.; Firestone, S.M. Q-Vax Q fever vaccine failures, Victoria, Australia 1994–2013. Vaccine 2017, 35, 7084–7087. [Google Scholar] [CrossRef]
- The Australian Immunisation Handbook, 10th ed.; Australian Government Department of Health and Ageing: Canberra, Australia, 2013. Available online: https://immunisationhandbook.health.gov.au/vaccine-preventable-diseases/q-fever (accessed on 5 October 2018).
- Gidding, H.F.; Wallace, C.; Lawrence, G.L.; McIntyre, P.B. Australia’s national Q fever vaccination program. Vaccine 2009, 27, 2037–2041. [Google Scholar] [CrossRef] [PubMed]
- Sellens, E.; Norris, J.M.; Dhand, N.K.; Heller, J.; Hayes, L.; Gidding, H.F.; Willaby, H.; Wood, N.; Bosward, K.L. Q Fever Knowledge, Attitudes and Vaccination Status of Australia’s Veterinary Workforce in 2014. PLoS ONE 2016, 11, e0146819. [Google Scholar] [CrossRef] [PubMed]
- Number of Notifications of Q Fever, Australia, in the Period of 1991 to 2016 and Year-to-Date Notifications for 2017. National Notifiable Diseases Surveillance System: Australian Govenernment Department of Health. 2017. Available online: http://www9.health.gov.au/cda/source/cda-index.cfm (accessed on 4 September 2017).
- Van der Hoek, W.; Dijkstra, F.; Schimmer, B.; Schneeberger, P.M.; Vellema, P.; Wijkmans, C. Q fever in the Netherlands: An update on the epidemiology and control measures. Eurosurveillance 2010, 15, 19520. [Google Scholar] [PubMed]
- Isken, L.D.; Kraaij-Dirkzwager, M.; Vermeer-de Bondt, P.E.; Rümke, H.C.; Wijkmans, C.; Opstelten, W.; Timen, A. Implementation of a Q fever vaccination program for high-risk patients in The Netherlands. Vaccine 2013, 31, 2617–2622. [Google Scholar] [CrossRef] [PubMed]
- Tozer, S.J.; Lambert, S.B.; Sloots, T.P.; Nissen, M.D. Q fever seroprevalence in metropolitan samples is similar to rural/remote samples in Queensland, Australia. Eur. J. Clin. Microbiol. Infect. Dis. 2011, 30, 1287–1293. [Google Scholar] [CrossRef] [PubMed]
- Islam, A.; Ferguson, J.; Givney, R.; Graves, S. Seroprevalence to Coxiella burnetii among residents of the Hunter New England region of New South Wales, Australia. Am. J. Trop. Med. Hyg. 2011, 84, 318–320. [Google Scholar] [CrossRef] [PubMed]
- Archer, B.N.; Hallahan, C.; Stanley, P.; Seward, K.; Lesjak, M.; Hope, K.; Brown, A. Atypical outbreak of Q fever affecting low-risk residents of a remote rural town in New South Wales. Commun. Dis. Intell. 2017, 41, E125–E133. [Google Scholar]
- Bond, K.; Vincent, G.; Wilks, C.; Franklin, L.; Sutton, B.; Stenos, J.; Cowan, R.; Lim, K.; Athan, E.; Harris, O. One Health approach to controlling a Q fever outbreak on an Australian goat farm. Epidemiol. Infect. 2016, 144, 1129–1141. [Google Scholar] [CrossRef] [PubMed]
- Lord, H.; Fletcher-Lartey, S.; Weerasinghe, G.; Chandra, M.; Egana, N.; Schembrib, N.; Conaty, S. AQ fever cluster among workers at an abattoir in south-western Sydney, Australia, 2015. West. Pac. Surveill. Response J. 2016, 7, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Marmion, B.P.; Ormsbee, R.A.; Kyrkou, M.; Wright, J.; Worswick, D.A.; Izzo, A.A.; Esterman, A.; Feery, B.; Shapiro, R.A. Vaccine prophylaxis of abattoir-associated Q fever: Eight years’ experience in Australian abattoirs. Epidemiol. Infect. 1990, 104, 275–287. [Google Scholar] [CrossRef]
- Ackland, J.R.; Worswick, D.A.; Marmion, B.P. Vaccine prophylaxis of Q Fever: A follow up study of the efficacy of Q Vax (CSL) 1985–1990. Med. J. Aust. 1990, 160, 704–708. [Google Scholar]
- Marmion, B.P.; Kyrkou, M.; Worswick, D.A.; Esterman, A.; Ormsbee, R.A.; Wright, J.; Cameron, S.; Feery, B.; Collins, W. Vaccine Prophylaxis of Abattoir-Associated Q Fever. Lancet 1984, 324, 1411–1414. [Google Scholar] [CrossRef]
- Schoffelen, T.; Wong, A.; Rümke, H.C.; Netea, M.G.; Timen, A.; van Deuren, M.; Vermeer-de Bondt, P.E. Adverse events and association with age, sex and immunological parameters of Q fever vaccination in patients at risk for chronic Q fever in the Netherlands 2011. Vaccine 2014, 32, 6622–6630. [Google Scholar] [CrossRef] [PubMed]
- Bentsi-Enchill, A.; Duclos, P.; Pal, S.; Zuber, P.; Bahri, P.; Ball, R.; Freitas Dias, M.; Keller-Stanislawski, B.; Kurz, X.; Bachtiar, N.S.; et al. CIOMS/WHO Working Group on Vaccine Pharmacovigilance: Definition and Application of Terms for Vaccine Pharmacovigilance; Bentsi-Enchil, Ed.; Council for International Organizations of Medical Sciences (CIOMS): Geneva, Switzerland, 2012. [Google Scholar]
- About the DAEN—Medicines; Australian Government Department of Health: Canberra, Australia, 2018. Available online: https://www.tga.gov.au/about-daen-medicines (accessed on 5 November 2018).
- Q-VAX® Q Fever Vaccine and Q-VAX® Skin Test (R100517 & 100518) Product Information—TGA Approved; Seqirus Pty Ltd.: Parkville, Australia, 2017; Available online: https://www.seqirus.com.au/docs/328/827/Q-VAX%20-%20Approved%20Product%20Information_v5_19%20Dec%202016.pdf (accessed on 5 October 2018).
- Cook, I.F. Sex differences in injection site reactions with human vaccines. Hum. Vaccines 2014, 5, 441–449. [Google Scholar] [CrossRef]
- Beyer, W.E.; Palache, A.M.; Kerstens, R.; Masurel, N. Gender differences in local and systemic reactions to inactivated influenza vaccine, established by a meta-analysis of fourteen independent studies. Eur. J. Clin. Microbiol. Infect. Dis. 1996, 15, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Fink, A.L.; Klein, S.L. Sex and Gender Impact Immune Responses to Vaccines among the Elderly. Physiology 2015, 30, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.L.; Jedlicka, A.; Pekosz, A. The Xs and Y of immune responses to viral vaccines. Lancet Infect. Dis. 2010, 10, 338–349. [Google Scholar] [CrossRef]
- Textoris, J.; Ban, L.H.; Capo, C.; Raoult, D.; Leone, M.; Mege, J.-L. Sex-Related Differences in Gene Expression Following Coxiella burnetii Infection in Mice: Potential Role of Circadian Rhythm. PLoS ONE 2010, 5, e12190. [Google Scholar] [CrossRef] [PubMed]
- Andoh, M.; Zhang, G.; Russell-Lodrigue, K.E.; Shive, H.R.; Weeks, B.R.; Samuel, J.E. T cells are essential for bacterial clearance, and gamma interferon, tumor necrosis factor alpha, and B cells are crucial for disease development in Coxiella burnetii infection in mice. Infect. Immun. 2007, 75, 3245–3255. [Google Scholar] [CrossRef] [PubMed]
- Leone, M.; Honstettre, A.; Lepidi, H.; Capo, C.; Bayard, F.; Raoult, D.; Mege, J.L. Effect of Sex on Coxiella burnetii Infection: Protective Role of 17β-Estradiol. J. Infect. Dis. 2002, 189, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Moro-García, M.A.; Alonso-Arias, R.; López-Larrea, C. When aging reaches CD4+ T-cells: Phenotypic and functional changes. Front. Immunol. 2013, 4, 107. [Google Scholar] [CrossRef] [PubMed]
- Gerolimatos, L.A.; Edelstein, B.A. Predictors of health anxiety among older and young adults. Int. Psychogeriatr. 2012, 24, 1998–2008. [Google Scholar] [CrossRef] [PubMed]
- Jackson, L.A.; Yu, O.; Belongia, E.A.; Hambidge, S.J.; Nelson, J.; Baxter, R.; Naleway, A.; Gay, C.; Nordin, J.; Baggs, J. Frequency of medically attended adverse events following tetanus and diphtheria toxoid vaccine in adolescents and young adults: A Vaccine Safety Datalink study. BMC Infect. Dis. 2009, 9, 165. [Google Scholar] [CrossRef] [PubMed]
- 2013 Special Courses. Available online: https://docs.education.gov.au/documents/2013-special-courses (accessed on 23 September 2018).
- Therapeutic Goods Administration Database of Adverse Event Notifications—Medicines; Australian Government Department of Health: Canberra, Australia, 2017. Available online: http://apps.tga.gov.au/PROD/DAEN/daen-entry.aspx (accessed on 11 October 2017).
| Grade | Severity | Description of Pain | Area of Erythema or Swelling |
|---|---|---|---|
| 1 | Mild | Pain to the touch, no obstruction of use | <2.5cm |
| 2 | Moderate | Pain on movement, some interference with normal activity | 2.5 to <7.5cm |
| 3 | Severe | Considerable pain in rest, obstruction of use | 7.5 to <15 cm |
| 4 | Extensive | - | 15 cm or greater |
| Variable | University of Sydney | University of Queensland | Charles Sturt University | Total Vaccinees |
|---|---|---|---|---|
| Sex | ||||
| Females n (%) | 194 (85.5) | 106 (80.9) | 124 (87.9) | 424 (85.0) |
| Males n (%) | 33 (14.5) | 25 (19.1) | 16 (11.3) | 74 (14.8) |
| Unspecified n (%) | 0 (0) | 0 (0) | 1 (<1) | 1 (<1) |
| Column Total | 227 (100) | 131 (100) | 141 (100) | 499 (100) |
| Age | ||||
| Range (years) | 17–43 | 17–29 | 17–38 | 17–43 |
| Mean (years) | 20.3 | 19.0 | 19.7 | 19.8 |
| Median (years) | 19.0 | 18.0 | 19.0 | 18.0 |
| Interquartile Range | 3.0 | 3.0 | 2.0 | 2.0 |
| Year of vaccination | ||||
| 2013 n (%) | 143 (63.0) | 0 (0) | 0 (0) | 143 (28.7) |
| 2014 n (%) | 75 (33.0) | 21 (16.0) | 19 (13.5) | 115 (23.0) |
| 2015 n (%) | 9 (4.0) | 110 (84.0) | 55 (39.0) | 174 (34.9) |
| 2016 n (%) | 0 (0) | 0 (0) | 67 (47.5) | 67 (13.4) |
| Column Total | 227 (100) | 131 (100) | 141 (100) | 499 (100) |
| All Respondents | Sex | ||
|---|---|---|---|
| Females | Males | ||
| Any Local Injection Site Reaction | |||
| Yes n (%) | 489 (98.0) | 420 (99.1) | 68 (91.9) |
| No n (%) | 10 (2.0) | 4 (<1) | 6 (8.1) |
| Column Total n (%) | 499 (100) | 424 (100) | 74 (100) |
| OR (95% CI; p-value) | - | 9.3 (2.5–33.8; <0.001) | ref |
| Injection Site Pain | |||
| Yes n (%) | 473 (94.8) | 411 (96.9) | 61 (82.4) |
| No n (%) | 26 (5.2) | 13 (3.1) | 13 (17.6) |
| Column Total n (%) | 499 (100) | 424 (100) | 74 (100) |
| OR (95% CI; p-value) | - | 6.7 (3.0–15.2; <0.001) | ref |
| Injection Site Swelling | |||
| Yes n (%) | 289 (57.9) | 257 (60.6) | 32 (43.2) |
| No n (%) | 208 (41.7) | 165 (38.9) | 42 (56.8) |
| Not Specified n (%) | 2 (<1) | 2 (0.5) | 0 (0) |
| Column Total n (%) | 499 (100) | 424 (100) | 74 (100) |
| OR (95% CI; p-value) | - | 2.5 (1.5–4.3; <0.001) | ref |
| Injection Site Erythema | |||
| Yes n (%) | 289 (57.9) | 257 (60.6) | 31 (41.9) |
| No n (%) | 207 (41.5) | 165 (38.9) | 42 (56.8) |
| Not Specified n (%) | 3 (<1) | 2 (<1) | 1 (1.4) |
| Column Total n (%) | 499 (100) | 424 (100) | 74 (100) |
| OR (95% CI; p-value) | - | 3.3 (1.9–5.7; <0.001) | ref |
| All Respondents | Sex | ||
|---|---|---|---|
| Females | Males | ||
| Any Systemic AEFI | |||
| Yes n (%) | 297 (59.5) | 263 (62.0) | 34 (45.9) |
| No n (%) | 202 (40.5) | 161 (38.0) | 40 (54.1) |
| Column Total n (%) | 499 (100) | 424 (100) | 74 (100) |
| OR (95% CI; p-value) | - | 1.9 (1.1–3.1; 0.016) | ref |
| Fever | |||
| Yes n (%) | 86 (17.2) | 76 (17.9) | 10 (13.5) |
| No n (%) | 409 (82.0) | 344 (81.1) | 64 (86.5) |
| Not Specified n (%) | 4 (<1) | 4 (<1) | 0 (0) |
| Column Total n (%) | 499 (100) | 424 (100) | 74 (100) |
| OR (95% CI; p-value) | - | 1.4 (0.7–2.8; 0.384) | ref |
| Headache | |||
| Yes n (%) | 219 (43.9) | 194 (45.8) | 25 (33.8) |
| No n (%) | 276 (55.3) | 226 (53.3) | 49 (66.2) |
| Not Specified n (%) | 4 (<1) | 4 (<1) | 0 (0) |
| Column Total n (%) | 499 (100) | 424 (100) | 74 (100) |
| OR (95% CI; p-value) | - | 1.6 (1.0–2.8; 0.058) | ref |
| Lethargy | |||
| Yes n (%) | 213 (42.7) | 190 (44.8) | 23 (31.1) |
| No n (%) | 283 (56.7) | 233 (55.0) | 49 (66.2) |
| Not Specified n (%) | 3 (<1) | 1 (<1) | 2 (2.7) |
| Column Total n (%) | 499 (100) | 424 (100) | 74 (100) |
| OR (95% CI; p-value) | - | 1.7 (1.0–3.0; 0.048) | ref |
| Joint Pain | |||
| Yes n (%) | 123 (24.6) | 112 (26.4) | 11 (14.9) |
| No n (%) | 374 (74.9) | 311 (73.3) | 62 (83.8) |
| Not Specified n (%) | 2 (<1) | 1 (<1) | 1 (1.4) |
| Column Total n (%) | 499 (100) | 424 (100) | 74 (100) |
| OR (95% CI; p-value) | - | 2.0 (1.0–3.9; 0.055) | ref |
| Independent Data | Clinical Trial Data | Registration Holder Surveillance Data | ||
|---|---|---|---|---|
| Current Study | Schoffelen et al. [36] (2014) | Marmion et al. [33] (1990) | Seqirus [39] (2017) | |
| Study population | Veterinary students; median age 18 years, predominantly female | Persons in community with high risk of Q fever due to comorbidities; median age 67 years | Abattoir workers; median age 29 years, predominantly male | Not applicable |
| Number of vaccinees for which AEFI results are reported | 499 | 970 | 464 | Not applicable |
| Any local or systemic AEFI | 98% | 82% | * | * |
| Any Local ISR | 98% | 80% | * | * |
| Injection Site Pain | 95% | * | 48% | ≥10% |
| Injection Site Swelling | 58% | * | * | ≥10% |
| Injection Site Erythema | 58% | * | 33% | ≥10% |
| Any Systemic AEFI | 60% | 43% | * | * |
| Headache | 44% | * | 9% | <10% and ≥1% |
| Lethargy | 43% | * | * | <1% and ≥0.1% |
| Joint Pain | 25% | * | * | <0.01% |
| Fever | 17% | 9% | 0.2% | <1% and ≥0.1% |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sellens, E.; Bosward, K.L.; Willis, S.; Heller, J.; Cobbold, R.; Comeau, J.L.; Norris, J.M.; Dhand, N.K.; Wood, N. Frequency of Adverse Events Following Q Fever Immunisation in Young Adults. Vaccines 2018, 6, 83. https://doi.org/10.3390/vaccines6040083
Sellens E, Bosward KL, Willis S, Heller J, Cobbold R, Comeau JL, Norris JM, Dhand NK, Wood N. Frequency of Adverse Events Following Q Fever Immunisation in Young Adults. Vaccines. 2018; 6(4):83. https://doi.org/10.3390/vaccines6040083
Chicago/Turabian StyleSellens, Emily, Katrina L. Bosward, Susan Willis, Jane Heller, Rowland Cobbold, Jeannette L. Comeau, Jacqueline M. Norris, Navneet K. Dhand, and Nicholas Wood. 2018. "Frequency of Adverse Events Following Q Fever Immunisation in Young Adults" Vaccines 6, no. 4: 83. https://doi.org/10.3390/vaccines6040083
APA StyleSellens, E., Bosward, K. L., Willis, S., Heller, J., Cobbold, R., Comeau, J. L., Norris, J. M., Dhand, N. K., & Wood, N. (2018). Frequency of Adverse Events Following Q Fever Immunisation in Young Adults. Vaccines, 6(4), 83. https://doi.org/10.3390/vaccines6040083






