Does the Immunocompetent Status of Cancer Patients Have an Impact on Therapeutic DC Vaccination Strategies?
Abstract
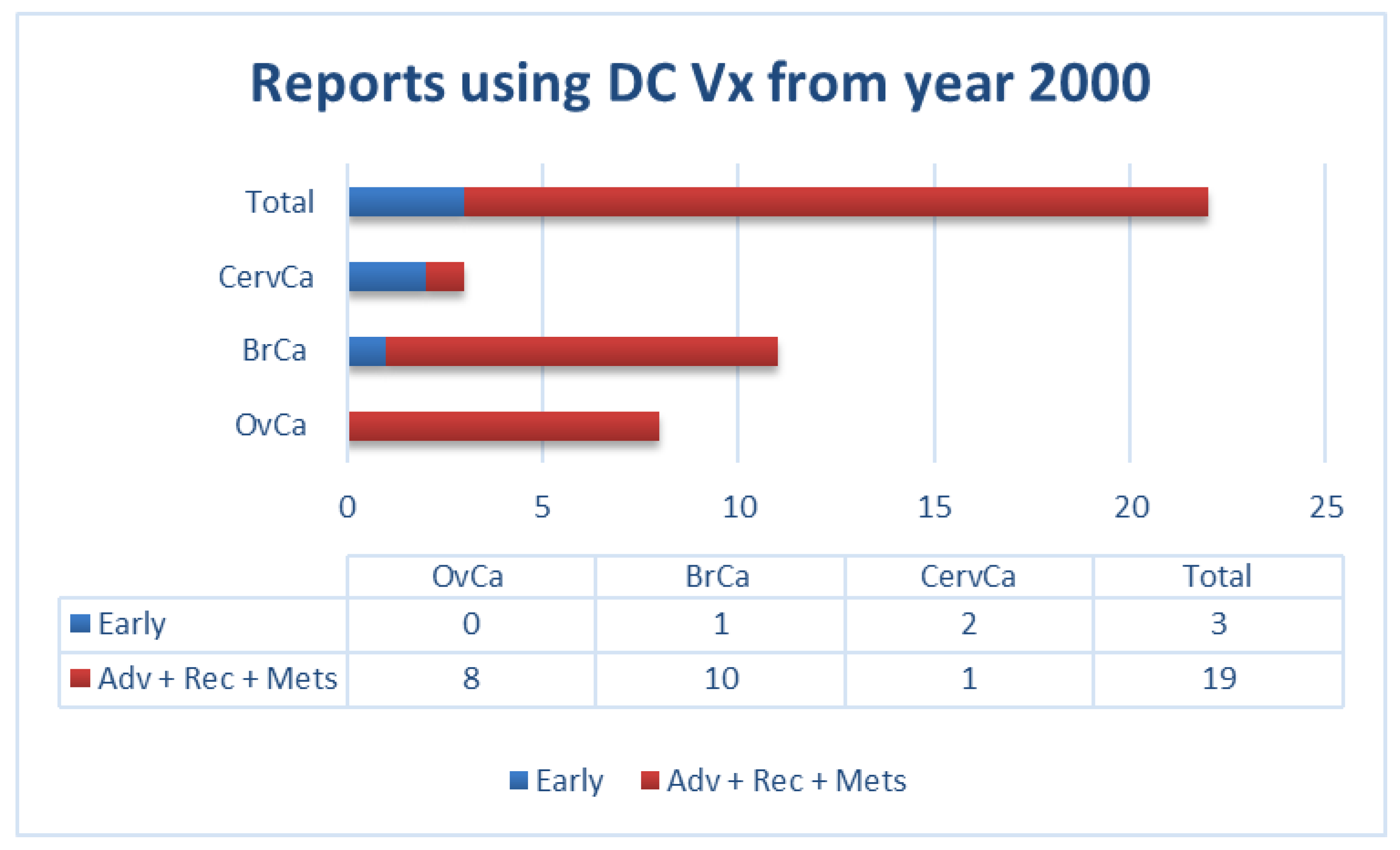
1. Therapeutic DC Vaccines in Cancer Patients: Context and Current Situation
- In melanoma, DC therapy had 8.5% ORR, similar to dacarbazine (standard of care), or ipilimumab (5–15%)
- In prostate cancer patients, ORR was 7.1% after DC vaccination, compared to 10% with conventional chemotherapeutic drugs
- In patients with malignant glioma, ORR after DC therapy was 15.6%
- In advanced renal cell cancer (RCC), ORR was 11.5%
2. Determinant Factors Impinging on the Efficacy of Therapeutic Vaccination
2.1. Tolerance Mechanisms Exerted by Tumors and Their Microevironment to Evade Immune Recognition
2.2. Reduced DC Fitness in Cancer Patients vs. Healthy Subjects
2.2.1. Peripheral Blood DC Defects in Cancer Patients
2.2.2. Defects of Monocyte-Derived DCs in Cancer Patients
2.3. Other Potential Factors Implicated in Reduced Immunocompetence
3. Proposed Actions to Counteract Decreased Immunocompetent Status in Cancer Patients
3.1. Patients’ Screening and Assessment
3.2. Improving DC Vaccine Formulation and Delivery Route
3.3. Combinatorial Approaches with Additional Therapeutic Agents
3.3.1. Immune Checkpoint Inhibitors
3.3.1.1 Anti-CTLA-4 Treatment
3.3.1.2. Anti-PD-1 and Anti-PD-L1 Treatment
3.3.1.3. Concomitant Blockade of CTLA-4 and PD-1/PD-L1
3.3.2. Other Potential Combinations to Enhance DC Vaccination
3.3.2.1. Inhibiting Tumor Angiogenesis and Improving Intratumor T-cell Infiltration
3.3.2.2. Inhibiting STAT-3 activity of tumors
3.3.2.3. Increasing Tumor Immunogenicity with Chemotherapeutic Drugs and Radiotherapy
3.3.2.4. Additional Adjuvants Specific for Therapeutic Vaccination Against Ovarian Cancer
3.4. Potential Supporting Actions that Could Add Therapeutic Benefit
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bol, K.F.; Schreibelt, G.; Gerritsen, W.R.; de Vries, I.J.M.; Figdor, C.G. Dendritic Cell–Based Immunotherapy: State of the Art and Beyond. Clin. Cancer Res. 2016, 22, 1897–1906. [Google Scholar] [CrossRef] [PubMed]
- Steinman, R.M.; Cohn, Z.A. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J. Exp. Med. 1973, 137, 1142–1162. [Google Scholar]
- O’Keeffe, M.; Mok, W.H.; Radford, K.J. Human dendritic cell subsets and function in health and disease. Cell. Mol. Life Sci. 2015, 72, 4309–4325. [Google Scholar] [CrossRef] [PubMed]
- Banchereau, J.; Briere, F.; Caux, C.; Davoust, J.; Lebecque, S.; Liu, Y.J.; Pulendran, B.; Palucka, K. Immunobiology of dendritic cells. Annu. Rev. Immunol. 2000, 18, 767–811. [Google Scholar] [CrossRef] [PubMed]
- Banchereau, J.; Palucka, A.K. Dendritic cells as therapeutic vaccines against cancer. Nat. Rev. Immunol. 2005, 5, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Sabado, R.L.; Balan, S.; Bhardwaj, N. Dendritic cell-based immunotherapy. Cell Res. 2017, 27, 74–95. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Manjili, M.H.; Subjeck, J.R.; Sarkar, D.; Fisher, P.B.; Wang, X.-Y. Therapeutic Cancer Vaccines: Past, Present and Future. Adv. Cancer Res. 2013, 119, 421–475. [Google Scholar] [PubMed]
- Draube, A.; Klein-González, N.; Mattheus, S.; Brillant, C.; Hellmich, M.; Engert, A.; von Bergwelt-Baildon, M. Dendritic Cell Based Tumor Vaccination in Prostate and Renal Cell Cancer: A Systematic Review and Meta-Analysis. PLoS ONE 2011, 6, e18801. [Google Scholar] [CrossRef] [PubMed]
- Boudewijns, S.; Westdorp, H.; Koornstra, R.H.T.; Aarntzen, E.H.J.G.; Schreibelt, G.; Creemers, J.H.A.; Punt, C.J.A.; Figdor, C.G.; de Vries, I.J.M.; Gerritsen, W.R.; et al. Immune-related Adverse Events of Dendritic Cell Vaccination Correlate With Immunologic and Clinical Outcome in Stage III and IV Melanoma Patients. J. Immunother. 2016, 39, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Beer, T.M.; Bernstein, G.T.; Corman, J.M.; Glode, L.M.; Hall, S.J.; Poll, W.L.; Schellhammer, P.F.; Jones, L.A.; Xu, Y.; Kylstra, J.W. Randomized trial of autologous cellular immunotherapy with sipuleucel-T in androgen-dependent prostate cancer. Clin. Cancer Res. 2011, 17, 4558–4567. [Google Scholar] [CrossRef] [PubMed]
- Small, E.J.; Schellhammer, P.F.; Higano, C.S.; Redfern, C.H.; Nemunaitis, J.J.; Valone, F.H.; Verjee, S.S.; Jones, L.A.; Hershberg, R.M. Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J. Clin. Oncol. 2006, 24, 3089–3094. [Google Scholar] [CrossRef] [PubMed]
- Kantoff, P.W.; Higano, C.S.; Shore, N.D.; Berger, E.R.; Small, E.J.; Penson, D.F.; Redfern, C.H.; Ferrari, A.C.; Dreicer, R.; Sims, R.B. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N. Engl. J. Med. 2010, 363, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Schadendorf, D.; Ugurel, S.; Schuler-Thurner, B.; Nestle, F.O.; Enk, A.; Brocker, E.B.; Grabbe, S.; Rittgen, W.; Edler, L.; Sucker, A.; et al. Dacarbazine (DTIC) versus vaccination with autologous peptide-pulsed dendritic cells (DC) in first-line treatment of patients with metastatic melanoma: A randomized phase III trial of the DC study group of the DeCOG. Ann. Oncol. 2006, 17, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Leonhartsberger, N.; Ramoner, R.; Falkensammer, C.; Rahm, A.; Gander, H.; Holtl, L.; Thurnher, M. Quality of life during dendritic cell vaccination against metastatic renal cell carcinoma. Cancer Immunol. Immunother. 2012, 61, 1407–1413. [Google Scholar] [CrossRef] [PubMed]
- Saxena, M.; Bhardwaj, N. Re-Emergence of Dendritic Cell Vaccines for Cancer Treatment. Trends Cancer 2018, 4, 119–137. [Google Scholar] [CrossRef] [PubMed]
- Anguille, S.; Smits, E.L.; Lion, E.; van Tendeloo, V.F.; Berneman, Z.N. Clinical use of dendritic cells for cancer therapy. Lancet. Oncol. 2014, 15, e257–e267. [Google Scholar] [CrossRef]
- Rosenberg, S.A.; Yang, J.C.; Restifo, N.P. Cancer immunotherapy: Moving beyond current vaccines. Nat. Med. 2004, 10, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Bol, K.F.; Aarntzen, E.H.; Hout, F.E.; Schreibelt, G.; Creemers, J.H.; Lesterhuis, W.J.; Gerritsen, W.R.; Grunhagen, D.J.; Verhoef, C.; Punt, C.J.; et al. Favorable overall survival in stage III melanoma patients after adjuvant dendritic cell vaccination. Oncoimmunology 2016, 5, e1057673. [Google Scholar] [CrossRef] [PubMed]
- Nakai, N.; Hartmann, G.; Kishimoto, S.; Katoh, N. Dendritic cell vaccination in human melanoma: Relationships between clinical effects and vaccine parameters. Pigment Cell Melanoma Res. 2010, 23, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Triozzi, P.L.; Khurram, R.; Aldrich, W.A.; Walker, M.J.; Kim, J.A.; Jaynes, S. Intratumoral injection of dendritic cells derived in vitro in patients with metastatic cancer. Cancer 2000, 89, 2646–2654. [Google Scholar] [CrossRef]
- Vonderheide, R.H.; Domchek, S.M.; Schultze, J.L.; George, D.J.; Hoar, K.M.; Chen, D.-Y.; Stephans, K.F.; Masutomi, K.; Loda, M.; Xia, Z.; et al. Vaccination of Cancer Patients Against Telomerase Induces Functional Antitumor CD8+ T Lymphocytes. Clin. Cancer Res. 2004, 10, 828–839. [Google Scholar] [CrossRef] [PubMed]
- Svane, I.M.; Pedersen, A.E.; Johnsen, H.E.; Nielsen, D.; Kamby, C.; Gaarsdal, E.; Nikolajsen, K.; Buus, S.; Claesson, M.H. Vaccination with p53-peptide-pulsed dendritic cells, of patients with advanced breast cancer: Report from a phase I study. Cancer Immunol. Immunother. 2004, 53, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Danet-Desnoyers, G.-a.H.; Luongo, J.L.; Bonnet, D.A.; Domchek, S.M.; Vonderheide, R.H. Telomerase vaccination has no detectable effect on SCID-repopulating and colony-forming activities in the bone marrow of cancer patients. Exp. Hematol. 2005, 33, 1275–1280. [Google Scholar] [CrossRef] [PubMed]
- Svane, I.M.; Pedersen, A.E.; Johansen, J.S.; Johnsen, H.E.; Nielsen, D.; Kamby, C.; Ottesen, S.; Balslev, E.; Gaarsdal, E.; Nikolajsen, K.; et al. Vaccination with p53 peptide-pulsed dendritic cells is associated with disease stabilization in patients with p53 expressing advanced breast cancer; monitoring of serum YKL-40 and IL-6 as response biomarkers. Cancer Immunol. Immunother. 2007, 56, 1485–1499. [Google Scholar] [CrossRef] [PubMed]
- Svane, I.M.; Pedersen, A.E.; Nikolajsen, K.; Zocca, M.-B. Alterations in p53-specific T cells and other lymphocyte subsets in breast cancer patients during vaccination with p53-peptide loaded dendritic cells and low-dose interleukin-2. Vaccine 2008, 26, 4716–4724. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.S.; Boyer, J.; Schullery, D.S.; Gimotty, P.A.; Gamerman, V.; Bender, J.; Levine, B.L.; Coukos, G.; Rubin, S.C.; Morgan, M.A.; et al. Phase I/II randomized trial of dendritic cell vaccination with or without cyclophosphamide for consolidation therapy of advanced ovarian cancer in first or second remission. Cancer Immunol. Immunother. 2012, 61, 629–641. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Chiba, A.; Izawa, H.; Yanagida, E.; Okamoto, M.; Shimodaira, S.; Yonemitsu, Y.; Shibamoto, Y.; Suzuki, N.; Nagaya, M. The feasibility and clinical effects of dendritic cell-based immunotherapy targeting synthesized peptides for recurrent ovarian cancer. J. Ovarian Res. 2014, 7, 48. [Google Scholar] [CrossRef] [PubMed]
- Brossart, P.; Wirths, S.; Stuhler, G.; Reichardt, V.L.; Kanz, L.; Brugger, W. Induction of cytotoxic T-lymphocyte responses in vivo after vaccinations with peptide-pulsed dendritic cells. Blood 2000, 96, 3102–3108. [Google Scholar] [PubMed]
- Santin, A.D.; Bellone, S.; Palmieri, M.; Zanolini, A.; Ravaggi, A.; Siegel, E.R.; Roman, J.J.; Pecorelli, S.; Cannon, M.J. Human Papillomavirus Type 16 and 18 E7-Pulsed Dendritic Cell Vaccination of Stage IB or IIA Cervical Cancer Patients: A Phase I Escalating-Dose Trial. J. Virol. 2008, 82, 1968–1979. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Santin, A.D.; Bellone, S.; Gupta, S.; Nakagawa, M. A novel CD4 T-cell epitope described from one of the cervical cancer patients vaccinated with HPV 16 or 18 E7-pulsed dendritic cells. Cancer Immunol. Immunother. 2009, 58, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Hernando, J.; Park, T.-W.; Kübler, K.; Offergeld, R.; Schlebusch, H.; Bauknecht, T. Vaccination with autologous tumour antigen-pulsed dendritic cells in advanced gynaecological malignancies: Clinical and immunological evaluation of a phase I trial. Cancer Immunol. Immunother. 2002, 51, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.; Kim, C.-S.; Kim, S.-B.; Kim, Y.-m.; Kwon, S.-W.; Kim, Y.; Kim, H.; Lee, H. Combination therapy of renal cell carcinoma or breast cancer patients with dendritic cell vaccine and IL-2: Results from a phase I/II trial. J. Transl. Med. 2011, 9, 178. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.J.; Ning, Y.L.; Han, Y.S.; Min, H.Y.; Ye, H.; Zhu, Y.L.; Qian, K.Q. Autologous dendritic cell vaccine for estrogen receptor (ER)/progestin receptor (PR) double-negative breast cancer. Cancer Immunol. Immunother. 2012, 61, 1415–1424. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.L.; Kandalaft, L.E.; Tanyi, J.; Hagemann, A.R.; Motz, G.T.; Svoronos, N.; Montone, K.; Mantia-Smaldone, G.M.; Smith, L.; Nisenbaum, H.L.; et al. A dendritic cell vaccine pulsed with autologous hypochlorous Acid-oxidized ovarian cancer lysate primes effective broad antitumor immunity: From bench to bedside. Clin. Cancer Res. 2013, 19, 4801–4815. [Google Scholar] [CrossRef] [PubMed]
- Kandalaft, L.E.; Chiang, C.L.; Tanyi, J.; Motz, G.; Balint, K.; Mick, R.; Coukos, G. A Phase I vaccine trial using dendritic cells pulsed with autologous oxidized lysate for recurrent ovarian cancer. J. Transl. Med. 2013, 11, 149. [Google Scholar] [CrossRef] [PubMed]
- Kandalaft, L.E.; Powell, D.J.; Chiang, C.L.; Tanyi, J.; Kim, S.; Bosch, M.; Montone, K.; Mick, R.; Levine, B.L.; Torigian, D.A.; et al. Autologous lysate-pulsed dendritic cell vaccination followed by adoptive transfer of vaccine-primed ex vivo co-stimulated T cells in recurrent ovarian cancer. Oncoimmunology 2013, 2, e22664. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, P.; Ganeshrajah, S.; Raghanvan, R.K.; Singh, S.S.; Thangarajan, R. Development and clinical evaluation of dendritic cell vaccines for HPV related cervical cancer—A feasibility study. Asian Pac. J. Cancer Prev. 2014, 15, 5909–5916. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.; Kim, Y.M.; Kim, S.B.; Kim, C.S.; Kwon, S.W.; Kim, Y.; Kim, H.; Lee, H. Therapeutic DC vaccination with IL-2 as a consolidation therapy for ovarian cancer patients: A phase I/II trial. Cell. Mol. Immunol. 2015, 12, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Tanyi, J.L.; Bobisse, S.; Ophir, E.; Tuyaerts, S.; Roberti, A.; Genolet, R.; Baumgartner, P.; Stevenson, B.J.; Iseli, C.; Dangaj, D.; et al. Personalized cancer vaccine effectively mobilizes antitumor T cell immunity in ovarian cancer. Sci. Transl. Med. 2018, 10, eaao5931. [Google Scholar] [CrossRef] [PubMed]
- Avigan, D.; Vasir, B.; Gong, J.; Borges, V.; Wu, Z.; Uhl, L.; Atkins, M.; Mier, J.; McDermott, D.; Smith, T.; et al. Fusion Cell Vaccination of Patients with Metastatic Breast and Renal Cancer Induces Immunological and Clinical Responses. Clin. Cancer Res. 2004, 10, 4699–4708. [Google Scholar] [CrossRef] [PubMed]
- Mellman, I.; Coukos, G.; Dranoff, G. Cancer immunotherapy comes of age. Nature 2011, 480, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Kooi, S.; Zhang, H.Z.; Patenia, R.; Edwards, C.L.; Platsoucas, C.D.; Freedman, R.S. HLA class I expression on human ovarian carcinoma cells correlates with T-cell infiltration in vivo and T-cell expansion in vitro in low concentrations of recombinant interleukin-2. Cell. Immunol. 1996, 174, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Yoshihama, S.; Roszik, J.; Downs, I.; Meissner, T.B.; Vijayan, S.; Chapuy, B.; Sidiq, T.; Shipp, M.A.; Lizee, G.A.; Kobayashi, K.S. NLRC5/MHC class I transactivator is a target for immune evasion in cancer. Proc. Natl. Acad. Sci. USA 2016, 113, 5999. [Google Scholar] [CrossRef] [PubMed]
- Maine, C.J.; Aziz, N.H.; Chatterjee, J.; Hayford, C.; Brewig, N.; Whilding, L.; George, A.J.; Ghaem-Maghami, S. Programmed death ligand-1 over-expression correlates with malignancy and contributes to immune regulation in ovarian cancer. Cancer Immunol. Immunother. 2014, 63, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Teng, F.; Kong, L.; Yu, J. PD-L1 expression in human cancers and its association with clinical outcomes. OncoTargets Ther. 2016, 9, 5023–5039. [Google Scholar]
- Heusschen, R.; Griffioen, A.W.; Thijssen, V.L. Galectin-9 in tumor biology: A jack of multiple trades. Biochim. Biophys. Acta-Rev. Cancer 2013, 1836, 177–185. [Google Scholar]
- Lahm, H.; André, S.; Hoeflich, A.; Fischer, J.R.; Sordat, B.; Kaltner, H.; Wolf, E.; Gabius, H.J. Comprehensive galectin fingerprinting in a panel of 61 human tumor cell lines by RT-PCR and its implications for diagnostic and therapeutic procedures. J. Cancer Res. Clin. Oncol. 2001, 127, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Kouo, T.; Huang, L.; Pucsek, A.B.; Cao, M.; Solt, S.; Armstrong, T.; Jaffee, E. Galectin-3 Shapes Antitumor Immune Responses by Suppressing CD8+ T Cells via LAG-3 and Inhibiting Expansion of Plasmacytoid Dendritic Cells. Cancer Immunol. Res. 2015, 3, 412. [Google Scholar] [CrossRef] [PubMed]
- Godin-Ethier, J.; Hanafi, L.-A.; Piccirillo, C.A.; Lapointe, R. Indoleamine 2,3-Dioxygenase Expression in Human Cancers: Clinical and Immunologic Perspectives. Clin. Cancer Res. 2011, 17, 6985–6991. [Google Scholar] [CrossRef] [PubMed]
- Hornyák, L.; Dobos, N.; Koncz, G.; Karányi, Z.; Páll, D.; Szabó, Z.; Halmos, G.; Székvölgyi, L. The role of indoleamine-2,3-dioxygenase in cancer development, diagnostics, and therapy. Front. Immunol. 2018, 9, 151. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yu, S.; Li, A.; Xu, H.; Han, X.; Wu, K. Targeting interlukin-6 to relieve immunosuppression in tumor microenvironment. Tumor Boil. 2017, 39. [Google Scholar] [CrossRef] [PubMed]
- Gastl, G.A.; Abrams, J.S.; Nanus, D.M.; Oosterkamp, R.; Silver, J.; Liu, F.; Chen, M.; Albino, A.P.; Bander, N.H. Interleukin-10 production by human carcinoma cell lines and its relationship to interleukin-6 expression. Int. J. Cancer 1993, 55, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Inaba, T.; Ino, K.; Kajiyama, H.; Yamamoto, E.; Shibata, K.; Nawa, A.; Nagasaka, T.; Akimoto, H.; Takikawa, O.; Kikkawa, F. Role of the immunosuppressive enzyme indoleamine 2,3-dioxygenase in the progression of ovarian carcinoma. Gynecol. Oncol. 2009, 115, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Curiel, T.J.; Coukos, G.; Zou, L.; Alvarez, X.; Cheng, P.; Mottram, P.; Evdemon-Hogan, M.; Conejo-Garcia, J.R.; Zhang, L.; Burow, M.; et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat. Med. 2004, 10, 942–949. [Google Scholar] [CrossRef] [PubMed]
- Landskron, G.; De La Fuente, M.; Thuwajit, P.; Thuwajit, C.; Hermoso, M.A. Chronic inflammation and cytokines in the tumor microenvironment. J Immunol Res 2014, 2014, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez-Vea, M.; Zuazo, M.; Gato, M.; Arasanz, H.; Fernández-Hinojal, G.; Escors, D.; Kochan, G. Myeloid-Derived Suppressor Cells in the Tumor Microenvironment: Current Knowledge and Future Perspectives. Arch. Immunol. Ther. Exp. 2017, 66, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Mota, F.; Rayment, N.; Chong, S.; Singer, A.; Chain, B. The antigen-presenting environment in normal and human papillomavirus (HPV)-related premalignant cervical epithelium. Clin. Exp. Immunol. 1999, 116, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Bouzin, C.; Brouet, A.; De Vriese, J.; Dewever, J.; Feron, O. Effects of vascular endothelial growth factor on the lymphocyte-endothelium interactions: Identification of caveolin-1 and nitric oxide as control points of endothelial cell anergy. J. Immunol. 2007, 178, 1505–1511. [Google Scholar] [CrossRef] [PubMed]
- Buckanovich, R.J.; Facciabene, A.; Kim, S.; Benencia, F.; Sasaroli, D.; Balint, K.; Katsaros, D.; O’Brien-Jenkins, A.; Gimotty, P.A.; Coukos, G. Endothelin B receptor mediates the endothelial barrier to T cell homing to tumors and disables immune therapy. Nat. Med. 2008, 14, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Reinartz, S.; Schumann, T.; Finkernagel, F.; Wortmann, A.; Jansen, J.M.; Meissner, W.; Krause, M.; Schwörer, A.M.; Wagner, W.; Müller-Brüsselbach, S.; et al. Mixed-polarization phenotype of ascites-associated macrophages in human ovarian carcinoma: Correlation of CD163 expression, cytokine levels and early relapse. Int. J. Cancer 2014, 134, 32–42. [Google Scholar] [CrossRef]
- Finkernagel, F.; Reinartz, S.; Lieber, S.; Adhikary, T.; Wortmann, A.; Hoffmann, N.; Bieringer, T.; Nist, A.; Stiewe, T.; Jansen, M.J.; et al. The transcriptional signature of human ovarian carcinoma macrophages is associated with extracellular matrix reorganization. Oncotarget 2016, 7, 75339. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.-W.; Chen, M.-W.; Hsiao, M.; Wang, S.; Chen, C.-A.; Hsiao, S.-M.; Chang, J.-S.; Lai, T.-C.; Rose-John, S.; Kuo, M.-L.; et al. IL-6 Trans-Signaling in Formation and Progression of Malignant Ascites in Ovarian Cancer. Cancer Res. 2011, 71, 424. [Google Scholar] [CrossRef] [PubMed]
- Yanaihara, N.; Anglesio, M.; Ochiai, K.; Hirata, Y.; Saito, M.; Nagata, C.; Iida, Y.; Takakura, S.; Yamada, K.; Tanaka, T.; et al. Cytokine gene expression signature in ovarian clear cell carcinoma. Int. J. Oncol. 2012, 41, 1094–1100. [Google Scholar] [CrossRef] [PubMed]
- Noy, R.; Pollard, J.W. Tumor-Associated Macrophages: From Mechanisms to Therapy. Immunity 2014, 41, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Mamessier, E.; Sylvain, A.; Thibult, M.-L.; Houvenaeghel, G.; Jacquemier, J.; Castellano, R.; Gonçalves, A.; André, P.; Romagné, F.; Thibault, G.; et al. Human breast cancer cells enhance self tolerance by promoting evasion from NK cell antitumor immunity. J. Clin. Investig. 2011, 121, 3609–3622. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Li, N.; Li, H.; Zhang, T.; Wang, F.; Li, Q. Increased prevalence of regulatory T cells in the tumor microenvironment and its correlation with TNM stage of hepatocellular carcinoma. J. Cancer Res. Clin. Oncol. 2010, 136, 1745–1754. [Google Scholar] [CrossRef] [PubMed]
- Liu, V.C.; Wong, L.Y.; Jang, T.; Shah, A.H.; Park, I.; Yang, X.; Zhang, Q.; Lonning, S.; Teicher, B.A.; Lee, C. Tumor evasion of the immune system by converting CD4+CD25- T cells into CD4+CD25+ T regulatory cells: Role of tumor-derived TGF-beta. J. Immunol. 2007, 178, 2883–2892. [Google Scholar] [CrossRef] [PubMed]
- Larmonier, N.; Marron, M.; Zeng, Y.; Cantrell, J.; Romanoski, A.; Sepassi, M.; Thompson, S.; Chen, X.; Andreansky, S.; Katsanis, E. Tumor-derived CD4(+)CD25(+) regulatory T cell suppression of dendritic cell function involves TGF-beta and IL-10. Cancer Immunol. Immunother. 2007, 56, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Curiel, T.J.; Wei, S.; Dong, H.; Alvarez, X.; Cheng, P.; Mottram, P.; Krzysiek, R.; Knutson, K.L.; Daniel, B.; Zimmermann, M.C.; et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat. Med. 2003, 9, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.; Oberle, N.; Krammer, P.H. Molecular Mechanisms of Treg-Mediated T Cell Suppression. Front. Immunol. 2012, 3, 51. [Google Scholar] [CrossRef] [PubMed]
- Gabrilovich, D.I.; Nagaraj, S. Myeloid-derived-suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009, 9, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Pastuła, A.; Marcinkiewicz, J. Myeloid-derived suppressor cells: A double-edged sword? Int. J. Exp. Pathol. 2011, 92, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Condamine, T.; Ramachandran, I.; Youn, J.-I.; Gabrilovich, D.I. Regulation of Tumor Metastasis by Myeloid-Derived Suppressor Cells. Ann. Rev. Med. 2015, 66, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.L.; Obermajer, N.; Odunsi, K.; Edwards, R.P.; Kalinski, P. Synergistic COX2 induction by IFNgamma and TNFalpha self-limits type-1 immunity in the human tumor microenvironment. Cancer Immunol. Res. 2016. [Google Scholar] [CrossRef]
- Pinzon-Charry, A.; Ho, C.S.K.; Laherty, R.; Maxwell, T.; Walker, D.; Gardiner, R.A.; O’Connor, L.; Pyke, C.; Schmidt, C.; Furnival, C.; et al. A Population of HLA-DR(+) Immature Cells Accumulates in the Blood Dendritic Cell Compartment of Patients with Different Types of Cancer. Neoplasia 2005, 7, 1112–1122. [Google Scholar] [CrossRef] [PubMed]
- Failli, A.; Legitimo, A.; Orsini, G.; Romanini, A.; Consolini, R. Numerical defect of circulating dendritic cell subsets and defective dendritic cell generation from monocytes of patients with advanced melanoma. Cancer Lett. 2013, 337, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Orsini, G.; Legitimo, A.; Failli, A.; Ferrari, P.; Nicolini, A.; Spisni, R.; Miccoli, P.; Consolini, R. Quantification of Blood Dendritic Cells in Colorectal Cancer Patients During the Course of Disease. Pathol. Oncol. Res. 2014, 20, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Legitimo, A.; Consolini, R.; Failli, A.; Orsini, G.; Spisni, R. Dendritic cell defects in the colorectal cancer. Hum. Vaccines Immunother. 2014, 10, 3224–3235. [Google Scholar] [CrossRef] [PubMed]
- Sciarra, A.; Lichtner, M.; Autran, G.A.; Mastroianni, C.; Rossi, R.; Mengoni, F.; Cristini, C.; Gentilucci, A.; Vullo, V.; Di Silverio, F. Characterization of circulating blood dendritic cell subsets DC123+ (lymphoid) and DC11C+ (myeloid) in prostate adenocarcinoma patients. Prostate 2007, 67, 1–7. [Google Scholar] [CrossRef]
- Hoffmann, T.K.; Müller-Berghaus, J.; Ferris, R.L.; Johnson, J.T.; Storkus, W.J.; Whiteside, T.L. Alterations in the frequency of dendritic cell subsets in the peripheral circulation of patients with squamous cell carcinomas of the head and neck. Clin. Cancer Res. 2002, 8, 1787–1793. [Google Scholar] [PubMed]
- Della Bella, S.; Gennaro, M.; Vaccari, M.; Ferraris, C.; Nicola, S.; Riva, A.; Clerici, M.; Greco, M.; Villa, M.L. Altered maturation of peripheral blood dendritic cells in patients with breast cancer. Br. J. Cancer 2003, 89, 1463–1472. [Google Scholar] [CrossRef] [PubMed]
- Gabrilovich, D.I.; Corak, J.; Ciernik, I.F.; Kavanaugh, D.Y.; Carbone, D.P. Decreased antigen presentation by dendritic cells in patients with breast cancer. Clin. Cancer Res. 1997, 3, 483–490. [Google Scholar] [PubMed]
- Tjomsland, V.; Spangeus, A.; Sandstrom, P.; Borch, K.; Messmer, D.; Larsson, M. Semi mature blood dendritic cells exist in patients with ductal pancreatic adenocarcinoma owing to inflammatory factors released from the tumor. PLoS ONE 2010, 5, e13441. [Google Scholar] [CrossRef] [PubMed]
- Ormandy, L.-A.; Farber, A.; Cantz, T.; Petrykowska, S.; Wedemeyer, H.; Horning, M.; Lehner, F.; Manns, M.-P.; Korangy, F.; Greten, T.-F. Direct ex vivo analysis of dendritic cells in patients with hepatocellular carcinoma. World J. Gastroenterol. 2006, 12, 3275–3282. [Google Scholar] [CrossRef] [PubMed]
- Tucci, M.; Stucci, S.; Passarelli, A.; Giudice, G.; Dammacco, F.; Silvestris, F. The immune escape in melanoma: Role of the impaired dendritic cell function. Expert Rev. Clin. Immunol. 2014, 10, 1395–1404. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.; Gilmour, J.W.; Imami, N.; Amjadi, P.; Henderson, D.C.; Allen-Mersh, T.G. Increased serum transforming growth factor-beta1 in human colorectal cancer correlates with reduced circulating dendritic cells and increased colonic Langerhans cell infiltration. Clin. Exp. Immunol. 2003, 134, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Della Porta, M.; Danova, M.; Rigolin, G.M.; Brugnatelli, S.; Rovati, B.; Tronconi, C.; Fraulini, C.; Rossi, A.R.; Riccardi, A.; Castoldi, G. Dendritic cells and vascular endothelial growth factor in colorectal cancer: Correlations with clinicobiological findings. Oncology 2005, 68, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Toyokawa, H.; Takai, S.; Satoi, S.; Yanagimoto, H.; Terakawa, N.; Araki, H.; Kwon, A.H.; Kamiyama, Y. Surgical influence of pancreatectomy on the function and count of circulating dendritic cells in patients with pancreatic cancer. Cancer Immunol. Immunother. 2006, 55, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, E.; Wollenberg, B.; Rothenfusser, S.; Wagner, M.; Wellisch, D.; Mack, B.; Giese, T.; Gires, O.; Endres, S.; Hartmann, G. Identification and functional analysis of tumor-infiltrating plasmacytoid dendritic cells in head and neck cancer. Cancer Res. 2003, 63, 6478–6487. [Google Scholar] [PubMed]
- Mahnke, K.; Schmitt, E.; Bonifaz, L.; Enk, A.H.; Jonuleit, H. Immature, but not inactive: The tolerogenic function of immature dendritic cells. Immunol. Cell Boil. 2002, 80, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, L.H. Dendritic cells in cancer immunotherapy clinical trials: Are we making progress? Front. Immunol. 2013, 4, 454. [Google Scholar] [CrossRef] [PubMed]
- Ohm, J.E.; Carborne, D.P. VEGF as a mediator of tumor-associated immunodeficiency. Immunol. Res. 2001, 23, 263–272. [Google Scholar] [CrossRef]
- Carmeliet, P. VEGF as a key mediator of angiogenesis in cancer. Oncology 2005, 69, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Gabrilovich, D.; Ishida, T.; Oyama, T.; Ran, S.; Kravtsov, V.; Nadaf, S.; Carbone, D.P. Vascular endothelial growth factor inhibits the development of dendritic cells and dramatically affects the differentiation of multiple hematopoietic lineages in vivo. Blood 1998, 92, 4150–4166. [Google Scholar] [PubMed]
- Gabrilovich, D.I.; Chen, H.L.; Girgis, K.R.; Cunningham, H.T.; Meny, G.M.; Nadaf, S.; Kavanaugh, D.; Carbone, D.P. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat. Med. 1996, 2, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Mimura, K.; Kono, K.; Takahashi, A.; Kawaguchi, Y.; Fujii, H. Vascular endothelial growth factor inhibits the function of human mature dendritic cells mediated by VEGF receptor-2. Cancer Immunol. Immunother. 2007, 56, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Beckebaum, S.; Zhang, X.; Chen, X.; Yu, Z.; Frilling, A.; Dworacki, G.; Grosse-Wilde, H.; Broelsch, C.E.; Gerken, G.; Cicinnati, V.R. Increased levels of interleukin-10 in serum from patients with hepatocellular carcinoma correlate with profound numerical deficiencies and immature phenotype of circulating dendritic cell subsets. Clin. Cancer Res. 2004, 10, 7260–7269. [Google Scholar] [CrossRef] [PubMed]
- Ratta, M.; Fagnoni, F.; Curti, A.; Vescovini, R.; Sansoni, P.; Oliviero, B.; Fogli, M.; Ferri, E.; Cuna, G.R.D.; Tura, S.; et al. Dendritic cells are functionally defective in multiple myeloma: The role of interleukin-6. Blood 2002, 100, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, U.; Li, M.; Zhang, R.; Chen, C.; Yao, Q. Elevated interleukin-6 and G-CSF in human pancreatic cancer cell conditioned medium suppress dendritic cell differentiation and activation. Cancer Res. 2007, 67, 5479–5488. [Google Scholar] [CrossRef] [PubMed]
- Cabillic, F.; Bouet-Toussaint, F.; Toutirais, O.; Rioux-Leclercq, N.; Fergelot, P.; De La Pintière, C.T.; Genetet, N.; Patard, J.J.; Catros-Quemener, V. Interleukin-6 and vascular endothelial growth factor release by renal cell carcinoma cells impedes lymphocyte-dendritic cell cross-talk. Clin. Exp. Immunol. 2006, 146, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Veglia, F.; Gabrilovich, D.I. Dendritic cells in cancer: The role revisited. Curr. Opin. Immunol. 2017, 45, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, G.M.; Galpin, K.J.C.; McCloskey, C.W.; Vanderhyden, B.C. The Tumor Microenvironment of Epithelial Ovarian Cancer and Its Influence on Response to Immunotherapy. Cancers 2018, 10, 242. [Google Scholar] [CrossRef] [PubMed]
- Tesone, A.J.; Rutkowski, M.R.; Brencicova, E.; Svoronos, N.; Perales-Puchalt, A.; Stephen, T.L.; Allegrezza, M.J.; Payne, K.K.; Nguyen, J.M.; Wickramasinghe, J.; et al. Satb1 Overexpression Drives Tumor-Promoting Activities in Cancer-Associated Dendritic Cells. Cell Rep. 2016, 14, 1774–1786. [Google Scholar] [CrossRef] [PubMed]
- Kalinski, P.; Hilkens, C.M.; Snijders, A.; Snijdewint, F.G.; Kapsenberg, M.L. IL-12-deficient dendritic cells, generated in the presence of prostaglandin E2, promote type 2 cytokine production in maturing human naive T helper cells. J. Immunol. 1997, 159, 28–35. [Google Scholar] [PubMed]
- Obermajer, N.; Muthuswamy, R.; Lesnock, J.; Edwards, R.P.; Kalinski, P. Positive feedback between PGE2 and COX2 redirects the differentiation of human dendritic cells toward stable myeloid-derived suppressor cells. Blood 2011, 118, 5498–5505. [Google Scholar] [CrossRef] [PubMed]
- Scarlett, U.K.; Rutkowski, M.R.; Rauwerdink, A.M.; Fields, J.; Escovar-Fadul, X.; Baird, J.; Cubillos-Ruiz, J.R.; Jacobs, A.C.; Gonzalez, J.L.; Weaver, J.; et al. Ovarian cancer progression is controlled by phenotypic changes in dendritic cells. J. Exp. Med. 2012, 209, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Huarte, E.; Cubillos-Ruiz, J.R.; Nesbeth, Y.C.; Scarlett, U.K.; Martinez, D.G.; Buckanovich, R.J.; Benencia, F.; Stan, R.V.; Keler, T.; Sarobe, P.; et al. Depletion of dendritic cells delays ovarian cancer progression by boosting antitumor immunity. Cancer Res. 2008, 68, 7684–7691. [Google Scholar] [CrossRef] [PubMed]
- Benencia, F.; Sprague, L.; McGinty, J.; Pate, M.; Muccioli, M. Dendritic Cells The Tumor Microenvironment and the Challenges for an Effective Antitumor Vaccination. J. Biomed. Biotechnol. 2012, 2012, 425476. [Google Scholar] [CrossRef] [PubMed]
- Kvistborg, P.; Bechmann, C.M.; Pedersen, A.W.; Toh, H.C.; Claesson, M.H.; Zocca, M.B. Comparison of monocyte-derived dendritic cells from colorectal cancer patients, non-small-cell-lung-cancer patients and healthy donors. Vaccine 2009, 28, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Orsini, G.; Legitimo, A.; Failli, A.; Ferrari, P.; Nicolini, A.; Spisni, R.; Miccoli, P.; Consolini, R. Defective generation and maturation of dendritic cells from monocytes in colorectal cancer patients during the course of disease. Intern. J. Mol. Sci. 2013, 14, 22022–22041. [Google Scholar] [CrossRef] [PubMed]
- Gervais, A.; Levêque, J.; Bouet-Toussaint, F.; Burtin, F.; Lesimple, T.; Sulpice, L.; Patard, J.-J.; Genetet, N.; Catros-Quemener, V. Dendritic cells are defective in breast cancer patients: A potential role for polyamine in this immunodeficiency. Breast Cancer Res. 2005, 7, R326–R335. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Goswami, S.; Bose, A.; Goswami, K.K.; Sarkar, K.; Chakraborty, K.; Chakraborty, T.; Pal, S.; Haldar, A.; Basu, P.; et al. Defective dendritic cell generation from monocytes is a potential reason for poor therapeutic efficacy of interferon α2b (IFNα2b) in cervical cancer. Transl. Res. 2011, 158, 200–213. [Google Scholar] [CrossRef] [PubMed]
- Lan, C.Y.; Liu, J.H.; Xia, J.C.; Zheng, L.M. Biological characteristics of dendritic cells derived from peripheral blood of patients with epithelial ovarian cancer. Ai Zheng 2009, 28, 132–137. [Google Scholar] [PubMed]
- Derhovanessian, E.; Solana, R.; Larbi, A.; Pawelec, G. Immunity, ageing and cancer. Immunity Ageing 2008, 5, 11. [Google Scholar] [CrossRef] [PubMed]
- Gruver, A.L.; Hudson, L.L.; Sempowski, G.D. Immunosenescence of ageing. J. Pathol 2007, 211, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Suchard, M. Immunosenescence: Ageing of the immune system. Immunol. Today 2015, 82, 28–31. [Google Scholar]
- Martin Lluesma, S.; Wolfer, A.; Harari, A.; Kandalaft, L. Cancer Vaccines in Ovarian Cancer: How Can We Improve? Biomedicines 2016, 4, 10. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.L.-L.; Coukos, G.; Kandalaft, L.E. Whole Tumor Antigen Vaccines: Where Are We? Vaccines 2015, 3, 344–372. [Google Scholar] [CrossRef] [PubMed]
- Apostolopoulos, V. Vaccine Delivery Methods into the Future. Vaccines 2016, 4, 9. [Google Scholar] [CrossRef] [PubMed]
- Seya, T.; Shime, H.; Takeda, Y.; Tatematsu, M.; Takashima, K.; Matsumoto, M. Adjuvant for vaccine immunotherapy of cancer—Focusing on Toll-like receptor 2 and 3 agonists for safely enhancing antitumor immunity. Cancer Sci. 2015, 106, 1659–1668. [Google Scholar] [CrossRef] [PubMed]
- Linton, P.J.; Dorshkind, K. Age-related changes in lymphocyte development and function. Nat. Immunol. 2004, 5, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Dunn-Walters, D.K.; Banerjee, M.; Mehr, R. Effects of age on antibody affinity maturation. Biochem. Soc. Trans. 2003, 31, 447–448. [Google Scholar] [CrossRef] [PubMed]
- Orsini, G.; Legitimo, A.; Failli, A.; Massei, F.; Biver, P.; Consolini, R. Enumeration of human peripheral blood dendritic cells throughout the life. Intern. Immunol. 2012, 24, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Salem, M.L. The use of dendritic cells for peptide-based vaccination in cancer immunotherapy. Methods Mol. Boil. 2014, 1139, 479–503. [Google Scholar] [CrossRef]
- Pol, J.; Bloy, N.; Buqué, A.; Eggermont, A.; Cremer, I.; Sautès-Fridman, C.; Galon, J.; Tartour, E.; Zitvogel, L.; Kroemer, G.; et al. Trial Watch: Peptide-based anticancer vaccines. OncoImmunology 2015, 4, e974411. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, M.; Nishimura, Y. The present status and future prospects of peptide-based cancer vaccines. Int. Immunol. 2016, 28, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Ophir, E.; Bobisse, S.; Coukos, G.; Harari, A.; Kandalaft, L.E. Personalized approaches to active immunotherapy in cancer. Biochim. Biophys. Acta 2015, 1865, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Choo, Y.S.; Koo, T.; Bang, S.; Oh, T.Y.; Wen, J.; Song, S.Y. Enhancement of antitumor immunity of dendritic cells pulsed with heat-treated tumor lysate in murine pancreatic cancer. Immunol. Lett. 2006, 103, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Cao, T.; Connolly, J.E.; Monnet, L.; Bennett, L.; Chapel, S.; Bagnis, C.; Mannoni, P.; Davoust, J.; Palucka, A.K.; et al. Hyperthermia enhances CTL cross-priming. J. Immunol. 2006, 176, 2134–2141. [Google Scholar] [CrossRef] [PubMed]
- Prokopowicz, Z.M.; Arce, F.; Biedron, R.; Chiang, C.L.; Ciszek, M.; Katz, D.R.; Nowakowska, M.; Zapotoczny, S.; Marcinkiewicz, J.; Chain, B.M. Hypochlorous acid: A natural adjuvant that facilitates antigen processing, cross-priming, and the induction of adaptive immunity. J. Immunol. 2010, 184, 824–835. [Google Scholar] [CrossRef] [PubMed]
- Mookerjee, A.; Graciotti, M.; Kandalaft, L. A cancer vaccine with dendritic cells differentiated with GM-CSF and IFNα and pulsed with a squaric acid treated cell lysate improves T cell priming and tumor growth control in a mouse model. Bioimpacts 2018, 8, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Kotera, Y.; Shimizu, K.; Mule, J.J. Comparative analysis of necrotic and apoptotic tumor cells as a source of antigen(s) in dendritic cell-based immunization. Cancer Res. 2001, 61, 8105–8109. [Google Scholar] [PubMed]
- Brusa, D.; Garetto, S.; Chiorino, G.; Scatolini, M.; Migliore, E.; Camussi, G.; Matera, L. Post-apoptotic tumors are more palatable to dendritic cells and enhance their antigen cross-presentation activity. Vaccine 2008, 26, 6422–6432. [Google Scholar] [CrossRef] [PubMed]
- Fucikova, J.; Moserova, I.; Truxova, I.; Hermanova, I.; Vancurova, I.; Partlova, S.; Fialova, A.; Sojka, L.; Cartron, P.F.; Houska, M.; et al. High hydrostatic pressure induces immunogenic cell death in human tumor cells. Int. J. Cancer 2014, 135, 1165–1177. [Google Scholar] [CrossRef] [PubMed]
- Vandenberk, L.; Belmans, J.; Van Woensel, M.; Riva, M.; Van Gool, S.W. Exploiting the Immunogenic Potential of Cancer Cells for Improved Dendritic Cell Vaccines. Front. Immunol. 2015, 6, 663. [Google Scholar] [CrossRef] [PubMed]
- Green, D.R.; Ferguson, T.; Zitvogel, L.; Kroemer, G. Immunogenic and tolerogenic cell death. Nat. Rev. Immunol. 2009, 9, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Krysko, D.V.; Garg, A.D.; Kaczmarek, A.; Krysko, O.; Agostinis, P.; Vandenabeele, P. Immunogenic cell death and DAMPs in cancer therapy. Nat. Rev. Cancer 2012, 12, 860–875. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G.; Galluzzi, L.; Kepp, O.; Zitvogel, L. Immunogenic Cell Death in Cancer Therapy. Annu. Rev. Immunol. 2013, 31, 51–72. [Google Scholar] [CrossRef] [PubMed]
- De Vries, I.J.M.; Krooshoop, D.J.E.B.; Scharenborg, N.M.; Lesterhuis, W.J.; Diepstra, J.H.S.; Van Muijen, G.N.P.; Strijk, S.P.; Ruers, T.J.; Boerman, O.C.; Oyen, W.J.G.; et al. Effective migration of antigen-pulsed dendritic cells to lymph nodes in melanoma patients is determined by their maturation state. Cancer Res. 2003, 63, 12–17. [Google Scholar] [PubMed]
- Ahmed, S.; Bae, Y.-S. Dendritic cell-based therapeutic cancer vaccines: Past, present and future. Clin. Exp. Vaccine Res. 2014, 3, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Reis E Sousa, C. Dendritic cells in a mature age. Nat. Rev. Immunol. 2006, 6, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.W.; Truong, T.; Bickham, K.; Fonteneau, J.F.; Larsson, M.; Da Silva, I.; Somersan, S.; Thomas, E.K.; Bhardwaj, N. A clinical grade cocktail of cytokines and PGE2 results in uniform maturation of human monocyte-derived dendritic cells: Implications for immunotherapy. Vaccine 2002, 20, 8–22. [Google Scholar] [CrossRef]
- Chiang, C.L.-L.; Maier, D.A.; Kandalaft, L.E.; Brennan, A.L.; Lanitis, E.; Ye, Q.; Levine, B.L.; Czerniecki, B.J.; Powell, D.J., Jr.; Coukos, G. Optimizing parameters for clinical-scale production of high IL-12 secreting dendritic cells pulsed with oxidized whole tumor cell lysate. J. Transl. Med. 2011, 9, 198. [Google Scholar] [CrossRef] [PubMed]
- Carreno, B.M.; Becker-Hapak, M.; Huang, A.; Chan, M.; Alyasiry, A.; Lie, W.R.; Aft, R.L.; Cornelius, L.A.; Trinkaus, K.M.; Linette, G.P. IL-12p70-producing patient DC vaccine elicits Tc1-polarized immunity. J. Clin. Investig. 2013, 123, 3383–3394. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-J.; Foon, K.A.; Mailliard, R.B.; Muthuswamy, R.; Kalinski, P. Type 1-polarized dendritic cells loaded with autologous tumor are a potent immunogen against chronic lymphocytic leukemia. J. Leukoc. Biol. 2008, 84, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Bonehill, A.; Van Nuffel, A.M.T.; Corthals, J.; Tuyaerts, S.; Heirman, C.; François, V.; Colau, D.; Van Der Bruggen, P.; Neyns, B.; Thielemans, K. Single-step antigen loading and activation of dendritic cells by mRNA electroporation for the purpose of therapeutic vaccination in melanoma patients. Clin. Cancer Res. 2009, 15, 3366–3375. [Google Scholar] [CrossRef] [PubMed]
- Van Nuffel, A.M.T.; Benteyn, D.; Wilgenhof, S.; Corthals, J.; Heirman, C.; Neyns, B.; Thielemans, K.; Bonehill, A. Intravenous and intradermal TriMix-dendritic cell therapy results in a broad T-cell response and durable tumor response in a chemorefractory stage IV-M1c melanoma patient. Cancer Immunol. Immunother. 2012, 61, 1033–1043. [Google Scholar] [CrossRef] [PubMed]
- Radice, E.; Bellone, G.; Miranda, V. Enhancement of the immunostimulatory functions of ex vivo-generated dendritic cells from early-stage colon cancer patients by consecutive exposure to low doses of sequential-kinetic-activated IL-4 and IL-12. A preliminary study. Transl. Oncol. 2015, 8, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Aarntzen, E.H.J.G.; Srinivas, M.; Bonetto, F.; Cruz, L.J.; Verdijk, P.; Schreibelt, G.; Van De Rakt, M.; Lesterhuis, W.J.; Van Riel, M.; Punt, C.J.A.; et al. Targeting of 111in-labeled dendritic cell human vaccines improved by reducing number of cells. Clin. Cancer Res. 2013, 19, 1525–1533. [Google Scholar] [CrossRef] [PubMed]
- Spranger, S.; Bao, R.; Gajewski, T.F. Melanoma-intrinsic β-catenin signalling prevents anti-tumour immunity. Nature 2015, 523, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Zong, J.; Keskinov, A.A.; Shurin, G.V.; Shurin, M.R. Tumor-derived factors modulating dendritic cell function. Cancer Immunol. Immunother. 2016, 65, 821–833. [Google Scholar] [CrossRef] [PubMed]
- Graciotti, M.; Berti, C.; Klok, H.A.; Kandalaft, L. The era of bioengineering: How will this affect the next generation of cancer immunotherapy? J. Transl. Med. 2017, 15, 142. [Google Scholar] [CrossRef] [PubMed]
- Nirschl, C.J.; Drake, C.G. Molecular Pathways: Co-Expression of Immune Checkpoint Molecules: Signaling Pathways and Implications for Cancer Immunotherapy. Clin. Cancer Res. 2013, 19, 4917–4924. [Google Scholar] [CrossRef] [PubMed]
- Leach, D.R.; Krummel, M.F.; Allison, J.P. Enhancement of antitumor immunity by CTLA-4 blockade. Science 1996, 271, 1734–1736. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, J.R.; Tykodi, S.S.; Chow, L.Q.; Hwu, W.J.; Topalian, S.L.; Hwu, P.; Drake, C.G.; Camacho, L.H.; Kauh, J.; Odunsi, K.; et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 2012, 366, 2455–2465. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.P.; Thomas, E.K.; Hu, S.L.; Hellström, I.; Hellström, K.E. Human papillomavirus type 16 nucleoprotein E7 is a tumor rejection antigen. Proc. Natl. Acad. Sci. USA 1991, 88, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Kast, W.M.; Brandt, R.M.; Sidney, J.; Drijfhout, J.W.; Kubo, R.T.; Grey, H.M.; Melief, C.J.; Sette, A. Role of HLA-A motifs in identification of potential CTL epitopes in human papillomavirus type 16 E6 and E7 proteins. J. Immunol. 1994, 152, 3904–3912. [Google Scholar] [PubMed]
- Longoria, T.C.; Eskander, R.N. Immunotherapy in endometrial cancer—An evolving therapeutic paradigm. Gynecol. Oncol. Res. Pract. 2015, 2, 11. [Google Scholar] [CrossRef] [PubMed]
- Soliman, H. Immunotherapy strategies in the treatment of breast cancer. Cancer Control. 2013, 20, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Ribas, A.; Camacho, L.H.; Lopez-Berestein, G.; Pavlov, D.; Bulanhagui, C.A.; Millham, R.; Comin-Anduix, B.; Reuben, J.M.; Seja, E.; Parker, C.A. Antitumor activity in melanoma and anti-self responses in a phase I trial with the anti-cytotoxic T lymphocyte–associated antigen 4 monoclonal antibody CP-675,206. J. Clin. Oncol. 2005, 23, 8968–8977. [Google Scholar] [CrossRef] [PubMed]
- Pierret, L.; Wilgenhof, S.; Corthals, J.; Roelandt, T.; Thielemans, K.; Neyns, B. Correlation between prior therapeutic dendritic cell vaccination and the outcome of patients with metastatic melanoma treated with ipilimumab. J. Clin. Oncol. 2009, 27, e20006. [Google Scholar]
- Ribas, A.; Comin-Anduix, B.; Chmielowski, B.; Jalil, J.; de la Rocha, P.; McCannel, T.A.; Ochoa, M.T.; Seja, E.; Villanueva, A.; Oseguera, D.K. Dendritic cell vaccination combined with CTLA4 blockade in patients with metastatic melanoma. Clin. Cancer Res. 2009, 15, 6267–6276. [Google Scholar] [CrossRef] [PubMed]
- Wilgenhof, S.; Corthals, J.; Heirman, C.; van Baren, N.; Lucas, S.; Kvistborg, P.; Thielemans, K.; Neyns, B. Phase II study of autologous monocyte-derived mRNA electroporated dendritic cells (TriMixDC-MEL) plus ipilimumab in patients with pretreated advanced melanoma. J. Clin. Oncol. 2016, 34, 1330–1338. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Lutz, E.; Uram, J.N.; Sugar, E.A.; Onners, B.; Solt, S.; Zheng, L.; Diaz, L.A.; Donehower, R.C.; Jaffee, E.M.; et al. Evaluation of Ipilimumab in combination with allogeneic pancreatic tumor cells transfected with a GM-CSF gene in previously treated pancreatic cancer. J. Immunother. 2013, 36, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Dummer, R.; Smylie, M.; Rutkowski, P.; et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N. Engl. J. Med. 2015, 373, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Schachter, J.; Long, G.V.; Arance, A.; Grob, J.J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.; Lotem, M.; et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2015, 372, 2521–2532. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.S.; D’Angelo, S.P.; Minor, D.; Hodi, F.S.; Gutzmer, R.; Neyns, B.; Hoeller, C.; Khushalani, N.I.; Miller, W.H., Jr.; Lao, C.D.; et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): A randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015, 16, 375–384. [Google Scholar] [CrossRef]
- Schachter, J.; Ribas, A.; Long, G.V.; Arance, A.; Grob, J.J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.; Lotem, M.; et al. Pembrolizumab versus ipilimumab for advanced melanoma: Final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006). Lancet 2017, 390, 1853–1862. [Google Scholar] [CrossRef]
- Varn, F.S.; Wang, Y.; Mullins, D.W.; Fiering, S.; Cheng, C. Systematic Pan-Cancer Analysis Reveals Immune Cell Interactions in the Tumor Microenvironment. Cancer Res. 2017, 77, 1271–1282. [Google Scholar] [CrossRef] [PubMed]
- Nakano, O.; Sato, M.; Naito, Y.; Suzuki, K.; Orikasa, S.; Aizawa, M.; Suzuki, Y.; Shintaku, I.; Nagura, H.; Ohtani, H. Proliferative activity of intratumoral CD8(+) T-lymphocytes as a prognostic factor in human renal cell carcinoma: Clinicopathologic demonstration of antitumor immunity. Cancer Res. 2001, 61, 5132–5136. [Google Scholar] [PubMed]
- Sato, E.; Olson, S.H.; Ahn, J.; Bundy, B.; Nishikawa, H.; Qian, F.; Jungbluth, A.A.; Frosina, D.; Gnjatic, S.; Ambrosone, C.; et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc. Natl. Acad. Sci. USA 2005, 102, 18538–18543. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, S.M.; Paish, E.C.; Powe, D.G.; Macmillan, R.D.; Grainge, M.J.; Lee, A.H.; Ellis, I.O.; Green, A.R. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J. Clin. Oncol. 2011, 29, 1949–1955. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Shen, Y.; Wen, S.; Yamada, S.; Jungbluth, A.A.; Gnjatic, S.; Bajorin, D.F.; Reuter, V.E.; Herr, H.; Old, L.J.; et al. CD8 tumor-infiltrating lymphocytes are predictive of survival in muscle-invasive urothelial carcinoma. Proc. Natl. Acad. Sci. USA 2007, 104, 3967–3972. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Chehrazi-Raffle, A.; Reddi, S.; Salgia, R. Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: A comprehensive review of registration trials and future considerations. J. Immunother. Cancer 2018, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Luke, J.J.; Edwards, R.; Hedvat, C.; Pandya, D.; Ely, S.; Meier, R.; McDonald, D.; Harbison, C.T.; Baxi, V.; Lee, G.; et al. 1135PDCharacterization of the immune tumor microenvironment (TME) to inform personalized medicine with immuno-oncology (IO) combinations. Ann. Oncol. 2018, 29. [Google Scholar] [CrossRef]
- Matulonis, U.A.; Chen, M.; Puhlmann, M.; Shentu, Y.; Ledermann, J. KEYNOTE-100: Phase 2 trial of pembrolizumab in patients with advanced recurrent ovarian cancer. Ann. Oncol. 2016, 27, 900TiP. [Google Scholar] [CrossRef]
- Matulonis, U.A.; Shapira-Frommer, R.; Santin, A.; Lisyanskaya, A.S.; Pignata, S.; Vergote, I.; Raspagliesi, F.; Sonke, G.S.; Birrer, M.; Provencher, D.M. Antitumor activity and safety of pembrolizumab in patients with advanced recurrent ovarian cancer: Interim results from the phase 2 KEYNOTE-100 study. J. Clin. Oncol. 2018, 36, 5511. [Google Scholar] [CrossRef]
- Liu, J.F.; Herold, C.; Luo, W.; Penson, R.; Horowitz, N.; Konstantinopoulos, P.; Castro, C.; Curtis, J.; Matulonis, U.A.; Cannistra, S.; et al. 937PDA phase II trial of combination nivolumab and bevacizumab in recurrent ovarian cancer. Ann. Oncol. 2018, 29. [Google Scholar] [CrossRef]
- Lee, J.M.; Annunziata, C.M.; Houston, N.; Kohn, E.C.; Lipkowitz, S.; Minasian, L.; Nichols, E.; Trepel, J.; Trewhitt, K.; Zia, F.; et al. 936PDA phase II study of durvalumab, a PD-L1 inhibitor and olaparib in recurrent ovarian cancer (OvCa). Ann. Oncol. 2018, 29. [Google Scholar] [CrossRef]
- O’Cearbhaill, R.E.; Wolfer, A.; Disilvestro, P.; O’Malley, D.M.; Sabbatini, P.; Shohara, L.; Schwarzenberger, P.O.; Ricciardi, T.; Macri, M.; Ryan, A.; et al. 945PA phase I/II study of chemo-immunotherapy with durvalumab (durva) and pegylated liposomal doxorubicin (PLD) in platinum-resistant recurrent ovarian cancer (PROC). Ann. Oncol. 2018, 29. [Google Scholar] [CrossRef]
- Matulonis, U.A.; Moore, K.N.; Martin, L.P.; Vergote, I.B.; Castro, C.; Gilbert, L.; Malek, K.; Birrer, M.J.; O’Malley, D.M. 949PMirvetuximab soravtansine, a folate receptor alpha (FRα)-targeting antibody-drug conjugate (ADC), with pembrolizumab in platinum-resistant ovarian cancer (PROC): Initial results of an expansion cohort from FORWARD II, a phase Ib study. Ann. Oncol. 2018, 29. [Google Scholar] [CrossRef]
- Oaknin, A.; Ellard, S.L.; Leath Iii, C.; Moreno, V.; Kristeleit, R.; Guo, W.; Lu, S.; Jenkins, D.; McEachern, K.; Yu Jen, K.; et al. 935PDPreliminary safety, efficacy, and PK/PD characterization from GARNET, a phase I clinical trial of the anti–PD-1 monoclonal antibody, TSR-042, in patients with recurrent or advanced MSI-H endometrial cancer. Ann. Oncol. 2018, 29. [Google Scholar] [CrossRef]
- Rischin, D.; Gil-Martin, M.; González-Martín, A.; Brana, I.; Hou, J.Y.; Cho, D.; Falchook, G.S.; Formenti, S.; Jabbour, S.; Moore, K.; et al. 958PCemiplimab, a human PD-1 monoclonal antibody, in patients (pts) with recurrent or metastatic cervical cancer: Interim data from phase I cohorts. Ann. Oncol. 2018, 29. [Google Scholar] [CrossRef]
- Nanda, R.; Chow, L.Q.; Dees, E.C.; Berger, R.; Gupta, S.; Geva, R.; Pusztai, L.; Pathiraja, K.; Aktan, G.; Cheng, J.D.; et al. Pembrolizumab in Patients With Advanced Triple-Negative Breast Cancer: Phase Ib KEYNOTE-012 Study. J. Clin. Oncol. 2016, 34, 2460–2467. [Google Scholar] [CrossRef] [PubMed]
- Emens, L.A.; Braiteh, F.S.; Cassier, P.; Delord, J.-P.; Eder, J.P.; Fasso, M.; Xiao, Y.; Wang, Y.; Molinero, L.; Chen, D.S.; et al. Abstract 2859: Inhibition of PD-L1 by MPDL3280A leads to clinical activity in patients with metastatic triple-negative breast cancer (TNBC). Cancer Res. 2015, 75, 2859. [Google Scholar] [CrossRef]
- Mayer, I.A.; Dent, R.; Tan, T.; Savas, P.; Loi, S. Novel Targeted Agents and Immunotherapy in Breast Cancer. Am. Soc. Clin. Oncol. Educ. Book 2017, 37, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.; Diamond, J.R.; Hamilton, E.P.; Pohlmann, P.R.; Tolaney, S.M.; Molinero, L.; He, X.; Waterkamp, D.; Funke, R.P.; Powderly, J.D. Phase Ib trial of atezolizumab in combination with nab-paclitaxel in patients with metastatic triple-negative breast cancer (mTNBC). J. Clin. Oncol. 2016, 34, 1009. [Google Scholar] [CrossRef]
- Devaux, A.; Canon, J.L.; Duhoux, F.P.; Delrée, P.; Galant, C.; Coulie, P.G.; Bar, I.; Constant, M.; Haussy, S.; Bricard, O.; et al. 281TiPA phase Ib/II study of durvalumab combined with dose-dense EC in neoadjuvant setting for patients with locally advanced luminal B HER2(-) or triple negative breast cancers (B-IMMUNE). Ann. Oncol. 2018, 29. [Google Scholar] [CrossRef]
- Nesselhut, J.; Marx, D.; Lange, H.; Regalo, G.; Cillien, N.; Chang, R.Y.; Nesselhut, T. Systemic treatment with anti-PD-1 antibody nivolumab in combination with vaccine therapy in advanced pancreatic cancer. J. Clin. Oncol. 2017, 34, 3092. [Google Scholar] [CrossRef]
- Carmen, O.M.; Luna, M.; Inmaculada, R.; Saray, G.; Elisabeth, P.-R.; Susana, I.; Ignacio, M.; Pedro, B. Antibody-dependent cell cytotoxicity: Immunotherapy strategies enhancing effector NK cells. Immunol. Cell Boil. 2017, 95, 347–355. [Google Scholar] [CrossRef]
- Rosenblatt, J.; Glotzbecker, B.; Mills, H.; Vasir, B.; Tzachanis, D.; Levine, J.D.; Joyce, R.M.; Wellenstein, K.; Keefe, W.; Schickler, M.; et al. PD-1 Blockade by CT-011, Anti-PD-1 Antibody, Enhances Ex Vivo T-cell Responses to Autologous Dendritic Cell/Myeloma Fusion Vaccine. J. immunother. 2011, 34, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Antonios, J.P.; Soto, H.; Everson, R.G.; Orpilla, J.; Moughon, D.; Shin, N.; Sedighim, S.; Yong, W.H.; Li, G.; Cloughesy, T.F.; et al. PD-1 blockade enhances the vaccination-induced immune response in glioma. JCI Insight 2016, 1, e87059. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Xi, H.; Ju, S.; Zhang, X. Blockade of PD-1/PD-L1 immune checkpoint during DC vaccination induces potent protective immunity against breast cancer in hu-SCID mice. Cancer Lett. 2013, 336, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Salmon, H.; Idoyaga, J.; Rahman, A.; Leboeuf, M.; Remark, R.; Jordan, S.; Casanova-Acebes, M.; Khudoynazarova, M.; Agudo, J.; Tung, N.; et al. Expansion and Activation of CD103(+) Dendritic Cell Progenitors at the Tumor Site Enhances Tumor Responses to Therapeutic PD-L1 and BRAF Inhibition. Immunity 2016, 44, 924–938. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.J.; Tan, S.H.; Lim, T.W.; Tan, T.J.Y.; Chay, W.Y.; Lim, E.H.; Soh, L.T.; Lim, S.L.; Chia, J.W.K. 979POregovomab (orego) and nivolumab (nivo) as a combinatorial immunotherapy strategy for recurrent epithelial ovarian cancer (rEOC): ORION-01 phase Ib cohort. Ann. Oncol. 2018, 29. [Google Scholar] [CrossRef]
- Curran, M.A.; Montalvo, W.; Yagita, H.; Allison, J.P. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc. Natl. Acad. Sci. USA 2010, 107, 4275–4280. [Google Scholar] [CrossRef] [PubMed]
- Alan Korman, B.C.; Wang, C.; Wu, L.; Cardarelli, P.; Selby, M. Activity of Anti-PD-1 in Murine Tumor Models: Role of “Host” PD-L1 and Synergistic Effect of Anti-PD-1 and Anti-CTLA-4. J. Immunol. 2007, 2007, S82. [Google Scholar]
- Duraiswamy, J.; Kaluza, K.M.; Freeman, G.J.; Coukos, G. Dual Blockade of PD-1 and CTLA-4 Combined with Tumor Vaccine Effectively Restores T Cell Rejection Function in Tumors. Cancer Res. 2013, 73, 3591–3603. [Google Scholar] [CrossRef] [PubMed]
- Shoushtari, A.N.; Friedman, C.F.; Navid-Azarbaijani, P.; Postow, M.A.; Callahan, M.K.; Momtaz, P.; Panageas, K.S.; Wolchok, J.D.; Chapman, P.B. Measuring Toxic Effects and Time to Treatment Failure for Nivolumab Plus Ipilimumab in Melanoma. JAMA Oncol. 2018, 4, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Motz, G.T.; Santoro, S.P.; Wang, L.-P.; Garrabrant, T.; Lastra, R.R.; Hagemann, I.S.; Lal, P.; Feldman, M.D.; Benencia, F.; Coukos, G. Tumor Endothelium FasL Establishes a Selective Immune Barrier Promoting Tolerance in Tumors. Nat. Med. 2014, 20, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Cannistra, S.A.; Matulonis, U.A.; Penson, R.T.; Hambleton, J.; Dupont, J.; Mackey, H.; Douglas, J.; Burger, R.A.; Armstrong, D.; Wenham, R.; et al. Phase II study of bevacizumab in patients with platinum-resistant ovarian cancer or peritoneal serous cancer. J. Clin. Oncol. 2007, 25, 5180–5186. [Google Scholar] [CrossRef] [PubMed]
- Burger, R.A.; Sill, M.W.; Monk, B.J.; Greer, B.E.; Sorosky, J.I. Phase II trial of bevacizumab in persistent or recurrent epithelial ovarian cancer or primary peritoneal cancer: A Gynecologic Oncology Group Study. J. Clin. Oncol. 2007, 25, 5165–5171. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.A.; Hirte, H.; Fleming, G.; Yang, D.; Tsao-Wei, D.D.; Roman, L.; Groshen, S.; Swenson, S.; Markland, F.; Gandara, D.; et al. Phase II clinical trial of bevacizumab and low-dose metronomic oral cyclophosphamide in recurrent ovarian cancer: A trial of the California, Chicago, and Princess Margaret Hospital phase II consortia. J. Clin. Oncol. 2008, 26, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Klug, F.; Prakash, H.; Huber, P.E.; Seibel, T.; Bender, N.; Halama, N.; Pfirschke, C.; Voss, R.H.; Timke, C.; Umansky, L.; et al. Low-dose irradiation programs macrophage differentiation to an iNOS(+)/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell 2013, 24, 589–602. [Google Scholar] [CrossRef] [PubMed]
- Herrera, F.G.; Bourhis, J.; Coukos, G. Radiotherapy combination opportunities leveraging immunity for the next oncology practice. CA Cancer J. Clin. 2017, 67, 65–85. [Google Scholar] [CrossRef] [PubMed]
- Kortylewski, M.; Jove, R.; Yu, H. Targeting STAT3 affects melanoma on multiple fronts. Cancer Metastasis Rev. 2005, 24, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Niu, G.; Wright, K.L.; Huang, M.; Song, L.; Haura, E.; Turkson, J.; Zhang, S.; Wang, T.; Sinibaldi, D.; Coppola, D.; et al. Constitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene 2002, 21, 2000–2008. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Niu, G.; Kortylewski, M.; Burdelya, L.; Shain, K.; Zhang, S.; Bhattacharya, R.; Gabrilovich, D.; Heller, R.; Coppola, D.; et al. Regulation of the innate and adaptive immune responses by Stat-3 signaling in tumor cells. Nat. Med. 2004, 10, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.L.A.; Hirpara, J.L.; Pervaiz, S.; Eu, J.-Q.; Sethi, G.; Goh, B.-C. Do STAT3 inhibitors have potential in the future for cancer therapy? Expert Opin. Investig. Drugs 2017, 26, 883–887. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, H.; Ohno, Y.; Toyoshima, Y.; Ohtake, J.; Homma, S.; Kawamura, H.; Takahashi, N.; Taketomi, A. Interleukin-6/STAT3 signaling as a promising target to improve the efficacy of cancer immunotherapy. Cancer Sci. 2017, 108, 1947–1952. [Google Scholar] [CrossRef] [PubMed]
- Umansky, V.; Blattner, C.; Gebhardt, C.; Utikal, J. The Role of Myeloid-Derived Suppressor Cells (MDSC) in Cancer Progression. Vaccines 2016, 4, 36. [Google Scholar] [CrossRef] [PubMed]
- Poschke, I.; Mao, Y.; Adamson, L.; Salazar-Onfray, F.; Masucci, G.; Kiessling, R. Myeloid-derived suppressor cells impair the quality of dendritic cell vaccines. Cancer Immunol. Immunother. 2012, 61, 827–838. [Google Scholar] [CrossRef] [PubMed]
- Khosravianfar, N.; Hadjati, J.; Namdar, A.; Boghozian, R.; Hafezi, M.; Ashourpour, M.; Kheshtchin, N.; Banitalebi, M.; Mirzaei, R.; Razavi, S.A. Myeloid-derived suppressor cells elimination by 5-fluorouracil increased dendritic cell-based vaccine function and improved immunity in tumor mice. Iran. J. Allergy Asthma Immunol. 2018, 17, 47–55. [Google Scholar] [PubMed]
- Iclozan, C.; Antonia, S.; Chiappori, A.; Chen, D.-T.; Gabrilovich, D. Therapeutic regulation of myeloid-derived suppressor cells and immune response to cancer vaccine in patients with extensive stage small cell lung cancer. Cancer Immunol. Immunother. 2013, 62, 909–918. [Google Scholar] [CrossRef] [PubMed]
- De Haas, N.; de Koning, C.; Spilgies, L.; de Vries, I.J.M.; Hato, S.V. Improving cancer immunotherapy by targeting the STATe of MDSCs. OncoImmunology 2016, 5, e1196312. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Senovilla, L.; Zitvogel, L.; Kroemer, G. The secret ally: Immunostimulation by anticancer drugs. Nat. Rev. Drug Discov. 2012, 11, 215–233. [Google Scholar] [CrossRef] [PubMed]
- Mikyskova, R.; Stepanek, I.; Indrova, M.; Bieblova, J.; Simova, J.; Truxova, I.; Moserova, I.; Fucikova, J.; Bartunkova, J.; Spisek, R.; et al. Dendritic cells pulsed with tumor cells killed by high hydrostatic pressure induce strong immune responses and display therapeutic effects both in murine TC-1 and TRAMP-C2 tumors when combined with docetaxel chemotherapy. Int. J. Oncol. 2016, 48, 953–964. [Google Scholar] [CrossRef] [PubMed]
- Martins, I.; Wang, Y.; Michaud, M.; Ma, Y.; Sukkurwala, A.Q.; Shen, S.; Kepp, O.; Metivier, D.; Galluzzi, L.; Perfettini, J.L.; et al. Molecular mechanisms of ATP secretion during immunogenic cell death. Cell Death Differ. 2014, 21, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.M.; Fowler, D.W.; Smith, P.; Dalgleish, A.G. Pre-treatment with chemotherapy can enhance the antigenicity and immunogenicity of tumours by promoting adaptive immune responses. Br. J. Cancer 2010, 102, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Truxova, I.; Hensler, M.; Skapa, P.; Halaska, M.J.; Laco, J.; Ryska, A.; Spisek, R.; Fucikova, J. Rationale for the Combination of Dendritic Cell-Based Vaccination Approaches With Chemotherapy Agents. Int. Rev. Cell Mol. Biol. 2017, 330, 115–156. [Google Scholar] [CrossRef] [PubMed]
- Ellebaek, E.; Engell-Noerregaard, L.; Iversen, T.Z.; Froesig, T.M.; Munir, S.; Hadrup, S.R.; Andersen, M.H.; Svane, I.M. Metastatic melanoma patients treated with dendritic cell vaccination, Interleukin-2 and metronomic cyclophosphamide: Results from a phase II trial. Cancer Immunol. Immunother. 2012, 61, 1791–1804. [Google Scholar] [CrossRef] [PubMed]
- Hegmans, J.P.; Veltman, J.D.; Lambers, M.E.; de Vries, I.J.; Figdor, C.G.; Hendriks, R.W.; Hoogsteden, H.C.; Lambrecht, B.N.; Aerts, J.G. Consolidative dendritic cell-based immunotherapy elicits cytotoxicity against malignant mesothelioma. Am. J. Respir. Crit. Care Med. 2010, 181, 1383–1390. [Google Scholar] [CrossRef] [PubMed]
- Krishnadas, D.K.; Shusterman, S.; Bai, F.; Diller, L.; Sullivan, J.E.; Cheerva, A.C.; George, R.E.; Lucas, K.G. A phase I trial combining decitabine/dendritic cell vaccine targeting MAGE-A1, MAGE-A3 and NY-ESO-1 for children with relapsed or therapy-refractory neuroblastoma and sarcoma. Cancer Immunol. Immunother. 2015, 64, 1251–1260. [Google Scholar] [CrossRef] [PubMed]
- Podrazil, M.; Horvath, R.; Becht, E.; Rozkova, D.; Bilkova, P.; Sochorova, K.; Hromadkova, H.; Kayserova, J.; Vavrova, K.; Lastovicka, J.; et al. Phase I/II clinical trial of dendritic-cell based immunotherapy (DCVAC/PCa) combined with chemotherapy in patients with metastatic, castration-resistant prostate cancer. Oncotarget 2015, 6, 18192–18205. [Google Scholar] [CrossRef] [PubMed]
- Golden, E.B.; Apetoh, L. Radiotherapy and immunogenic cell death. Semin. Radiat Oncol 2015, 25, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Demaria, S.; Coleman, C.N.; Formenti, S.C. Radiotherapy: Changing the Game in Immunotherapy. Trends Cancer 2016, 2, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Ghoneum, A.; Afify, H.; Salih, Z.; Kelly, M.; Said, N. Role of tumor microenvironment in ovarian cancer pathobiology. Oncotarget 2018, 9, 22832–22849. [Google Scholar] [CrossRef] [PubMed]
- Goff, B.A.; Mandel, L.S.; Drescher, C.W.; Urban, N.; Gough, S.; Schurman, K.M.; Patras, J.; Mahony, B.S.; Andersen, M.R. Development of an ovarian cancer symptom index. Cancer 2007, 109, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Cubillos-Ruiz, J.R.; Engle, X.; Scarlett, U.K.; Martinez, D.; Barber, A.; Elgueta, R.; Wang, L.; Nesbeth, Y.; Durant, Y.; Gewirtz, A.T.; et al. Polyethylenimine-based siRNA nanocomplexes reprogram tumor-associated dendritic cells via TLR5 to elicit therapeutic antitumor immunity. J. Clin. Investig. 2009, 119, 2231–2244. [Google Scholar] [CrossRef]
- Scarlett, U.K.; Cubillos-Ruiz, J.R.; Nesbeth, Y.C.; Martinez, D.G.; Engle, X.; Gewirtz, A.T.; Ahonen, C.L.; Conejo-Garcia, J.R. In situ stimulation of CD40 and Toll-like receptor 3 transforms ovarian cancer-infiltrating dendritic cells from immunosuppressive to immunostimulatory cells. Cancer Res. 2009, 69, 7329–7337. [Google Scholar] [CrossRef] [PubMed]
- Piersma, S.J. Immunosuppressive tumor microenvironment in cervical cancer patients. Cancer Microenviron. 2011, 4, 361–375. [Google Scholar] [CrossRef] [PubMed]
- Aspinall, R.; Lang, P.O. Interventions to restore appropriate immune function in the elderly. Immun Ageing 2018, 15, 5. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.A.; Brondstetter, T.I.; English, C.A.; Lee, H.E.; Virts, E.L.; Thoman, M.L. IL-7 Gene Therapy in Aging Restores Early Thymopoiesis without Reversing Involution. J. Immunol. 2004, 173, 4867–4874. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, L.A.; Lo, J.C.; Gotway, M.B.; Mulligan, K.; Barbour, J.D.; Schmidt, D.; Grant, R.M.; Halvorsen, R.A.; Schambelan, M.; McCune, J.M. Increased thymic mass and circulating naive CD4 T cells in HIV-1-infected adults treated with growth hormone. AIDS 2002, 16, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Aspinall, R.; Govind, S.; Lapenna, A.; Lang, P.O. Dose response kinetics of CD8 lymphocytes from young animals transfused into old animals and challenged with influenza. Immunit Ageing 2013, 10, 34. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.O.; Siska, P.J.; Contreras, D.C.; Rathmell, J.C. Nutrients and the Microenvironment to Feed a T Cell Army. Semin. Immunol. 2016, 28, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Marko, M.G.; Ahmed, T.; Bunnell, S.C.; Wu, D.; Chung, H.; Huber, B.T.; Meydani, S.N. Age-associated decline in effective immune synapse formation of CD4(+) T cells is reversed by vitamin E supplementation. J. Immunol. 2007, 178, 1443–1449. [Google Scholar] [CrossRef] [PubMed]
- Albers, R.; van der Wielen, R.P.; Brink, E.J.; Hendriks, H.F.; Dorovska-Taran, V.N.; Mohede, I.C. Effects of cis-9, trans-11 and trans-10, cis-12 conjugated linoleic acid (CLA) isomers on immune function in healthy men. Eur. J. Clin. Nutr. 2003, 57, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Fulop, T.; Dupuis, G.; Fortin, C.; Douziech, N.; Larbi, A. T cell response in aging: Influence of cellular cholesterol modulation. Adv. Exp. Med. Boil. 2006, 584, 157–169. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martin Lluesma, S.; Graciotti, M.; Chiang, C.L.-L.; Kandalaft, L.E. Does the Immunocompetent Status of Cancer Patients Have an Impact on Therapeutic DC Vaccination Strategies? Vaccines 2018, 6, 79. https://doi.org/10.3390/vaccines6040079
Martin Lluesma S, Graciotti M, Chiang CL-L, Kandalaft LE. Does the Immunocompetent Status of Cancer Patients Have an Impact on Therapeutic DC Vaccination Strategies? Vaccines. 2018; 6(4):79. https://doi.org/10.3390/vaccines6040079
Chicago/Turabian StyleMartin Lluesma, Silvia, Michele Graciotti, Cheryl Lai-Lai Chiang, and Lana E. Kandalaft. 2018. "Does the Immunocompetent Status of Cancer Patients Have an Impact on Therapeutic DC Vaccination Strategies?" Vaccines 6, no. 4: 79. https://doi.org/10.3390/vaccines6040079
APA StyleMartin Lluesma, S., Graciotti, M., Chiang, C. L.-L., & Kandalaft, L. E. (2018). Does the Immunocompetent Status of Cancer Patients Have an Impact on Therapeutic DC Vaccination Strategies? Vaccines, 6(4), 79. https://doi.org/10.3390/vaccines6040079




