Fast Tracks and Roadblocks for Zika Vaccines
Abstract
1. History of Zika Virus
2. Treatment
3. Challenges for a Zika Vaccine
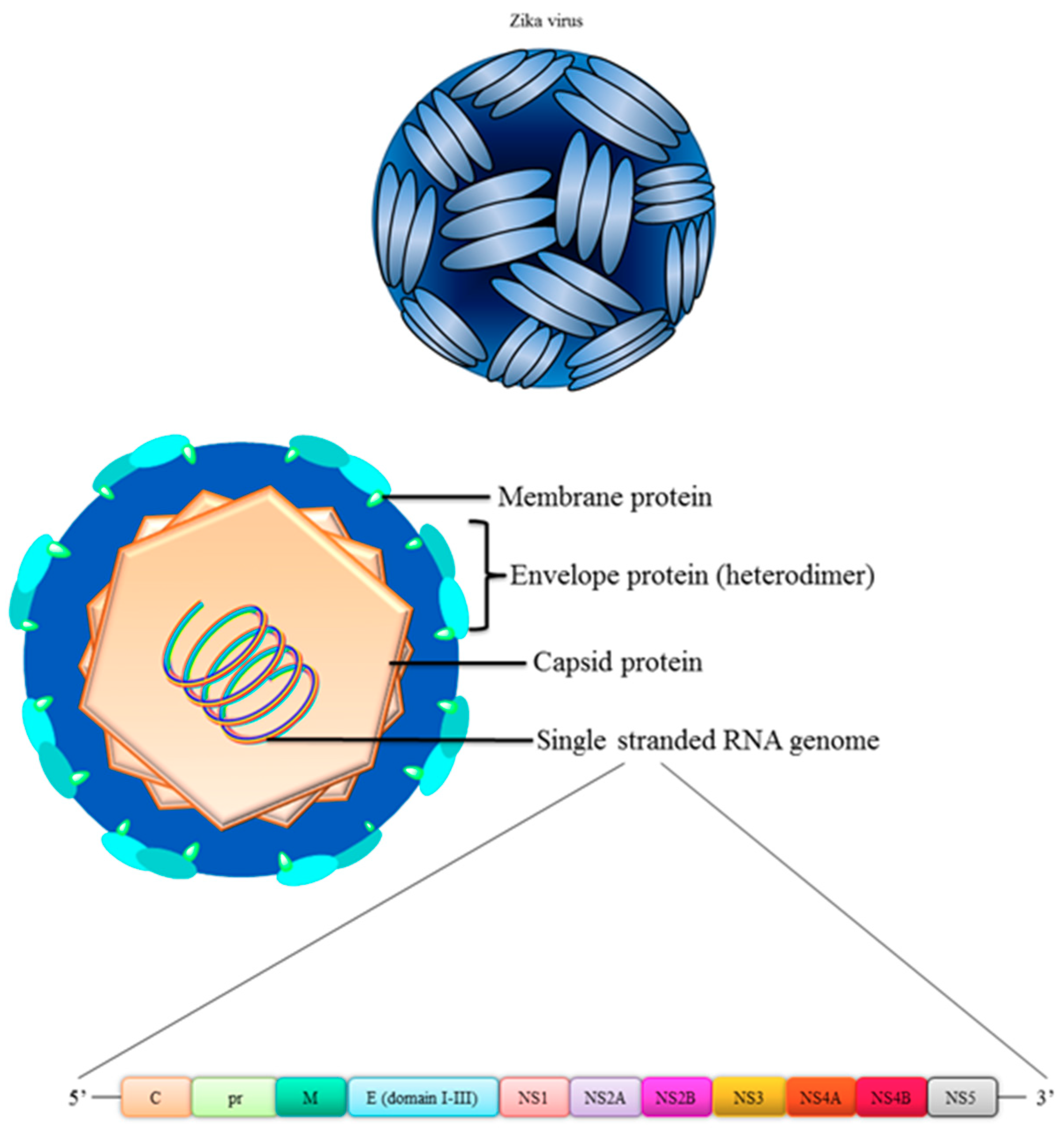
4. Structure of Zika Virus
5. Immunity Induced by ZIKV Vaccines
6. Vaccine Formulations
6.1. Live Attenuated Virus (LAV) Vaccine
6.2. Messenger RNA (mRNA) Vaccines
6.3. DNA Vaccines
6.4. Adenovirus Vector Based
6.5. Subunit Vaccines
6.6. Combinatorial Vaccines
7. The Pros and Cons of Various Vaccine Platforms
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dick, G.W.; Kitchen, S.F.; Haddow, A.J. Zika virus. Isolations and serological specificity. Trans. R. Soc. Trop. Med. Hyg. 1952, 46, 509–520. [Google Scholar] [CrossRef]
- Macnamara, F.N. Zika virus: A report on three cases of human infection during an epidemic of jaundice in Nigeria. Trans. R. Soc. Trop. Med. Hyg. 1954, 48, 139–145. [Google Scholar] [CrossRef]
- Duffy, M.R.; Chen, T.-H.; Hancock, W.T.; Powers, A.M.; Kool, J.L.; Lanciotti, R.S.; Pretrick, M.; Marfel, M.; Holzbauer, S.; Dubray, C.; et al. Zika Virus Outbreak on Yap Island, Federated States of Micronesia. N. Engl. J. Med. 2009, 360, 2536–2543. [Google Scholar] [CrossRef] [PubMed]
- Foy, B.D.; Kobylinski, K.C.; Foy, J.L.C.; Blitvich, B.J.; Travassos da Rosa, A.; Haddow, A.D.; Lanciotti, R.S.; Tesh, R.B. Probable non–vector-borne transmission of Zika virus, Colorado, USA. Emerg. Infect. Dis. 2011, 17, 880–882. [Google Scholar] [CrossRef] [PubMed]
- Cao-Lormeau, V.M.; Musso, D. Emerging arboviruses in the Pacific. Lancet 2014, 384, 1571–1572. [Google Scholar] [CrossRef]
- Rodriguez-Morales, A.J.; Villamil-Gomez, W.E.; Franco-Paredes, C. The arboviral burden of disease caused by co-circulation and co-infection of dengue, chikungunya and Zika in the Americas. Travel Med. Infect. Dis. 2016, 14, 177–179. [Google Scholar] [CrossRef] [PubMed]
- Musso, D.; Nhan, T.; Robin, E.; Roche, C.; Bierlaire, D.; Zisou, K.; Shan Yan, A.; Cao-Lormeau, L.M.; Broult, J. Potential for Zika virus transmission through blood transfusion demonstrated during an outbreak in French Polynesia, November 2013 to February 2014. Euro. Surveill. 2014, 10, 10. [Google Scholar] [CrossRef]
- Besnard, M.; Lastere, S.; Teissier, A.; Cao-Lormeau, V.; Musso, D. Evidence of perinatal transmission of Zika virus, French Polynesia, December 2013 and February 2014. Euro. Surveill. 2014, 19, 3. [Google Scholar] [CrossRef]
- Besnard, M.; Eyrolle-Guignot, D.; Guillemette-Artur, P.; Lastère, S.; Bost-Bezeaud, F.; Marcelis, L.; Abadie, V.; Garel, C.; Moutard, M.L.; Jouannic, J.M.; et al. Congenital cerebral malformations and dysfunction in fetuses and newborns following the 2013 to 2014 Zika virus epidemic in French Polynesia. Euro. Surveill. 2016, 21, 31. [Google Scholar] [CrossRef] [PubMed]
- Schuler-Faccini, L.; Riberio, E.M.; Feitosa, I.M.; Horovitz, D.D.; Cavalcanti, D.P.; Pessoa, A.; Doriqui, M.J.; Neri, J.I.; Neto, J.M.; Wanderley, H.Y.; et al. Brazilian Medical Genetics Society–Zika embryopathy Task Force. Possible association between Zika virus infection and microcephaly—Brazil, 2015. MMWR 2016, 65, 59–62. [Google Scholar] [PubMed]
- Alves, M.P.; Vielle, N.J.; Thiel, V.; Pfaender, S. Research Models and Tools for the Identification of Antivirals and Therapeutics against Zika Virus Infection. Viruses 2018, 10, E593. [Google Scholar] [CrossRef] [PubMed]
- Devilliers, J. Repurposing drugs for use against Zika virus infection. SAR QSAR Environ. Res. 2018, 29, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Zou, J.; Shan, C.; Shi, P.Y. Small molecules and antibodies for Zika therapy. J. Infect. Dis. 2018, 216, S945–S950. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/zika/symptoms/index.html (accessed on 20 October 2018).
- Tripathi, S.; Balasubramaniam, V.R.; Brown, J.A.; Mena, I.; Grant, A.; Bardina, S.V.; Maringer, K.; Schwarz, M.C.; Maestre, A.M.; Sourisseau, M.; et al. A novel Zika virus mouse model reveals strain specific differences in virus pathogenesis and host inflammatory immune responses. PLoS Pathog. 2017, 13, e1006258. [Google Scholar] [CrossRef] [PubMed]
- Lazear, H.M.; Govero, J.; Smith, A.M.; Platt, D.J.; Fernandez, E.; Miner, J.J.; Diamond, M.S. A Mouse Model of Zika Virus Pathogenesis. Cell Host Microbe 2016, 19, 720–730. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, W.; Xiong, S. A novel tuberculosis DNA vaccine in an HIV-1 p24 protein backbone confers protection against Mycobacterium tuberculosis and simultaneously elicits robust humoral and cellular responses to HIV-1. Clin. Vaccine Immunol. 2012, 19, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Plitnick, L.M. Chapter 9—Global Regulatory Guidelines for Vaccines. In Nonclinical Development of Novel Biologics, Biosimilars, Vaccines and Specialty Biologics; Plitnick, L.M., Herzyk, D.J., Eds.; Academic Press: San Diego, CA, USA, 2013; pp. 225–241. [Google Scholar]
- Lee, C.Y.P.; Ng, L.F.P. Zika virus: From an obscurity to a priority. Microbes Infect. 2018. [Google Scholar] [CrossRef] [PubMed]
- Szaba, F.M.; Tighe, M.; Kummer, L.W.; Lanzer, K.G.; Ward, J.M.; Lanthier, P.; Kim, I.J.; Kuki, A.; Blackman, M.A.; Thomas, S.J.; et al. Zika virus infection in immunocompetent pregnant mice causes fetal damage and placental pathology in the absence of fetal infection. PLoS Pathog. 2018, 14, e1006994. [Google Scholar] [CrossRef] [PubMed]
- Hadinegoro, S.; Arredondo-García, J.L.; Capeding, M.R.; Deseda, C.; Chotpitayasunondh, T.; Dietze, R.; Muhammad Ismail, H.I.; Reynales, H.; Limkittikul, K.; Rivera-Medina, D.M.; et al. Efficacy and long-term safety of a Dengue vaccine in regions of endemic disease. N. Engl. J. Med. 2015, 373, 1195–1206. [Google Scholar] [CrossRef] [PubMed]
- Sevvana, M.; Long, F.; Miller, A.S.; Klose, T.; Buda, G.; Sun, L.; Kuhn, R.J.; Rossman, M.G. Refinement and analysis of the mature Zika virus cryo-EM structure at 3.1 Å resolution. Structure 2018, 26, 1169–1177. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Thurmond, S.; Islas, L.; Hui, K.; Hai, R. Zika virus genome biology and molecular pathogenesis. Emerg. Microbes Infect. 2017, 6, e13. [Google Scholar] [CrossRef]
- Kuno, G.; Chang, G.J. Full-length sequencing and genomic characterization of Bagaza, Kedougou, and Zika viruses. Arch. Virol. 2007, 152, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Janeway, C.A.; Travers, P.; Walport, M.; Shlomchik, M. Immunobiology: The Immune System in Health and Disease, 5th ed.; Garland Science: New York, NY, USA, 2001. [Google Scholar]
- Stettler, K.; Beltramello, M.; Espinosa, D.A.; Graham, V.; Cassotta, A.; Bianchi, S.; Vanzetta, F.; Minola, A.; Jaconi, S.; Mele, F.; et al. Specificity, cross-reactivity, and function of antibodies elicited by Zika virus infection. Science 2016, 353, 823–826. [Google Scholar] [CrossRef] [PubMed]
- Magnani, D.M.; Rogers, T.F. Neutralizing human monoclonal antibodies prevent Zika virus infection in macaques. Sci. Transl. Med. 2017, 9, eaan8184. [Google Scholar] [CrossRef]
- Robbiani, D.F.; Bozzacco, L.; Keeffe, J.R.; Khouri, R.; Olsen, P.C.; Gazumyan, A.; Schaefer-Babajew, D.; Avila-Rios, S.; Nogueira, L.; Patel, R.; et al. Recurrent potent human neutralizing antibodies to Zika virus in Brazil and Mexico. Cell 2017, 169, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Aberle, J.H.; Koblischke, M.; Stiasny, K. CD4 T cell responses to flaviviruses. J. Clin. Virol. 2018, 108, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Barrett, A.D.; Teuwen, D.E. Yellow fever vaccine-how does it work and why do rare cases of serious adverse events take place? Curr. Opin. Immunol. 2009, 21, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Bonaldo, M.C.; Sequeira, P.C.; Galler, R. The yellow fever 17D virus as a platform for new live attenuated vaccines. Hum. Vaccin. Immunother. 2014, 10, 1256–1265. [Google Scholar] [CrossRef] [PubMed]
- Kwek, S.S.; Watanabe, S.; Chan, K.R.; Ong, E.Z.; Tan, H.C.; Ng, W.C.; Nguyen, M.T.X.; Gan, E.S.; Zhang, S.L.; Chan, K.W.K.; et al. A systematic approach to the development of a safe live attenuated Zika vaccine. Nat. Commun. 2018, 9, 1031. [Google Scholar] [CrossRef] [PubMed]
- Shan, C.; Xie, X.; Muruato, A.E.; Rossi, S.L.; Roundy, C.M.; Azar, S.R.; Yang, Y.; Tesh, R.B.; Bourne, N.; Barrett, A.D.; et al. An infectious cDNA clone of Zika virus to study viral virulence, mosquito transmission, and antiviral inhibitors. Cell Host Microbe 2016, 19, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Shan, C.; Muruato, A.E.; Nunes, B.T.D.; Luo, H.; Xie, X.; Medeiros, D.B.A.; Wakamiya, M.; Tesh, R.B.; Barrett, A.D.; Wang, T.; et al. A live-attenuated Zika virus vaccine candidate induces sterilizing immunity in mouse models. Nat. Med. 2017, 23, 763–767. [Google Scholar] [CrossRef] [PubMed]
- Shan, C.; Muruato, A.E.; Jagger, B.W.; Richner, J.; Nunes, B.T.D.; Medeiros, D.B.A.; Xie, X.; Nunes, J.G.C.; Morabito, K.M.; Kong, W.P.; et al. A single-dose live-attenuated vaccine prevents Zika virus pregnancy transmission and testis damage. Nat. Commun. 2017, 8, 676. [Google Scholar] [CrossRef] [PubMed]
- Lundstrom, K. Latest development on RNA-based drugs and vaccines. Future Sci. OA. 2018, 4, FSO300. [Google Scholar] [CrossRef]
- Burgess, D.J. Remember your driver. Nat. Rev. Mol. Cell. Biol. 2012, 13, 65. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Pelc, R.S.; Muramatsu, H.; Andersen, H.; DeMaso, C.R.; Dowd, K.A.; Sutherland, L.L.; Scearce, R.M.; Parks, R.; et al. Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination. Nature 2017, 543, 248–251. [Google Scholar] [CrossRef] [PubMed]
- Richner, J.M.; Himansu, S.; Dowd, K.A.; Butler, S.L.; Salazar, V.; Fox, J.M.; Julander, J.G.; Tang, W.W.; Shresta, S.; Pierson, T.C.; et al. Modified mRNA Vaccines Protect against Zika Virus Infection. Cell 2017, 168, 1114–1125. [Google Scholar] [CrossRef] [PubMed]
- Cherif, M.S.; Shuaibu, M.N.; Kurosaki, T.; Helegbe, G.K.; Kikuchi, M.; Yanagi, T.; Tsuboi, T.; Sasaki, H.; Hirayama, K. Immunogenicity of novel nanoparticle-coated MSP-1 C-terminus malaria DNA vaccine using different routes of administration. Vaccine 2011, 29, 9038–9050. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.C.; Lin, C.N.; Peng, S.Y.; Kang, T.F.; Lee, K.M. Combined IL-12 plasmid and recombinant SjGST enhance the protective and anti-pathology effect of SjGST DNA vaccine against schistosoma japonicum. PLoS Negl. Trop. Dis. 2016, 10, e0004459. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, M.T.; Eyles, J.E.; Baillie, L.W.; Keane-Myers, A.M. Immunogenicity and efficacy of an anthrax/plague DNA fusion vaccine in a mouse model. FEMS Immunol. Med. Microbiol. 2012, 65, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, J.J.; Friedman, A.; Martinez, D.; Montgomery, D.L.; Shiver, J.W.; Motzel, S.L.; Ulmer, J.B.; Liu, M.A. Preclinical efficacy of a prototype DNA vaccine: Enhanced protection against antigenic drift in influenza virus. Nat. Med. 1995, 1, 583–587. [Google Scholar] [CrossRef] [PubMed]
- Porter, K.R.; Ewing, D.; Chen, L.; Wu, S.J.; Hayes, C.G.; Ferrari, M.; Teneza-Mora, N.; Raviprakash, K. Immunogenicity and protective efficacy of a vaxfectin-adjuvanted tetravalent dengue DNA vaccine. Vaccine 2012, 30, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Larocca, R.A.; Abbink, P.; Peron, J.P.; Zanotto, P.M.; Iampietro, M.J.; Badamchi-Zadeh, A.; Boyd, M.; Ng'ang'a, D.; Kirilova, M.; Nityanandam, R.; et al. Vaccine protection against Zika virus from Brazil. Nature 2016, 536, 474–478. [Google Scholar] [CrossRef] [PubMed]
- Abbink, P.; Larocca, R.A.; De La Barrera, R.A.; Bricault, C.A.; Moseley, E.T.; Boyd, M.; Kirilova, M.; Li, Z.; Ng'ang'a, D.; Nanayakkara, O.; et al. Protective efficacy of multiple vaccine platforms against Zika virus challenge in rhesus monkeys. Science 2016, 353, 1129–1132. [Google Scholar] [CrossRef] [PubMed]
- Muthumani, K.; Griffin, B.D.; Agarwal, S.; Kudchodkar, S.B.; Reuschel, E.L.; Choi, H.; Kraynyak, K.A.; Duperret, E.K.; Keaton, A.A.; Chung, C.; et al. In vivo protection against ZIKV infection and pathogenesis through passive antibody transfer and active immunisation with a prMEnv DNA vaccine. NPJ Vaccines 2016, 1, 16021. [Google Scholar] [CrossRef] [PubMed]
- Tebas, P.; Roberts, C.C.; Muthumani, K.; Reuschel, E.L.; Kudchodkar, S.B.; Zaidi, F.I.; White, S.; Khan, A.S.; Racine, T.; Choi, H.; et al. Safety and immunogenicity of an anti-Zika virus DNA vaccine-Preliminary report. N. Engl. J. Med. 2017. [Google Scholar] [CrossRef] [PubMed]
- Dowd, K.A.; Ko, S.Y.; Morabito, K.M.; Yang, E.S.; Pelc, R.S.; DeMaso, C.R.; Castilho, L.R.; Abbink, P.; Boyd, M.; Nityanandam, R.; et al. Rapid development of a DNA vaccine for Zika virus. Science 2016, 354, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Gaudinski, M.R.; Houser, K.V.; Morabito, K.M.; Hu, Z.; Yamshchikov, G.; Rothwell, R.S.; Berkowitz, N.; Mendoza, F.; Saunders, J.G.; Novik, L.; et al. Safety, tolerability, and immunogenicity of two Zika virus DNA vaccine candidates in healthy adults: Randomised, open-label, phase 1 clinical trials. Lancet 2018, 391, 552–562. [Google Scholar] [CrossRef]
- Xu, K.; Song, Y.; Dai, L.; Zhang, Y.; Lu, X.; Xie, Y.; Zhang, H.; Cheng, T.; Wang, Q.; Huang, Q.; et al. Recombinant chimpanzee adenovirus vaccine AdC7-M/E protects against Zika virus infection and testis damage. J. Virol. 2018, 92, 6. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Chan, J.F.; Poon, V.K.; Wu, S.; Chan, C.C.; Hou, L.; Yip, C.C.; Ren, C.; Cai, J.P.; Zhao, M.; et al. Immunization with a novel human type 5 adenovirus-vectored vaccine expressing the premembrane and envelope proteins of Zika virus provides consistent and sterilizing protection in multiple immunocompetent and immunocompromised animal models. J. Infect. Dis. 2018, 218, 365–377. [Google Scholar] [CrossRef] [PubMed]
- To, A.; Medina, L.O.; Mfuh, K.O.; Lieberman, M.M.; Wong, T.A.S.; Namekar, M.; Nakano, E.; Lai, C.Y.; Kimar, M.; Nerukar, V.R.; et al. Recombinant Zika virus subunits are immunogenic and efficacious in mice. mSphere 2018, 3, e00576-17. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Dent, M.; Lai, H.; Sun, H.; Chen, Q. Immunization of Zika virus envelope protein domain III induces specific and neutralizing immune responses against Zika virus. Vaccine 2017, 35, 4287–4294. [Google Scholar] [CrossRef] [PubMed]
- Tai, W.; He, L.; Wang, Y.; Sun, S.; Zhao, G.; Luo, C.; Li, P.; Zhao, H.; Fremont, D.H.; Li, F.; et al. Critical neutralizing fragment of Zika virus EDIII elicits cross-neutralization and protection against divergent Zika viruses. Emerg. Microbes Infect. 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, A.; Aguilar, P.V.; Bopp, N.E.; Yarovinsky, T.O.; Weaver, S.C.; Rose, J.K. A recombinant virus vaccine that protects against both Chikungunya and Zika virus infections. Vaccine 2018, 36, 3894–3900. [Google Scholar] [CrossRef] [PubMed]
- Hanley, K.A. The double-edged sword: How evolution can make or break a live-attenuated virus vaccine. Evolution 2011, 4, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Mead, P.S.; Duggal, N.K.; Hook, S.A.; Delorey, M.; Fischer, M.; Olzenak McGuire, D.; Becksted, H.; Max, R.J.; Anishchenko, M.; Schwartz, A.M.; et al. Zika virus shedding in semen of symptomatic infected men. NEJM 2018, 378, 1377–1385. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.H. DNA vaccines: Roles against diseases. Germs 2013, 3, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.A. The Elements of Immunology; Chennai Microprint: Tamil Nadu, India, 2009; Chapter 16; pp. 343–359. [Google Scholar]
- Nascimento, I.P.; Leite, L.C. Recombinant vaccines and the development of new vaccine strategies. Braz. J. Med. Biol. Res. 2012, 45, 1102–1111. [Google Scholar] [CrossRef]
- Smaill, F.; Jeyanathan, M.; Smieja, M.; Medina, M.F.; Thanthrige-Don, N.; Zganiacz, A.; Yin, C.; Heriazon, A.; Damjanovic, D.; Puri, L.; et al. A human type 5 adenovirus-based tuberculosis vaccine induces robust T cell responses in humans despite preexisting anti-adenovirus immunity. Sci. Transl. Med. 2013, 5, 205ra134. [Google Scholar] [CrossRef] [PubMed]
- Griffin, B.D.; Muthumani, K.; Warner, B.M.; Majer, A.; Hagan, M.; Audet, J.; Stein, D.R.; Ranadheera, C.; Racine, T.; De La Vega, M.A.; et al. DNA vaccination protects mice against Zika virus-induced damage to the testes. Nat. Commun. 2017, 8, 15743. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Liao, X.; Fan, D.; Wang, L.; Song, J.; Feng, K.; Li, M.; Wang, P.; Chem, H.; An, J. Maternal immunization with a DNA vaccine candidate elicits specific passive protection against post-natal Zika virus infection in immunocompetent BALB/c mice. Vaccine 2018, 36, 3522–3532. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Kum, D.B.; Xia, H.; Luo, H.; Shan, C.; Zou, J.; Muruato, A.E.; Medeiros, D.B.A.; Nunes, B.T.D.; Dallmeier, K.; et al. A single-dose live-attenuated Zika virus vaccine with controlled infection rounds that protects against vertical transmission. Cell Host Microbe 2018, 24, 487–499.e5. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Xie, X.; Luo, H.; Shan, C.; Muruato, A.E.; Weaver, S.C.; Wang, T.; Shi, P.Y. A single-dose plasmid-launched live-attenuated Zika vaccine induces protective immunity. EBioMedicine 2018. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Ke, X.; Wang, T.; Tan, Z.; Luo, D.; Miao, Y.; Sun, J.; Zhang, Y.; Liu, Y.; Hu, Q.; et al. Zika virus attenuation by codon pair deoptimization induces sterilizing immunity in mouse models. J. Virol. 2018, 92, e00701-18. [Google Scholar] [CrossRef] [PubMed]
- Touret, F.; Gilles, M.; Klitting, R.; Aubry, F.; de Lamballerie, X.; Nougairède, A. Live Zika virus chimeric vaccine candidate based on a yellow fever 17-D attenuated backbone. Emerg. Microbes Infect. 2018, 7, 161. [Google Scholar] [CrossRef] [PubMed]
- Giel-Moloney, M.; Goncalvez, A.P.; Catalan, J.; Lecouturier, V.; Girerd-Chambaz, Y.; Diaz, F.; Maldonado-Arocho, F.; Gomila, R.C.; Bernard, M.C.; Oomen, R.; et al. Chimeric yellow fever 17D-Zika virus (ChimeriVax-Zika) as a live-attenuated Zika virus vaccine. Sci. Rep. 2018, 8, 13206. [Google Scholar] [CrossRef] [PubMed]
- Cox, F.; van der Fits, L.; Abbink, P.; Larocca, R.A.; van Huizen, E.; Saeland, E.; Verhagen, J.; Peterson, R.; Tolboom, J.; Kaufmann, B.; et al. Adenoviral vector type 26 encoding Zika virus (ZIKV) M-Env antigen induces humoral and cellular immune responses and protects mice and nonhuman primates against ZIKV challenge. PLoS ONE 2018, 13, e0202820. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Yang, R.; Liu, Z.; Li, M.; Liu, H.; Jin, X. Recombinant Zika virus envelope protein elicited protective immunity against Zika virus in immunocompetent mice. PLoS ONE 2018, 13, e0194860. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, D.; Mendy, J.; Manayani, D.; Vang, L.; Wang, C.; Richard, T.; Guenther, B.; Aruri, J.; Avanzini, J.; Garduno, F.; et al. Passive transfer of immune sera induced by a Zika virus-like particle vaccine protects AG129 mice against lethal Zika virus challenge. EBioMedicine 2018, 27, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Boigard, H.; Alimova, A.; Martin, G.R.; Katz, A.; Gottlieb, P.; Galarza, J.M. Zika virus-like particle (VLP) based vaccine. PLoS Negl. Trop. Dis. 2017, 11, e0005608. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Li, C.; Afridi, S.K.; Zu, S.; Xu, J.W.; Quanquin, N.; Yang, H.; Cheng, G.; Xu, Z. E90 subunit vaccine protects mice from Zika virus infection and microcephaly. Acta. Neuropathol. Commun. 2018, 10, 77. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Qu, L.; Ye, X.; Yi, C.; Zheng, X.; Hao, M.; Su, W.; Yao, Z.; Chen, P.; Zhang, S.; et al. Incorporation of NS1 and prM/M are important to confer effective protection of adenovirus-vectored Zika virus vaccine carrying E protein. NPJ Vaccines 2018, 3, 29. [Google Scholar] [CrossRef] [PubMed]
- Emanuel, J.; Callison, J.; Dowd, K.A.; Pierson, T.C.; Feldmann, H.; Marzi, A. A VSV-based Zika virus vaccine protects mice from lethal challenge. Sci. Rep. 2018, 8, 11043. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Yu, J.; Lu, M.; Ma, Y.; Attia, Z.; Shan, C.; Xue, M.; Liang, X.; Craig, K.; Makadiya, N.; et al. A Zika virus vaccine expressing premembrane-envelope-NS1 polyprotein. Nat. Commun. 2018, 9, 3067. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Camacho, C.; Abbink, P.; Larocca, R.A.; Dejnirattisai, W.; Boyd, M.; Badamchi-Zadeh, A.; Wallace, Z.R.; Doig, J.; Velazquez, R.S.; Neto, R.D.L.; et al. Rational Zika vaccine design via the modulation of antigen membrane anchors in chimpanzee adenoviral vectors. Nat. Commun. 2018, 9, 2441. [Google Scholar] [CrossRef] [PubMed]
- Martins, P.; Machado, D.; Theizen, T.H.; Guarnieri, J.P.O.; Bernardes, B.G.; Gomide, G.P.; Corat, M.A.F.; Abbehausen, C.; Módena, J.L.P.; Melo, C.F.O.R.; et al. Outer membrane vesicles from Neisseria Meningitidis (Proteossome) used for nanostructured Zika virus vaccine production. Sci. Rep. 2018, 8, 8290. [Google Scholar] [CrossRef] [PubMed]
- Prow, N.A.; Liu, L.; Nakayama, E.; Cooper, T.H.; Yan, K.; Eldi, P.; Hazlewood, J.E.; Tang, B.; Le, T.T.; Setoh, Y.X.; et al. A vaccinia-based single vector construct multi-pathogen vaccine protects against both Zika and chikungunya viruses. Nat. Commun. 2018, 9, 1230. [Google Scholar] [CrossRef] [PubMed]
- Li, X.F.; Dong, H.L.; Wang, H.J.; Huang, X.Y.; Qiu, Y.F.; Ji, X.; Ye, Q.; Li, C.; Liu, Y.; Deng, Y.Q.; et al. Development of a chimeric Zika vaccine using a licensed live-attenuated flavivirus vaccine as backbone. Nat. Commun. 2018, 9, 673. [Google Scholar] [CrossRef] [PubMed]
- Brault, A.C.; Domi, A.; McDonald, E.M.; Talmi-Frank, D.; McCurley, N.; Basu, R.; Robinson, H.L.; Hellerstein, M.; Duggal, N.K.; Bowen, R.A.; et al. A Zika vaccine targeting NS1 protein protects immunocompetent adult mice in a lethal challenge model. Sci. Rep. 2017, 7, 14769. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Sun, H.; Lai, H.; Hurtado, J.; Chen, Q. Plant-produced Zika virus envelope protein elicits neutralizing immune responses that correlate with protective immunity against Zika virus in mice. Plant Biotechnol. 2018, 16, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Zhang, T.; Zhang, Y.; Wang, H.; Deng, F. Zika virus Baculovirus-expressed virus-like particles induce neutralizing antibodies in mice. Virol. Sin. 2018, 33, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Chahal, J.S.; Fang, T.; Woodham, A.W.; Khan, O.F.; Ling, J.; Anderson, D.G.; Ploegh, H.L. An RNA nanoparticle vaccine against Zika virus elicits antibody and CD8+ T cell responses in a mouse model. Sci. Rep. 2017, 7, 252. [Google Scholar] [CrossRef]
- Qu, P.; Zhang, W.; Li, D.; Zhang, C.; Liu, Q.; Zhang, X.; Wang, X.; Dai, W.; Xu, Y.; Leng, Q.; et al. Insect cell-produced recombinant protein subunit vaccines protect against Zika virus infection. Antiviral. Res. 2018, 154, 97–103. [Google Scholar] [CrossRef] [PubMed]
| Vaccine | Type of Vaccine | Sponsor | Phase |
|---|---|---|---|
| ZIKA-001 * | DNA plasmid vaccine | GeneOne Life Science | Phase 1 |
| GLS-5700 * [48] | DNA plasmid vaccine | GeneOne Life Science | Phase 1 |
| mRNA-1325* | mRNA vaccine | Moderna Therapeutics | Phase 1/2 |
| MV-ZIKA * | Recombinant measles-Zika vaccine | Themis Bioscience | Phase 1 |
| VRC-ZKADNA090-00-VP * [50] | DNA vaccine | National Institutes of Allergy and Infectious Diseases (NIAID) | Phase 1/2 |
| ZPIV * | ZIKV purified inactivated vaccine | NIAID | Phase 1 |
| PIZV * | ZIKV purified inactivated vaccine | Takeda | Phase 1 |
| VLA1601 * | ZIKV purified inactivated vaccine+alum | Valneva Austria GmbH | Phase 1 |
| VRC-ZKADNA085-00-VP * [50] | DNA vaccine | NIAID | Phase 1 |
| rZIKV/D4Δ30-713 * | Live attenuated ZIKV vaccine | NIAID | Phase 1 |
| Type of vaccine | Target | Phase | |
| DNA Vaccine | Pre-membrane/Membrane (PrM) Envelope (E) protein [45,46,47,48,49,50,63,64] | Pre-clinical mouse model NHP | |
| Live attenuated Zika vaccine | Whole virus [32,33,34,35,65,66,67] | Pre-clinical mouse model NHP | |
| Live ZIKV chimeric vaccine (YF 17-D attenuated backbone) | Whole virus [68,69] | Pre-clinical mouse model | |
| Adenoviral vector type 26 | Whole virus [70] | Pre-clinical mouse model NHP | |
| Recombinant protein | E protein [54,55,71,72,73] Capsid (C) PrME + NS2B/NS3 proteins [74] | Pre-clinical mouse model | |
| Adenoviral vector type 2 | PrME protein + NS1 protein [75] | Pre-clinical mouse model | |
| Recombinant VSV | Membrane (M) E [56] PrME [76] PrME + NS1 [77] | Pre-clinical mouse model | |
| Chimpanzee adenoviral vector | PrME protein [78] | Pre-clinical mouse model | |
| Outer membrane vesicles from Neisseria Meningitidis (Proteosome) | Whole virus [79] | Pre-clinical mouse model | |
| “Sementis Copenhagen Vector” (SCV) system | PrME protein [80] | Pre-clinical mouse model | |
| Human adenoviral vector type 5 | PrME protein [52] | Pre-clinical mouse model | |
| Recombinant chimeric ZIKV vaccine candidate (JEV backbone) | PrME protein [81] | Pre-clinical mouse model | |
| Recombinant chimpanzee adenovirus type 7 | PrME protein [51] | Pre-clinical mouse model | |
| Modified Vaccinia Ankara (MVA) vector | NS1 protein [82] | Pre-clinical mouse model | |
| Plant produced ZIKV E protein | E protein [83] | Pre-clinical mouse model | |
| Virus like particles | PrME protein [84] | Pre-clinical mouse model | |
| RNA nanoparticle vaccine | PrME protein [85] | Pre-clinical mouse model | |
| Insect cell-produced subunit vaccine | E protein [53,86] | Pre-clinical mouse model | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghaffar, K.A.; Ng, L.F.P.; Renia, L. Fast Tracks and Roadblocks for Zika Vaccines. Vaccines 2018, 6, 77. https://doi.org/10.3390/vaccines6040077
Ghaffar KA, Ng LFP, Renia L. Fast Tracks and Roadblocks for Zika Vaccines. Vaccines. 2018; 6(4):77. https://doi.org/10.3390/vaccines6040077
Chicago/Turabian StyleGhaffar, Khairunnisa Abdul, Lisa F.P. Ng, and Laurent Renia. 2018. "Fast Tracks and Roadblocks for Zika Vaccines" Vaccines 6, no. 4: 77. https://doi.org/10.3390/vaccines6040077
APA StyleGhaffar, K. A., Ng, L. F. P., & Renia, L. (2018). Fast Tracks and Roadblocks for Zika Vaccines. Vaccines, 6(4), 77. https://doi.org/10.3390/vaccines6040077






