Respiratory Tract Deposition and Distribution Pattern of Microparticles in Mice Using Different Pulmonary Delivery Techniques
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Mouse Dosing
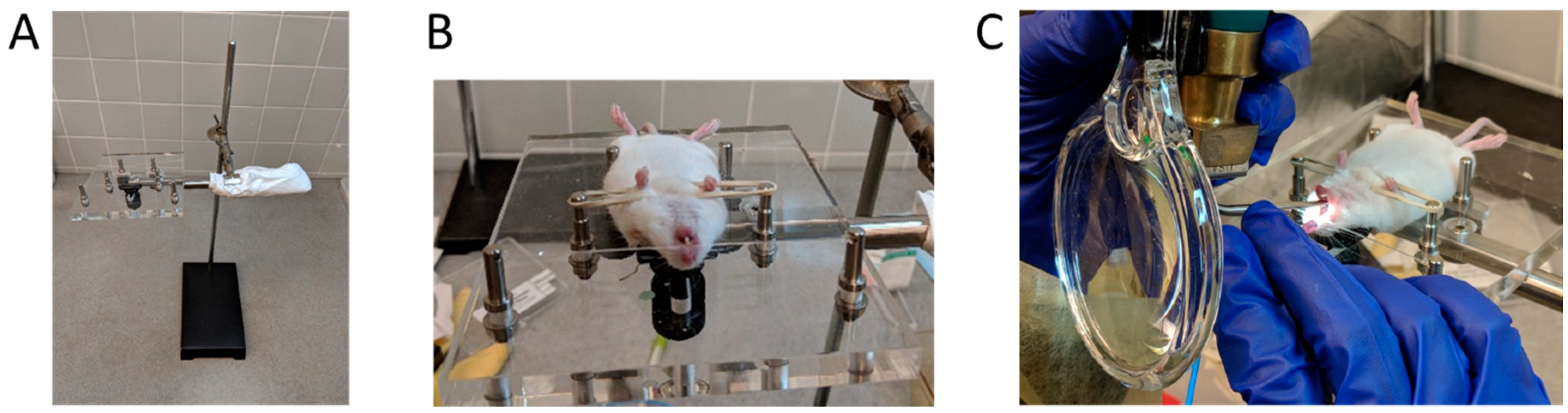
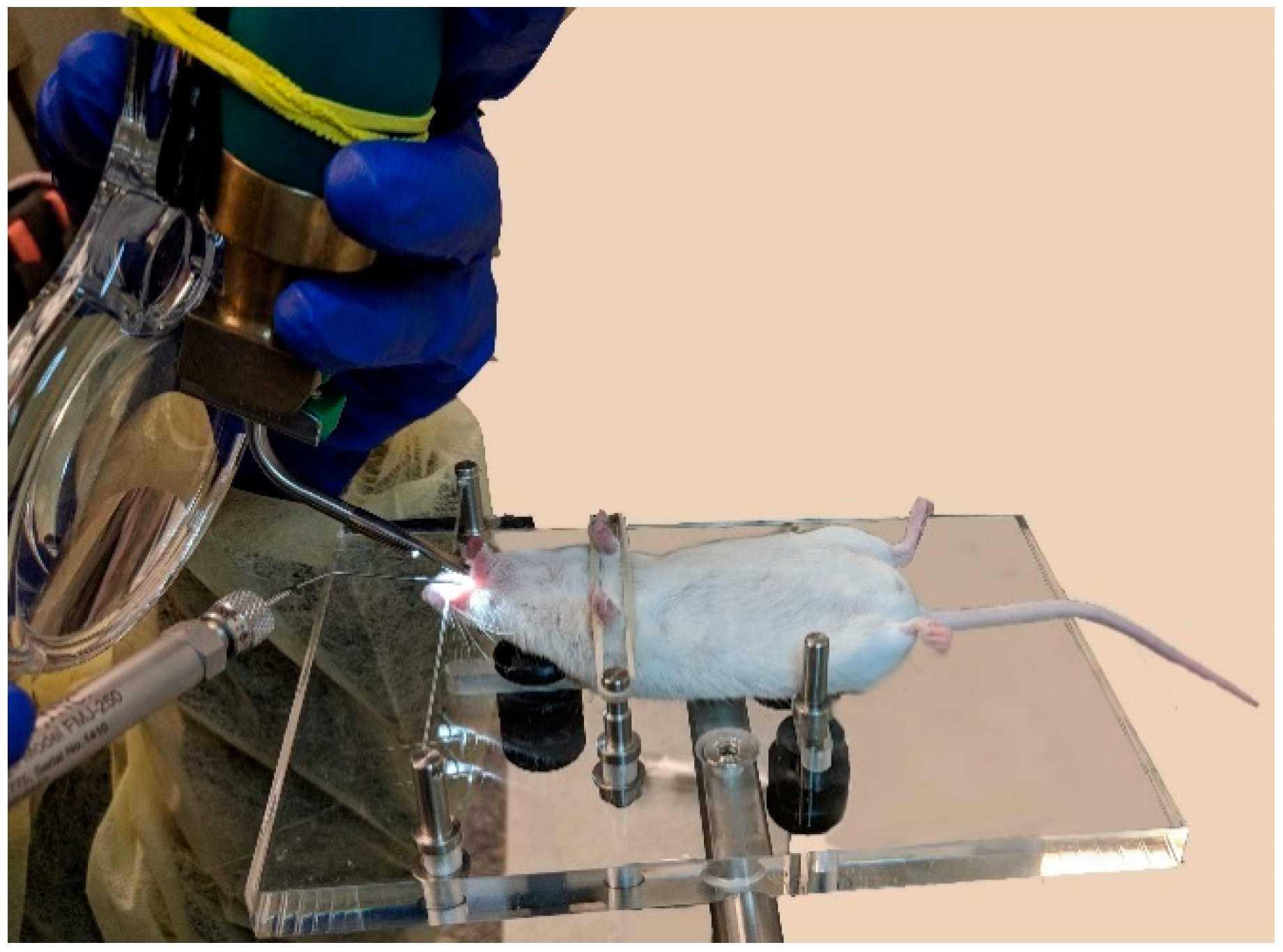
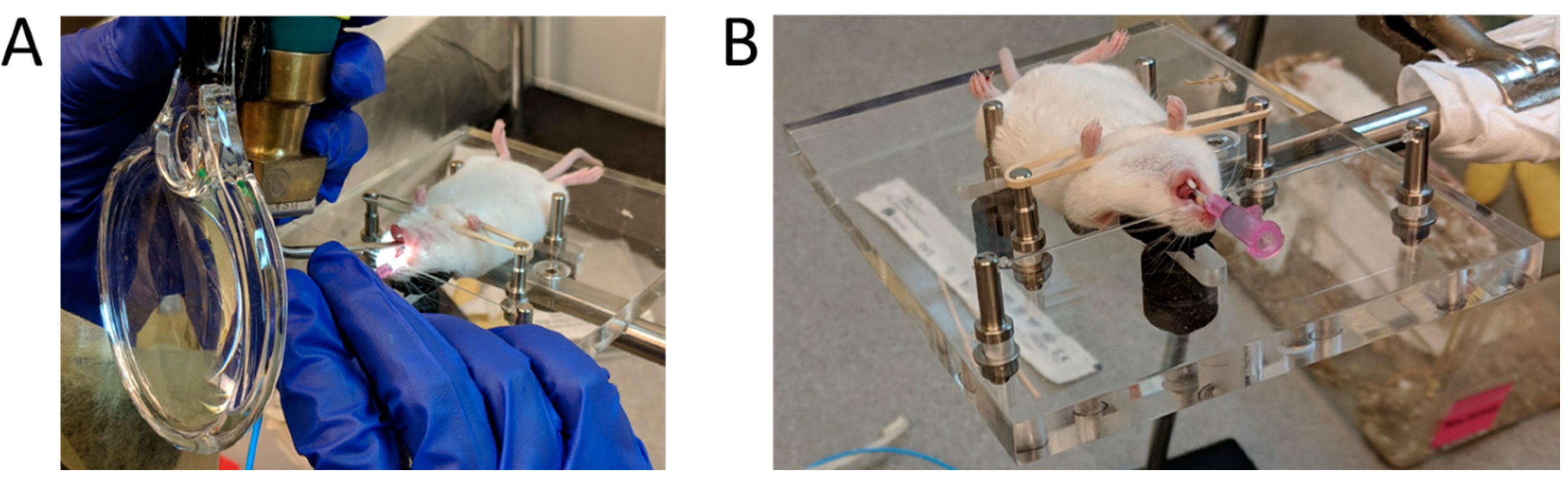
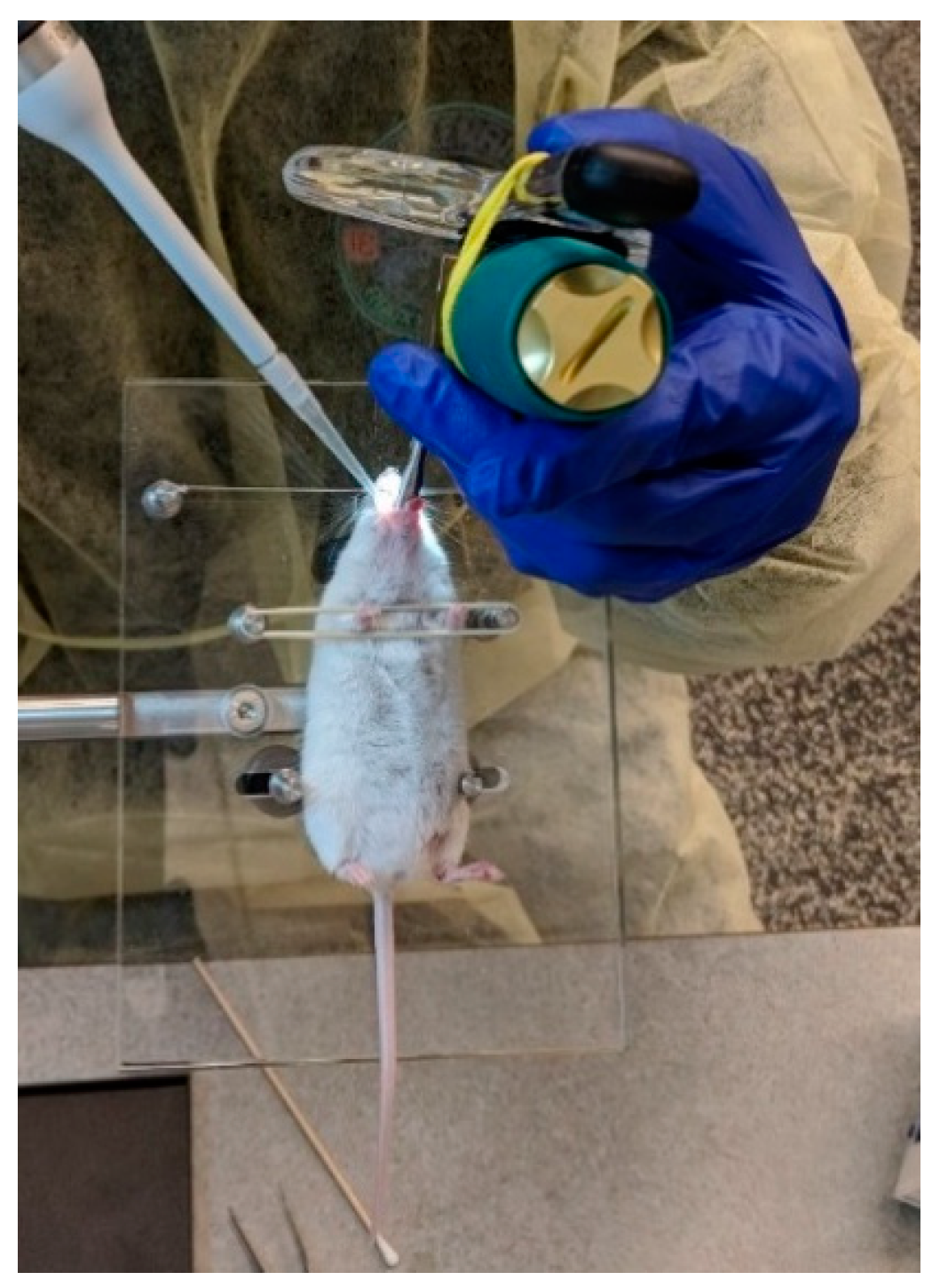
2.2.1. MicroSprayer® Aerosolizer
2.2.2. BioLite Intubation System
2.2.3. Oropharyngeal Aspiration
2.3. Fluorescence Imaging
2.4. Statistical Analysis
3. Results
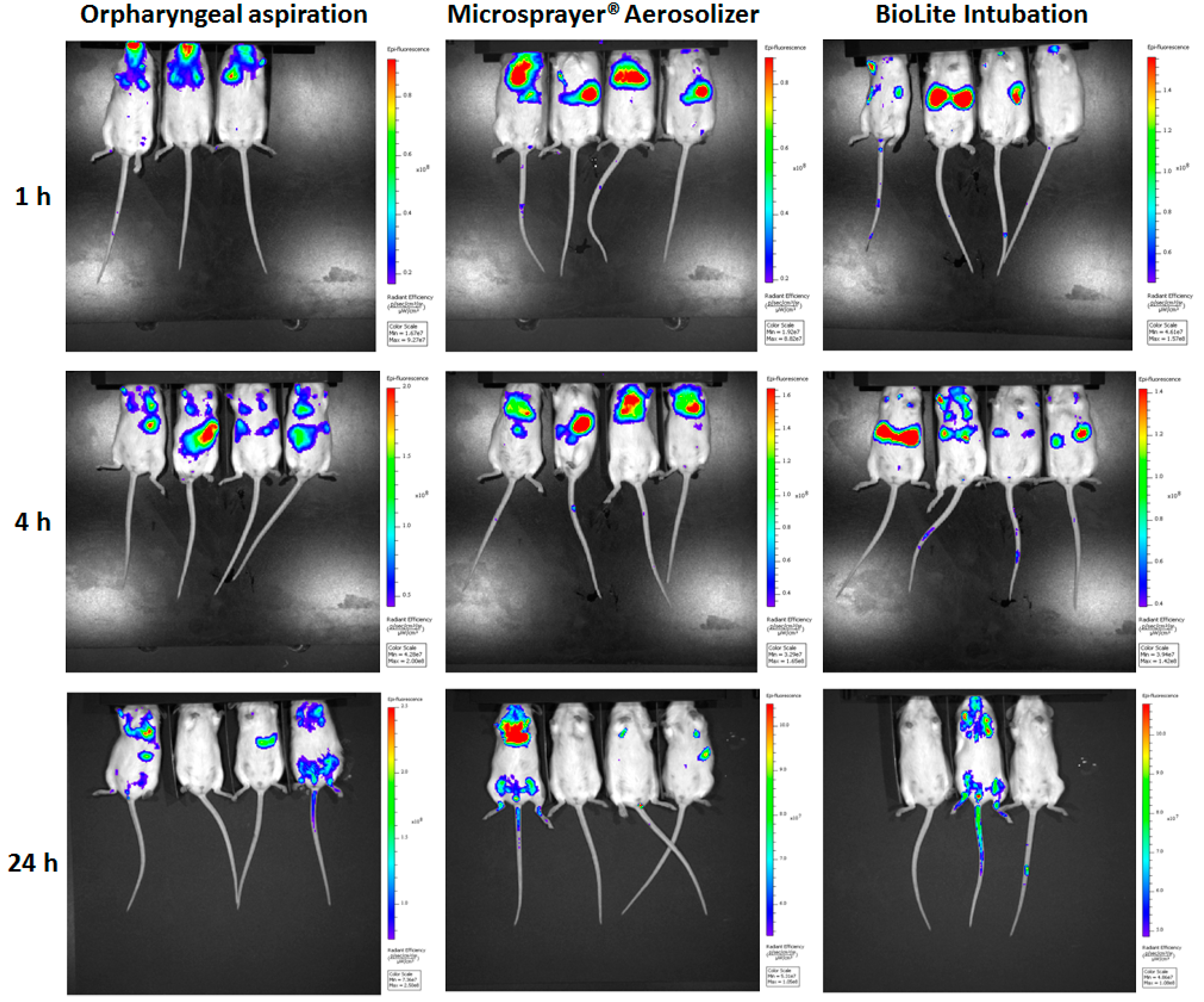
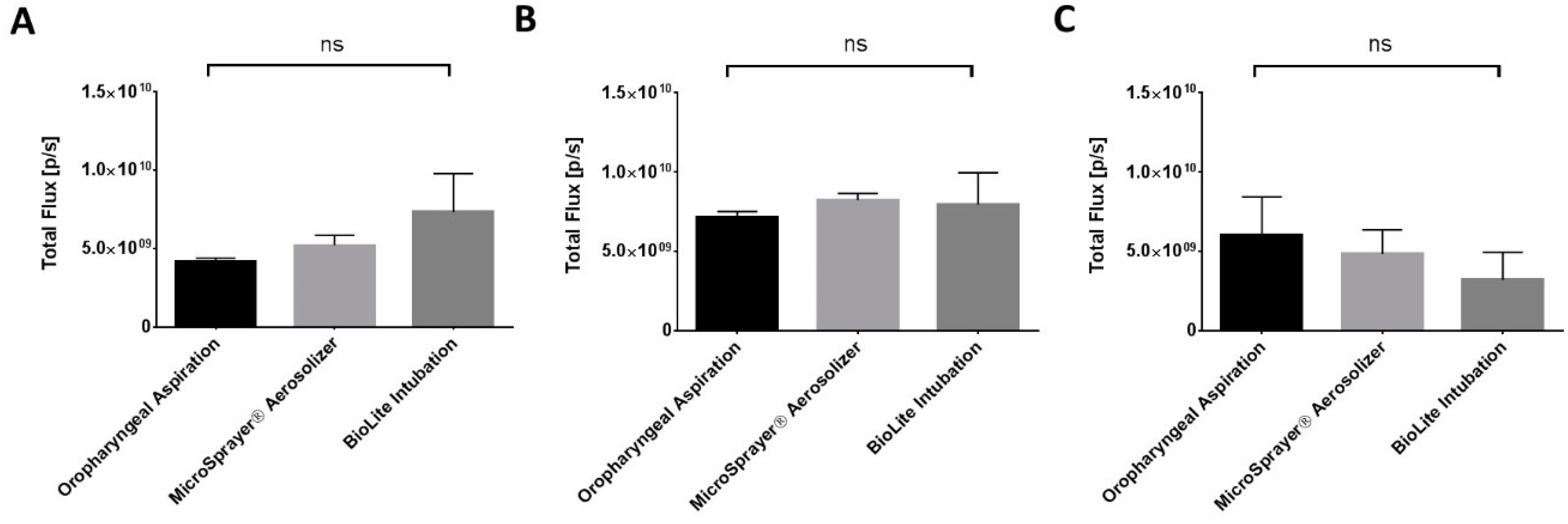
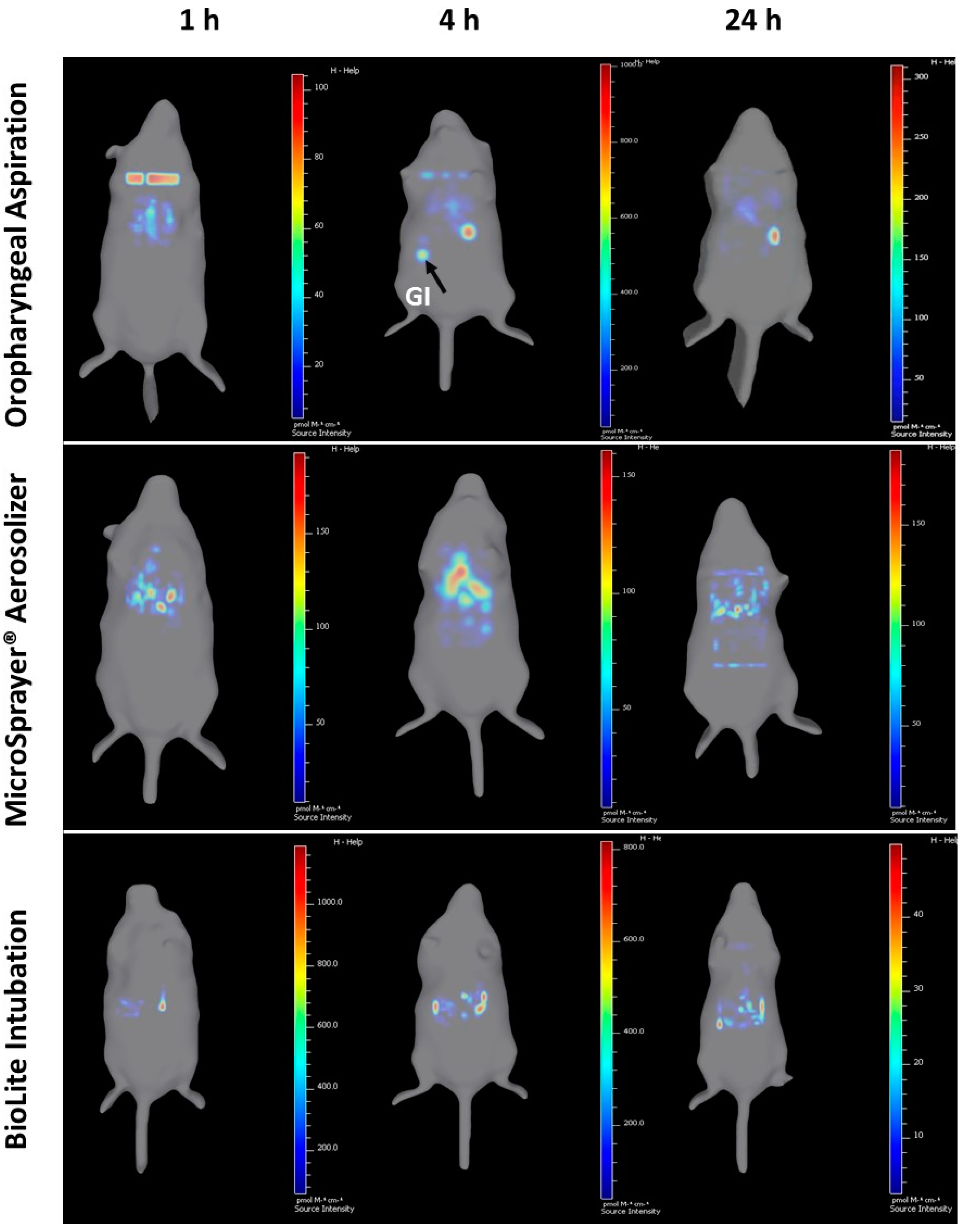
3.1. Whole Animal Imaging
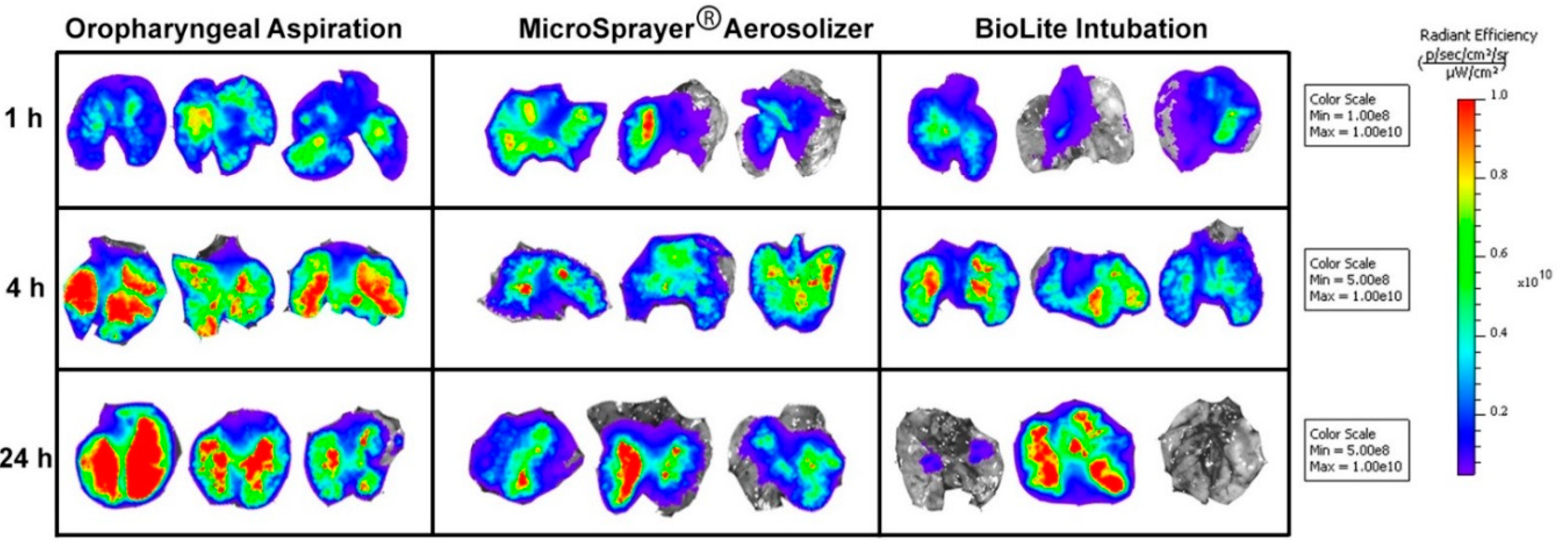
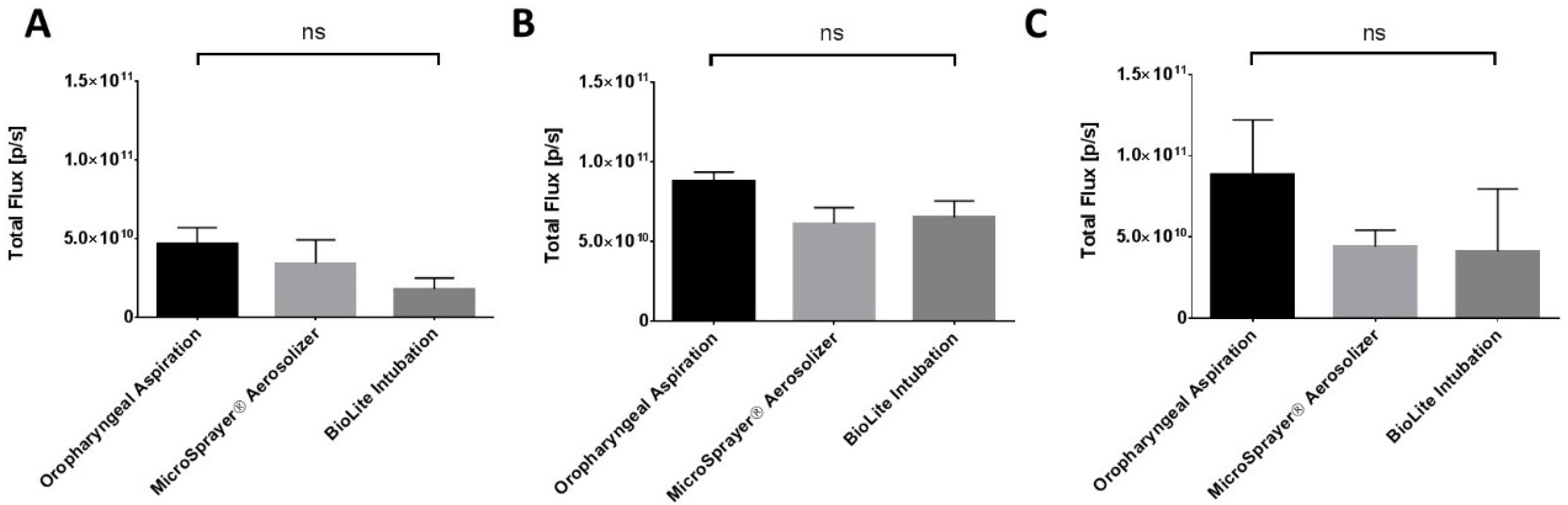
3.2. Lung Imaging
3.3. Three-Dimensional Longitudinal Imaging
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kunda, N.K.; Somavarapu, S.; Gordon, S.B.; Hutcheon, G.A.; Saleem, I.Y. Nanocarriers Targeting Dendritic Cells for Pulmonary Vaccine Delivery. Pharm. Res. 2013, 30, 325–341. [Google Scholar] [CrossRef] [PubMed]
- Eleftheriadis, G.K.; Akrivou, M.; Bouropoulos, N.; Tsibouklis, J.; Vizirianakis, I.S.; Fatouros, D.G. Polymer–Lipid Microparticles for Pulmonary Delivery. Langmuir 2018, 34, 3438–3448. [Google Scholar] [CrossRef] [PubMed]
- Price, D.N.; Stromberg, L.R.; Kunda, N.K.; Muttil, P. In vivo pulmonary delivery and magnetic-targeting of dry powder nano-in-microparticles. Mol. Pharm. 2017, 14, 4741–4750. [Google Scholar] [CrossRef] [PubMed]
- Cauley, L.S.; Lefrançois, L. Guarding the perimeter: Protection of the mucosa by tissue-resident memory T cells. Mucosal Immunol. 2013, 6, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Walrath, J.; Zukowski, L.; Krywiak, A.; Silver, R.F. Resident Th1-like effector memory cells in pulmonary recall responses to Mycobacterium tuberculosis. Am. J. Respir. Cell Mol. Biol. 2005, 33, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.L.; Bickham, K.L.; Thome, J.J.; Kim, C.Y.; D’Ovidio, F.; Wherry, E.J.; Farber, D.L. Lung niches for the generation and maintenance of tissue-resident memory T cells. Mucosal Immunol. 2014, 7, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Price, D.N.; Kusewitt, D.F.; Lino, C.A.; McBride, A.A.; Muttil, P. Oral Tolerance to Environmental Mycobacteria Interferes with Intradermal, but Not Pulmonary, Immunization against Tuberculosis. PLoS Pathog. 2016, 12, e1005614. [Google Scholar] [CrossRef] [PubMed]
- O’Hagan, D.T.; Singh, M.; Ulmer, J.B. Microparticle-based technologies for vaccines. Methods 2006, 40, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Thiele, L.; Merkle, H.; Walter, E. Phagocytosis and Phagosomal Fate of Surface-Modified Microparticles in Dendritic Cells and Macrophages. Pharm. Res. 2003, 20, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Jorquera, P.A.; Tripp, R.A. Synthetic Biodegradable Microparticle and Nanoparticle Vaccines against the Respiratory Syncytial Virus. Vaccines 2016, 4, 45. [Google Scholar] [CrossRef] [PubMed]
- Gamvrellis, A.; Leong, D.; Hanley, J.C.; Xiang, S.D.; Mottram, P.; Plebanski, M. Vaccines that facilitate antigen entry into dendritic cells. Immunol. Cell Biol. 2004, 82, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Pandya, S.M.; Mohammad, I.; Agrawal, A.K.; Mohan, M.; Misra, A. Particulate Pulmonary Delivery Systems Containing Anti-Tuberculosis Agents. Crit. Rev. Ther. Drug Carr. Syst. 2013, 30, 277–291. [Google Scholar] [CrossRef]
- El-Sherbiny, I.M.; El-Baz, N.M.; Yacoub, M.H. Inhaled nano-and microparticles for drug delivery. Glob. Cardiol. Sci. Pract. 2015. [Google Scholar] [CrossRef] [PubMed]
- Abdelaziz, H.M.; Gaber, M.; Abd-Elwakil, M.M.; Mabrouk, M.T.; Elgohary, M.M.; Kamel, N.M.; Kabary, D.M.; Freag, M.S.; Samaha, M.W.; Mortada, S.M.; et al. Inhalable particulate drug delivery systems for lung cancer therapy: Nanoparticles, microparticles, nanocomposites and nanoaggregates. J. Control. Release 2018, 269, 374–392. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Garcia-Contreras, L.; Muttil, P.; Padilla, D.; Xu, D.; Liu, J.; Braunstein, M.; McMurray, D.; Hickey, A. Pulmonary Immunization Using Antigen 85-B Polymeric Microparticles to Boost Tuberculosis Immunity. AAPS J. 2010, 12, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, T.C.; Oliveira, M.L.S.; Soares-Schanoski, A.; Chavez-Rico, S.L.; Figueiredo, D.B.; Gonçalves, V.M.; Ferreira, D.M.; Kunda, N.K.; Saleem, I.Y.; Miyaji, E.N. Mucosal immunization with PspA (Pneumococcal surface protein A)-adsorbed nanoparticles targeting the lungs for protection against pneumococcal infection. PLoS ONE 2018, 13, e0191692. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Muttil, P.; Verma, R.K.; Kumar, K.; Yadav, A.B.; Sharma, R.; Misra, A. A hand-held apparatus for “nose-only” exposure of mice to inhalable microparticles as a dry powder inhalation targeting lung and airway macrophages. Eur. J. Pharm. Sci. 2008, 34, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Contreras, L.; Wong, Y.-L.; Muttil, P.; Padilla, D.; Sadoff, J.; Derousse, J.; Germishuizen, W.A.; Goonesekera, S.; Elbert, K.; Bloom, B.R.; et al. Immunization by a bacterial aerosol. Proc. Natl. Acad. Sci. USA 2008, 105, 4656–4660. [Google Scholar] [CrossRef] [PubMed]
- Wylie, J.L.; House, A.; Mauser, P.J.; Sellers, S.; Terebetski, J.; Wang, Z.; Ehrick, J.D. Inhaled formulation and device selection: Bridging the gap between preclinical species and first-in-human studies. Ther. Deliv. 2018, 9, 387–404. [Google Scholar] [CrossRef] [PubMed]
- Price, D.N.; Kunda, N.K.; Muttil, P. Challenges Associated with the Pulmonary Delivery of Therapeutic Dry Powders for Preclinical Testing. KONA Powder Part J. 2018. [Google Scholar] [CrossRef]
- De Vooght, V.; Vanoirbeek, J.A.J.; Haenen, S.; Verbeken, E.; Nemery, B.; Hoet, P.H.M. Oropharyngeal aspiration: An alternative route for challenging in a mouse model of chemical-induced asthma. Toxicology 2009, 259, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.A.; Vanbever, R. Preclinical models for pulmonary drug delivery. Expert Opin. Drug Deliv. 2009, 6, 1231–1245. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Buff, S.M.; Baatz, J.E.; Virella-Lowell, I. Oral instillation with surfactant phospholipid: A reliable alternative to intratracheal injection in mouse studies. Lab. Anim. 2008, 42, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Bale, S.; Sunkoju, M.; Reddy, S.S.; Swamy, V.; Godugu, C. Oropharyngeal aspiration of bleomycin: An alternative experimental model of pulmonary fibrosis developed in Swiss mice. Indian J. Pharmacol. 2016, 48, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Morello, M.; Krone, C.L.; Dickerson, S.; Howerth, E.; Germishuizen, W.A.; Wong, Y.-L.L.; Edwards, D.; Bloom, B.R.; Hondalus, M.K. Dry-powder pulmonary insufflation in the mouse for application to vaccine or drug studies. Tuberculosis 2009, 89, 371–377. [Google Scholar] [CrossRef] [PubMed]
- McBride, A.A.; Price, D.N.; Muttil, P. Pulmonary Delivery of Magnetically Targeted Nano-in-Microparticles BT—Cancer Nanotechnology: Methods and Protocols; Zeineldin, R., Ed.; Springer: New York, NY, USA, 2017; pp. 369–378. ISBN 978-1-4939-6646-2. [Google Scholar]
- Price, D.N.; Muttil, P. Delivery of Therapeutics to the Lung. In Lung Innate Immunity and Inflammation: Methods and Protocols, Methods in Molecular Biology; Alper, S., Janssen, W., Eds.; Springer Science + Business Media; LLC: Berlin, Germany, 2018; Volume 1809, pp. 415–429. [Google Scholar]
- Kunda, N.K.; Price, D.N.; Muttil, P. Microparticle Distribution in Spleen, Liver, and Kidney after Pulmonary Administration in Mice Using Different Pulmonary Delivery Techniques. Unpublished work. 2018. [Google Scholar]
- Stein, S.W.; Thiel, C.G. The History of Therapeutic Aerosols: A Chronological Review. J. Aerosol Med. Pulm. Drug Deliv. 2017, 30, 20–41. [Google Scholar] [CrossRef] [PubMed]
- Hoppentocht, M.; Hagedoorn, P.; Frijlink, H.W.; de Boer, A.H. Technological and practical challenges of dry powder inhalers and formulations. Adv. Drug Deliv. Rev. 2014, 75, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Rao, G.V.S.; Tinkle, S.; Weissman, D.; Antonini, J.; Kashon, M.; Salmen, R.; Battelli, L.; Willard, P.; Hubbs, A.; Hoover, M. Efficacy of a Technique for Exposing the Mouse Lung to Particles Aspirated from the Pharynx. J. Toxicol. Environ. Health Part A 2003, 66, 1441–1452. [Google Scholar] [CrossRef] [PubMed]
- Lakatos, H.F.; Burgess, H.A.; Thatcher, T.H.; Redonnet, M.R.; Hernady, E.; Williams, J.P.; Sime, P.J. Oropharyngeal Aspiration of a Silica Suspension Produces a Superior Model of Silicosis in the Mouse When Compared to Intratracheal Instillation. Exp. Lung Res. 2006, 32, 181–199. [Google Scholar] [CrossRef] [PubMed]
- Robbe, A.; Tassin, A.; Carpentier, J.; Declèves, A.-E.; Mekinda Ngono, Z.L.; Nonclercq, D.; Legrand, A. Intratracheal Bleomycin Aerosolization: The Best Route of Administration for a Scalable and Homogeneous Pulmonary Fibrosis Rat Model? BioMed Res. Int. 2015, 2015, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Vartiainen, V.; Raula, J.; Bimbo, L.M.; Viinamäki, J.; Backman, J.T.; Ugur, N.; Kauppinen, E.; Sutinen, E.; Joensuu, E.; Koli, K.; et al. Pulmonary administration of a dry powder formulation of the antifibrotic drug tilorone reduces silica-induced lung fibrosis in mice. Int. J. Pharm. 2018, 544, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Chakravarthy, K.V.; Davidson, B.A.; Helinski, J.D.; Ding, H.; Law, W.-C.; Yong, K.-T.; Prasad, P.N.; Knight, P.R. Doxorubicin-conjugated quantum dots to target alveolar macrophages and inflammation. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Juarrero, M.; Woolhiser, L.K.; Brooks, E.; DeGroote, M.A.; Lenaerts, A.J. Mouse model for efficacy testing of antituberculosis agents via intrapulmonary delivery. Antimicrob. Agents Chemother. 2012, 56, 3957–3959. [Google Scholar] [CrossRef] [PubMed]
- Gutbier, B.; Kube, S.M.; Reppe, K.; Santel, A.; Lange, C.; Kaufmann, J.; Suttorp, N.; Witzenrath, M. RNAi-mediated suppression of constitutive pulmonary gene expression by small interfering RNA in mice. Pulm. Pharmacol. Ther. 2010, 23, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Muttil, P.; Prego, C.; Garcia-Contreras, L.; Pulliam, B.; Fallon, J.; Wang, C.; Hickey, A.; Edwards, D. Immunization of Guinea Pigs with Novel Hepatitis B Antigen as Nanoparticle Aggregate Powders Administered by the Pulmonary Route. AAPS J. 2010, 12, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, A.; Padilla-Carlin, D.; Kelly, C.; Hickey, A.J.; Taggart, C.; McElvaney, N.G.; Cryan, S.-A. The Effect of Liposome Encapsulation on the Pharmacokinetics of Recombinant Secretory Leukocyte Protease Inhibitor (rSLPI) Therapy after Local Delivery to a Guinea Pig Asthma Model. Pharm. Res. 2011, 28, 2233–2245. [Google Scholar] [CrossRef] [PubMed]
- Rajapaksa, A.E.; Ho, J.J.; Qi, A.; Bischof, R.; Nguyen, T.-H.; Tate, M.; Piedrafita, D.; McIntosh, M.P.; Yeo, L.Y.; Meeusen, E.; et al. Effective pulmonary delivery of an aerosolized plasmid DNA vaccine via surface acoustic wave nebulization. Respir. Res. 2014, 15, 60. [Google Scholar] [CrossRef] [PubMed]
- Sadhukha, T.; Wiedmann, T.S.; Panyam, J. Inhalable magnetic nanoparticles for targeted hyperthermia in lung cancer therapy. Biomaterials 2013, 34, 5163–5171. [Google Scholar] [CrossRef] [PubMed]
| Oropharyngeal Aspiration | MicroSprayer® Aerosolizer | BioLite Intubation System | |
|---|---|---|---|
| Ease of Administration | Easy to use with minimal expertise; only requires a pipette (a small animal laryngoscope facilitates visualization of the oropharynx/trachea) | Technical expertise needed; requires a small animal laryngoscope, however, the device is currently discontinued | Technical expertise required; requires a small animal laryngoscope and the purchase of a BioLite Intubation System |
| Drug suspension/solution is placed at the back of the oropharynx; mice are forced to breathe by occluding nose with a fingertip, facilitating drug delivery into the lungs | The delivery tube is inserted gently into the trachea and the drug suspension/solution is forced into the lungs | The intubation catheter is gently inserted into the trachea with the help of a fiber-optic stylet/guide wire. The stylet is slowly removed and a drug suspension/solution loaded syringe is attached to the catheter and delivered by compressing the syringe plunger | |
| Respiratory Tract Deposition and Distribution | Possible deposition in the oral cavity, in addition to trachea, and the lungs | Showed deposition in the trachea and the lungs (no deposition in the oral cavity as the delivery tube is inserted into the trachea) | Showed deposition in the trachea and the lungs (no deposition in the oral cavity as the intubation catheter is inserted into the trachea) |
| 3D imaging shows microparticles reaching the GI tract at 4 and 24 h, indicating GI deposition along with tracheal and lung deposition | 3D imaging shows majority of the microparticle deposition in trachea and the lungs | 3D imaging shows majority of the microparticle deposition in trachea and the lungs | |
| No significant differences in total flux/deposition of microparticles at 1, 4, and 24 h in whole animal and excised lungs, liver, spleen, and kidneys | |||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kunda, N.K.; Price, D.N.; Muttil, P. Respiratory Tract Deposition and Distribution Pattern of Microparticles in Mice Using Different Pulmonary Delivery Techniques. Vaccines 2018, 6, 41. https://doi.org/10.3390/vaccines6030041
Kunda NK, Price DN, Muttil P. Respiratory Tract Deposition and Distribution Pattern of Microparticles in Mice Using Different Pulmonary Delivery Techniques. Vaccines. 2018; 6(3):41. https://doi.org/10.3390/vaccines6030041
Chicago/Turabian StyleKunda, Nitesh K., Dominique N. Price, and Pavan Muttil. 2018. "Respiratory Tract Deposition and Distribution Pattern of Microparticles in Mice Using Different Pulmonary Delivery Techniques" Vaccines 6, no. 3: 41. https://doi.org/10.3390/vaccines6030041
APA StyleKunda, N. K., Price, D. N., & Muttil, P. (2018). Respiratory Tract Deposition and Distribution Pattern of Microparticles in Mice Using Different Pulmonary Delivery Techniques. Vaccines, 6(3), 41. https://doi.org/10.3390/vaccines6030041






