Immune History and Influenza Vaccine Effectiveness
Abstract
1. Introduction
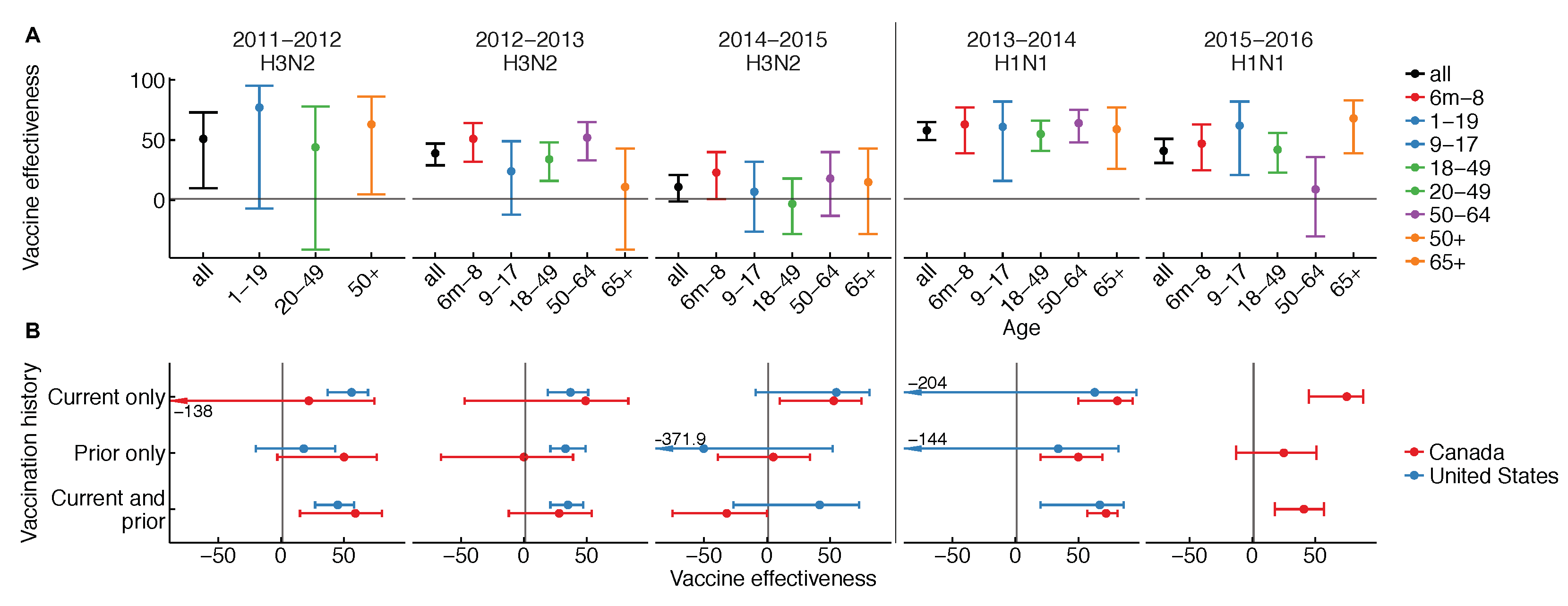
2. What Do We Expect, and What Do We See?
3. Are Our Measures of Vaccine Effectiveness Part of the Problem?
4. Biological Explanations: Original Antigenic Sin
5. Other Biological Explanations
6. Future Directions
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| OAS | Original antigenic sin |
| OR | Odds ratio |
| TND | Test negative design |
| VE | Vaccine efficacy or effectiveness (defined here as the vaccine direct effect on susceptibility to infection and/or disease) |
Appendix A
| Season | VE Type | Notes | Source |
|---|---|---|---|
| 2011–2012 | Age | All ages | Canada [91] |
| Vaccination history | ≥9 years, all flu | U.S. [92] | |
| ≥2 years | Canada [91] | ||
| 2012–2013 | Age | All ages | U.S. [93] |
| Vaccination history | ≥9 years | U.S. [93] | |
| ≥9 years | Canada [67] | ||
| 2013-2014 | Age | ≥6 months | U.S. [94] |
| Vaccination history | ≥9 years | U.S. [95] | |
| ≥2 years | Canada [96] | ||
| 2014–2015 | Age | ≥6 months | U.S. [97] |
| Vaccination history | ≥18 years | U.S. [98] | |
| ≥2 years | Canada [17] | ||
| 2015–2016 | Age | ≥6 months | U.S. [97] |
| Vaccination history | ≥9 years | Canada [12] |
Appendix B. Measuring VE against Influenza
References
- Tricco, A.C.; Chit, A.; Soobiah, C.; Hallett, D.; Meier, G.; Chen, M.H.; Tashkandi, M.; Bauch, C.T.; Loeb, M. Comparing influenza vaccine efficacy against mismatched and matched strains: A systematic review and meta-analysis. BMC Med. 2013, 11, 153. [Google Scholar] [CrossRef] [PubMed]
- Erbelding, E.J.; Post, D.; Stemmy, E.; Roberts, P.C.; Augustine, A.D.; Ferguson, S.; Paules, C.I.; Graham, B.S.; Fauci, A.S. A Universal Influenza Vaccine: The Strategic Plan for the National Institute of Allergy and Infectious Diseases. J. Infect. Dis. 2018. [Google Scholar] [CrossRef] [PubMed]
- Belongia, E.A.; Kieke, B.A.; Donahue, J.G.; Greenlee, R.T.; Balish, A.; Foust, A.; Lindstrom, S.; Shay, D.K. Effectiveness of inactivated influenza vaccines varied substantially with antigenic match from the 2004–2005 season to the 2006–2007 season. J. Infect. Dis. 2009, 199, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Keitel, W.A.; Cate, T.R.; Couch, R.B.; Huggins, L.L.; Hess, K.R. Efficacy of repeated annual immunization with inactivated influenza virus vaccines over a five year period. Vaccine 1997, 15, 1114–1122. [Google Scholar] [CrossRef]
- Zost, S.J.; Parkhouse, K.; Gumina, M.E.; Kim, K.; Perez, S.D.; Wilson, P.C.; Treanor, J.J.; Sant, A.J.; Cobey, S.; Hensley, S.E. Contemporary H3N2 influenza viruses have a glycosylation site that alters binding of antibodies elicited by egg-adapted vaccine strains. Proc. Natl. Acad. Sci. USA 2017, 114, 12578–12583. [Google Scholar] [CrossRef] [PubMed]
- Stacey, H.D.; Barjesteh, N.; Mapletoft, J.P.; Miller, M.S. “Gnothi Seauton”: Leveraging the Host Response to Improve Influenza Virus Vaccine Efficacy. Vaccines 2018, 6, 23. [Google Scholar] [CrossRef] [PubMed]
- Belongia, E.A.; Simpson, M.D.; King, J.P.; Sundaram, M.E.; Kelley, N.S.; Osterholm, M.T.; McLean, H.Q. Variable influenza vaccine effectiveness by subtype: A systematic review and meta-analysis of test-negative design studies. Lancet Infect. Dis. 2016, 16, 942–951. [Google Scholar] [CrossRef]
- Ramsay, L.C.; Buchan, S.A.; Stirling, R.G.; Cowling, B.J.; Feng, S.; Kwong, J.C.; Warshawsky, B.F. The impact of repeated vaccination on influenza vaccine effectiveness: A systematic review and meta-analysis. BMC Med. 2017, 15, 159. [Google Scholar] [CrossRef] [PubMed]
- Belongia, E.A.; Skowronski, D.M.; McLean, H.Q.; Chambers, C.; Sundaram, M.E.; De Serres, G. Repeated annual influenza vaccination and vaccine effectiveness: Review of evidence. Expert Rev. Vaccines 2017, 16, 723–736. [Google Scholar] [CrossRef] [PubMed]
- Ohmit, S.E.; Petrie, J.G.; Malosh, R.E.; Cowling, B.J.; Thompson, M.G.; Shay, D.K.; Monto, A.S. Influenza vaccine effectiveness in the community and the household. Clin. Infect. Dis. 2013, 56, 1363–1369. [Google Scholar] [CrossRef] [PubMed]
- Flannery, B.; Smith, C.; Garten, R.J.; Levine, M.Z.; Chung, J.R.; Jackson, M.L.; Jackson, L.A.; Monto, A.S.; Martin, E.T.; Belongia, E.A.; et al. Influence of Birth Cohort on Effectiveness of 2015–2016 Influenza Vaccine Against Medically Attended Illness Due to 2009 Pandemic Influenza A (H1N1) Virus in the United States. J. Infect. Dis. 2018. [Google Scholar] [CrossRef] [PubMed]
- Skowronski, D.M.; Chambers, C.; Sabaiduc, S.; De Serres, G.; Winter, A.L.; Dickinson, J.A.; Gubbay, J.B.; Drews, S.J.; Martineau, C.; Charest, H.; et al. Beyond Antigenic Match: Possible Agent-Host and Immuno-epidemiological Influences on Influenza Vaccine Effectiveness During the 2015–2016 Season in Canada. J. Infect. Dis. 2017, 216, 1487–1500. [Google Scholar] [CrossRef] [PubMed]
- Flannery, B.; Chung, J.R.; Thaker, S.N.; Monto, A.S.; Martin, E.T.; Belongia, E.A.; McLean, H.Q.; Gaglani, M.; Murthy, K.; Zimmerman, R.K.; et al. Interim Estimates of 2016–2017 Seasonal Influenza Vaccine Effectiveness- United States, February 2017. Morb. Mortal. Wkly. Rep. 2017, 66, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Flannery, B.; Chung, J.R.; Belongia, E.A.; McLean, H.Q.; Gaglani, M.; Murthy, K.; Zimmerman, R.K.; Nowalk, M.P.; Jackson, M.L.; Jackson, L.A.; et al. Interim estimates of 2017–18 seasonal influenza vaccine effectiveness—United States, February 2018. Am. J. Transplant. 2018, 18, 1020–1025. [Google Scholar] [CrossRef] [PubMed]
- McLean, H.Q.; Thompson, M.G.; Sundaram, M.E.; Meece, J.K.; McClure, D.L.; Friedrich, T.C.; Belongia, E.A. Impact of repeated vaccination on vaccine effectiveness against influenza A (H3N2) and B during 8 seasons. Clin. Infect. Dis. 2014, 59, 1375–1385. [Google Scholar] [CrossRef] [PubMed]
- Saito, N.; Komori, K.; Suzuki, M.; Kishikawa, T.; Yasaka, T.; Ariyoshi, K. Dose-Dependent Negative Effects of Prior Multiple Vaccinations against Influenza A and Influenza B among School Children: A Study of Kamigoto Island in Japan during the 2011/12, 2012/13 and 2013/14 Influenza Seasons. Clin. Infect. Dis. 2018, ciy202. [Google Scholar] [CrossRef]
- Skowronski, D.M.; Chambers, C.; Sabaiduc, S.; De Serres, G.; Winter, A.L.; Dickinson, J.A.; Krajden, M.; Gubbay, J.B.; Drews, S.J.; Martineau, C.; et al. A perfect storm: Impact of genomic variation and serial vaccination on low influenza vaccine effectiveness during the 2014–2015 season. Clin. Infect. Dis. 2016, 63, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Skowronski, D.M.; De Serres, G.; Crowcroft, N.S.; Janjua, N.Z.; Boulianne, N.; Hottes, T.S.; Rosella, L.C.; Dickinson, J.A.; Gilca, R.; Sethi, P.; et al. Association between the 2008–09 seasonal influenza vaccine and pandemic H1N1 illness during spring–summer 2009: Four observational studies from Canada. PLoS Med. 2010, 7, e1000258. [Google Scholar] [CrossRef] [PubMed]
- Cowling, B.J.; Ng, S.; Ma, E.S.; Cheng, C.K.; Wai, W.; Fang, V.J.; Chan, K.H.; Ip, D.K.; Chiu, S.S.; Peiris, J.M.; et al. Protective efficacy of seasonal influenza vaccination against seasonal and pandemic influenza virus infection during 2009 in Hong Kong. Clin. Infect. Dis. 2010, 51, 1370–1379. [Google Scholar] [CrossRef] [PubMed]
- Carcione, D.; Giele, C.; Goggin, L.; Kwan, K.; Smith, D.; Dowse, G.; Mak, D.B.; Effler, P. Association between 2009 seasonal influenza vaccine and influenza-like illness during the 2009 pandemic: Preliminary results of a large household transmission study in Western Australia. Eurosurveillance 2010, 15, 19616. [Google Scholar] [PubMed]
- Jefferies, S.; Earl, D.; Berry, N.; Blackmore, T.; Rooker, S.; Raymond, N.; Pritchard, A.; Weatherall, M.; Beasley, R.; Perrin, K. Effectiveness of the 2009 seasonal influenza vaccine against pandemic influenza A (H1N1) 2009 in healthcare workers in New Zealand, June–August 2009. Eurosurveillance 2011, 16, 19761. [Google Scholar] [PubMed]
- Halloran, M.E.; Haber, M.; Longini, I.M., Jr.; Struchiner, C.J. Direct and indirect effects in vaccine efficacy and effectiveness. Am. J. Epidemiol. 1991, 133, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Foppa, I.M.; Haber, M.; Ferdinands, J.M.; Shay, D.K. The case test-negative design for studies of the effectiveness of influenza vaccine. Vaccine 2013, 31, 3104–3109. [Google Scholar] [CrossRef] [PubMed]
- Broome, C.V.; Facklam, R.R.; Fraser, D.W. Pneumococcal disease after pneumococcal vaccination: An alternative method to estimate the efficacy of pneumococcal vaccine. N. Engl. J. Med. 1980, 303, 549–552. [Google Scholar] [CrossRef] [PubMed]
- Wacholder, S.; Silverman, D.T.; McLaughlin, J.K.; Mandel, J.S. Selection of controls in case-control studies: II. Types of controls. Am. J. Epidemiol. 1992, 135, 1029–1041. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.L.; Nelson, J.C. The test-negative design for estimating influenza vaccine effectiveness. Vaccine 2013, 31, 2165–2168. [Google Scholar] [CrossRef] [PubMed]
- Foppa, I.M.; Ferdinands, J.M.; Chaves, S.S.; Haber, M.J.; Reynolds, S.B.; Flannery, B.; Fry, A.M. The case test-negative design for studies of the effectiveness of influenza vaccine in inpatient settings. Int. J. Epidemiol. 2016, 45, 2052–2059. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.; Rodrigues, L.; Fine, P. Assessment of the protective efficacy of vaccines against common diseases using case-control and cohort studies. Int. J. Epidemiol. 1984, 13, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Noronha, C.P.; Struchiner, C.J.; Halloran, M.E. Assessment of the direct effectiveness of BC meningococcal vaccine in Rio de Janeiro, Brazil: A case-control study. Int. J. Epidemiol. 1995, 24, 1050–1057. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, S.G.; Tchetgen Tchetgen, E.J.; Cowling, B.J. Theoretical basis of the test-negative study design for assessment of influenza vaccine effectiveness. Am. J. Epidemiol. 2016, 184, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Lipsitch, M.; Jha, A.; Simonsen, L. Observational studies and the difficult quest for causality: Lessons from vaccine effectiveness and impact studies. Int. J. Epidemiol. 2016, 45, 2060–2074. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; An, Q.; Ainslie, K.E.; Haber, M.; Orenstein, W.A. A comparison of the test-negative and the traditional case-control study designs for estimation of influenza vaccine effectiveness under nonrandom vaccination. BMC Infect. Dis. 2017, 17, 757. [Google Scholar] [CrossRef] [PubMed]
- Ainslie, K.E.; Shi, M.; Haber, M.; Orenstein, W.A. On the bias of estimates of influenza vaccine effectiveness from test–negative studies. Vaccine 2017, 35, 7297–7301. [Google Scholar] [CrossRef] [PubMed]
- Lewnard, J.A.; Tedijanto, C.; Cowling, B.J.; Lipsitch, M. Quantifying biases in test-negative studies of vaccine effectiveness. bioRxiv 2018, 237503. [Google Scholar] [CrossRef]
- Westreich, D.; Hudgens, M.G. Invited commentary: Beware the test-negative design. Am. J. Epidemiol. 2016, 184, 354–356. [Google Scholar] [CrossRef] [PubMed]
- Ferdinands, J.M.; Foppa, I.M.; Fry, A.M.; Flannery, B.L.; Belongia, E.A.; Jackson, M.L. Re: “Invited Commentary: Beware the Test-Negative Design”. Am. J. Epidemiol. 2017, 185, 613. [Google Scholar] [CrossRef] [PubMed]
- Small, P.A.; Cronin, B.J. The Advisory Committee on Immunization Practices’ controversial recommendation against the use of live attenuated influenza vaccine is based on a biased study design that ignores secondary protection. Vaccine 2017, 8, 1110–1112. [Google Scholar] [CrossRef] [PubMed]
- Belongia, E.A.; Karron, R.A.; Reingold, A.; Walter, E.B.; Bennett, N.M. The Advisory Committee on Immunization Practices recommendation regarding the use of live influenza vaccine: A rejoinder. Vaccine 2017. [Google Scholar] [CrossRef] [PubMed]
- Demicheli, V.; Jefferson, T.; Di Pietrantonj, C.; Ferroni, E.; Thorning, S.; Thomas, R.E.; Rivetti, A. Vaccines for preventing influenza in the elderly. Cochrane Libr. 2018. [Google Scholar] [CrossRef] [PubMed]
- Demicheli, V.; Jefferson, T.; Ferroni, E.; Rivetti, A.; Pietrantonj, C.D. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst. Rev. 2018. [Google Scholar] [CrossRef] [PubMed]
- Jefferson, T.; Rivetti, A.; Pietrantonj, C.D.; Demicheli, V. Vaccines for preventing influenza in healthy children. Cochrane Database Syst. Rev. 2018. [Google Scholar] [CrossRef] [PubMed]
- Simonsen, L.; Reichert, T.A.; Viboud, C.; Blackwelder, W.C.; Taylor, R.J.; Miller, M.A. Impact of influenza vaccination on seasonal mortality in the US elderly population. Arch. Int. Med. 2005, 165, 265–272. [Google Scholar]
- Bar-Zeev, N.; Kapanda, L.; Tate, J.E.; Jere, K.C.; Iturriza-Gomara, M.; Nakagomi, O.; Mwansambo, C.; Costello, A.; Parashar, U.D.; Heyderman, R.S.; et al. Effectiveness of a monovalent rotavirus vaccine in infants in Malawi after programmatic roll-out: An observational and case-control study. Lancet Infect. Dis. 2015, 15, 422–428. [Google Scholar] [CrossRef]
- Lopman, B.A.; Pitzer, V.E. Waxing Understanding of Waning Immunity. J. Infect. Dis. 2018, 217, 851–853. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.G.M.; Gordon, S.B.; Lalloo, D.G. Clinical trials: The mathematics of falling vaccine efficacy with rising disease incidence. Vaccine 2016, 34, 3007. [Google Scholar] [CrossRef] [PubMed]
- O’Hagan, J.J.; Hernán, M.A.; Walensky, R.P.; Lipsitch, M. Apparent Declining Efficacy in Randomized Trials: Examples of the RV144 HIV Vaccine and CAPRISA 004 Microbicide Trials. AIDS 2012, 26, 123. [Google Scholar] [CrossRef] [PubMed]
- Young, B.; Sadarangani, S.; Jiang, L.; Wilder-Smith, A.; Chen, M.I. The Duration of Influenza Vaccine Effectiveness: A Systematic Review, Meta-analysis and Meta-regression of Test-Negative Design Case-control Studies. J. Infect. Dis. 2017. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Cowling, B.J.; Kelly, H.; Sullivan, S.G. Estimating Influenza Vaccine Effectiveness in the Test-negative Design Using Alternative Control Groups—A Systematic Review and Meta-analysis. Am. J. Epidemiol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Davenport, F.M.; Hennessy, A.V. A serologic recapitulation of past experiences with influenza A; Antibody response to monovalent vaccine. J. Exp. Med. 1956, 104, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Davenport, F.M.; Hennessy, A.V. Predetermination by infection and by vaccination of antibody response to influenza virus vaccines. J. Exp. Med. 1957, 106, 835–850. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Li, L.; Ye, Z.; Li, X.; Plant, E.P.; Zoueva, O.; Zhao, Y.; Jing, X.; Lin, Z.; Kawano, T.; et al. Differential effects of prior influenza exposures on H3N2 cross-reactivity of human postvaccination sera. Clin. Infect. Dis. 2017, 65, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Andrews, S.F.; Huang, Y.; Kaur, K.; Popova, L.I.; Ho, I.Y.; Pauli, N.T.; Dunand, C.J.H.; Taylor, W.M.; Lim, S.; Huang, M.; et al. Immune history profoundly affects broadly protective B cell responses to influenza. Sci. Transl. Med. 2015, 7, 316ra192. [Google Scholar] [CrossRef] [PubMed]
- Wrammert, J.; Smith, K.; Miller, J.; Langley, W.A.; Kokko, K.; Larsen, C.; Zheng, N.Y.; Mays, I.; Garman, L.; Helms, C.; et al. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature 2008, 453, 667. [Google Scholar] [CrossRef] [PubMed]
- Wrammert, J.; Koutsonanos, D.; Li, G.M.; Edupuganti, S.; Sui, J.; Morrissey, M.; McCausland, M.; Skountzou, I.; Hornig, M.; Lipkin, W.I.; et al. Broadly cross-reactive antibodies dominate the human B cell response against 2009 pandemic H1N1 influenza virus infection. J. Exp. Med. 2011, jem-20101352. [Google Scholar] [CrossRef]
- Miller, M.S.; Gardner, T.J.; Krammer, F.; Aguado, L.C.; Tortorella, D.; Basler, C.F.; Palese, P. Neutralizing antibodies against previously encountered influenza virus strains increase over time: A longitudinal analysis. Sci. Transl. Med. 2013, 5, 198ra107. [Google Scholar] [CrossRef] [PubMed]
- Lessler, J.; Riley, S.; Read, J.M.; Wang, S.; Zhu, H.; Smith, G.J.; Guan, Y.; Jiang, C.Q.; Cummings, D.A. Evidence for antigenic seniority in influenza A (H3N2) antibody responses in southern China. PLoS Pathog. 2012, 8, e1002802. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Q.; Wohlbold, T.J.; Zheng, N.Y.; Huang, M.; Huang, Y.; Neu, K.E.; Lee, J.; Wan, H.; Rojas, K.T.; Kirkpatrick, E.; et al. Influenza Infection in Humans Induces Broadly Cross-Reactive and Protective Neuraminidase-Reactive Antibodies. Cell 2018, 173, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Myers, J.L.; Bostick, D.L.; Sullivan, C.B.; Madara, J.; Linderman, S.L.; Liu, Q.; Carter, D.M.; Wrammert, J.; Esposito, S.; et al. Immune history shapes specificity of pandemic H1N1 influenza antibody responses. J. Exp. Med. 2013, jem-20130212. [Google Scholar] [CrossRef] [PubMed]
- Fonville, J.M.; Wilks, S.; James, S.L.; Fox, A.; Ventresca, M.; Aban, M.; Xue, L.; Jones, T.; Le, N.; Pham, Q.; et al. Antibody landscapes after influenza virus infection or vaccination. Science 2014, 346, 996–1000. [Google Scholar] [CrossRef] [PubMed]
- Kucharski, A.J.; Lessler, J.; Read, J.M.; Zhu, H.; Jiang, C.Q.; Guan, Y.; Cummings, D.A.; Riley, S. Estimating the life course of influenza A (H3N2) antibody responses from cross-sectional data. PLoS Biol. 2015, 13, e1002082. [Google Scholar] [CrossRef] [PubMed]
- Linderman, S.L.; Hensley, S.E. Antibodies with ‘original antigenic sin’ properties are valuable components of secondary immune responses to influenza viruses. PLoS Pathog. 2016, 12, e1005806. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Davis, W.G.; Sambhara, S.; Jacob, J. Strategies to alleviate original antigenic sin responses to influenza viruses. Proc. Natl. Acad. Sci. USA 2012, 109, 13751–13756. [Google Scholar] [CrossRef] [PubMed]
- Cobey, S.; Hensley, S.E. Immune history and influenza virus susceptibility. Curr. Opin. Virol. 2017, 22, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Gostic, K.M.; Ambrose, M.; Worobey, M.; Lloyd-Smith, J.O. Potent protection against H5N1 and H7N9 influenza via childhood hemagglutinin imprinting. Science 2016, 354, 722–726. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, A.; Acosta, E.; Hallman, S.; Bourbeau, R.; Dillon, L.Y.; Ouellette, N.; Earn, D.J.; Herring, D.A.; Inwood, K.; Madrenas, J.; et al. Pandemic Paradox: Early Life H2N2 Pandemic Influenza Infection Enhanced Susceptibility to Death during the 2009 H1N1 Pandemic. mBio 2018, 9, e02091-17. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.J.; Forrest, S.; Ackley, D.H.; Perelson, A.S. Variable efficacy of repeated annual influenza vaccination. Proc. Natl. Acad. Sci. USA 1999, 96, 14001–14006. [Google Scholar] [CrossRef] [PubMed]
- Skowronski, D.M.; Chambers, C.; De Serres, G.; Sabaiduc, S.; Winter, A.L.; Dickinson, J.A.; Gubbay, J.B.; Fonseca, K.; Drews, S.J.; Charest, H.; et al. Serial vaccination and the antigenic distance hypothesis: Effects on influenza vaccine effectiveness during A (H3N2) epidemics in Canada, 2010–2011 to 2014–2015. J. Infect. Dis. 2017, 215, 1059–1099. [Google Scholar] [CrossRef] [PubMed]
- Cobey, S.; Gouma, S.; Parkhouse, K.; Chambers, B.S.; Ertl, H.C.; Schmader, K.E.; Halpin, R.A.; Lin, X.; Stockwell, T.B.; Das, S.R.; et al. Poor immunogenicity, not vaccine strain egg adaptation, may explain the low H3N2 influenza vaccine effectiveness in 2012–2013. Clin. Infect. Dis. 2018, ciy097. [Google Scholar] [CrossRef] [PubMed]
- Flannery, B.; Zimmerman, R.K.; Gubareva, L.V.; Garten, R.J.; Chung, J.R.; Nowalk, M.P.; Jackson, M.L.; Jackson, L.A.; Monto, A.S.; Ohmit, S.E.; et al. Enhanced genetic characterization of influenza A (H3N2) viruses and vaccine effectiveness by genetic group, 2014–2015. J. Infect. Dis. 2016, 214, 1010–1019. [Google Scholar] [CrossRef] [PubMed]
- Linderman, S.L.; Chambers, B.S.; Zost, S.J.; Parkhouse, K.; Li, Y.; Herrmann, C.; Ellebedy, A.H.; Carter, D.M.; Andrews, S.F.; Zheng, N.Y.; et al. Potential antigenic explanation for atypical H1N1 infections among middle-aged adults during the 2013–2014 influenza season. Proc. Natl. Acad. Sci. USA 2014, 111, 15798–15803. [Google Scholar] [CrossRef] [PubMed]
- Ndifon, W. A simple mechanistic explanation for original antigenic sin and its alleviation by adjuvants. J. R. Soc. Interface 2015, 12, 20150627. [Google Scholar] [CrossRef] [PubMed]
- Van der Most, R.G.; Roman, F.P.; Innis, B.; Hanon, E.; Vaughn, D.W.; Gillard, P.; Walravens, K.; Wettendorff, M. Seeking help: B cells adapting to flu variability. Sci. Transl. Med. 2014, 6, 246ps8. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, K.R.; Watanabe, A.; Kuraoka, M.; Do, K.T.; McGee, C.E.; Sempowski, G.D.; Kepler, T.B.; Schmidt, A.G.; Kelsoe, G.; Harrison, S.C. Memory B Cells that Cross-React with Group 1 and Group 2 Influenza A Viruses Are Abundant in Adult Human Repertoires. Immunity 2018, 48, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Nachbagauer, R.; Choi, A.; Izikson, R.; Cox, M.M.; Palese, P.; Krammer, F. Age dependence and isotype specificity of influenza virus hemagglutinin stalk-reactive antibodies in humans. MBio 2016, 7, e01996-15. [Google Scholar] [CrossRef] [PubMed]
- Khurana, S.; Verma, N.; Yewdell, J.W.; Hilbert, A.K.; Castellino, F.; Lattanzi, M.; Del Giudice, G.; Rappuoli, R.; Golding, H. MF59 adjuvant enhances diversity and affinity of antibody-mediated immune response to pandemic influenza vaccines. Sci. Transl. Med. 2011, 3, 85ra48. [Google Scholar] [CrossRef] [PubMed]
- Couch, R.B.; Atmar, R.L.; Franco, L.M.; Quarles, J.M.; Wells, J.; Arden, N.; Niño, D.; Belmont, J.W. Antibody correlates and predictors of immunity to naturally occurring influenza in humans and the importance of antibody to the neuraminidase. J. Infect. Dis. 2013, 207, 974–981. [Google Scholar] [CrossRef] [PubMed]
- Petrie, J.G.; Malosh, R.E.; Cheng, C.K.; Ohmit, S.E.; Martin, E.T.; Johnson, E.; Truscon, R.; Eichelberger, M.C.; Gubareva, L.V.; Fry, A.M.; et al. The Household Influenza Vaccine Effectiveness Study: Lack of Antibody Response and Protection Following Receipt of 2014–2015 Influenza Vaccine. Clin. Infect. Dis. 2017, 65, 1644–1651. [Google Scholar] [CrossRef] [PubMed]
- Ohmit, S.E.; Petrie, J.G.; Cross, R.T.; Johnson, E.; Monto, A.S. Influenza hemagglutination-inhibition antibody titer as a correlate of vaccine-induced protection. J. Infect. Dis. 2011, 204, 1879–1885. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, R.M.; Grill, D.E.; Oberg, A.L.; Tosh, P.K.; Ovsyannikova, I.G.; Poland, G.A. Profiles of influenza A/H1N1 vaccine response using hemagglutination-inhibition titers. Hum. Vaccines Immunother. 2015, 11, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, K.; Viboud, C.; Simonsen, L. Antibody response to influenza vaccination in the elderly: A quantitative review. Vaccine 2006, 24, 1159–1169. [Google Scholar] [CrossRef] [PubMed]
- Neidich, S.D.; Green, W.D.; Rebeles, J.; Karlsson, E.A.; Schultz-Cherry, S.; Noah, T.L.; Chakladar, S.; Hudgens, M.G.; Weir, S.S.; Beck, M.A. Increased risk of influenza among vaccinated adults who are obese. Int. J. Obes. 2017, 41, 1324. [Google Scholar] [CrossRef] [PubMed]
- Omer, S.B.; Phadke, V.K.; Bednarczyk, R.A.; Chamberlain, A.T.; Brosseau, J.L.; Orenstein, W.A. Impact of statins on influenza vaccine effectiveness against medically attended acute respiratory illness. J. Infect. Dis. 2015, 213, 1216–1223. [Google Scholar] [CrossRef] [PubMed]
- McLean, H.Q.; Chow, B.D.; VanWormer, J.J.; King, J.P.; Belongia, E.A. Effect of statin use on influenza vaccine effectiveness. J. Infect. Dis. 2016, 214, 1150–1158. [Google Scholar] [CrossRef] [PubMed]
- Izurieta, H.S.; Chillarige, Y.; Kelman, J.A.; Forshee, R.; Qiang, Y.; Wernecke, M.; Ferdinands, J.M.; Lu, Y.; Wei, Y.; Xu, W.; et al. Statin use and risks of influenza-related outcomes among older adults receiving standard-dose or high-dose influenza vaccines through Medicare during 2010–2015. Clin. Infect. Dis. 2018. [Google Scholar] [CrossRef] [PubMed]
- Furman, D.; Jojic, V.; Sharma, S.; Shen-Orr, S.S.; Angel, C.J.; Onengut-Gumuscu, S.; Kidd, B.A.; Maecker, H.T.; Concannon, P.; Dekker, C.L.; et al. Cytomegalovirus infection enhances the immune response to influenza. Sci. Transl. Med. 2015, 7, 281ra43. [Google Scholar] [CrossRef] [PubMed]
- Trzonkowski, P.; Myśliwska, J.; Szmit, E.; Wieckiewicz, J.; Łukaszuk, K.; Brydak, L.B.; Machała, M.; Myśliwski, A. Association between cytomegalovirus infection, enhanced proinflammatory response and low level of anti-hemagglutinins during the anti-influenza vaccination—An impact of immunosenescence. Vaccine 2003, 21, 3826–3836. [Google Scholar] [CrossRef]
- Klein, S.L.; Pekosz, A. Sex-based biology and the rational design of influenza vaccination strategies. J. Infect. Dis. 2014, 209, S114–S119. [Google Scholar] [CrossRef] [PubMed]
- HIPC-CHI Signatures Project Team; HIPC-I Consortium. Multicohort analysis reveals baseline transcriptional predictors of influenza vaccination responses. Sci. Immunol. 2017, 2. [Google Scholar] [CrossRef]
- Wen, F.T.; Malani, A.; Cobey, S. Vaccination and the evolution of seasonal influenza. bioRxiv 2017, 162545. [Google Scholar] [CrossRef]
- Cai, F.Y.; Fussell, T.; Cobey, S.E.; Lipsitch, M. Use of an individual-based model of pneumococcal carriage for planning a randomized trial of a vaccine. bioRxiv 2018, 258871. [Google Scholar] [CrossRef]
- Skowronski, D.M.; Janjua, N.Z.; Sabaiduc, S.; De Serres, G.; Winter, A.L.; Gubbay, J.B.; Dickinson, J.A.; Fonseca, K.; Charest, H.; Bastien, N.; et al. Influenza A/subtype and B/lineage effectiveness estimates for the 2011–2012 trivalent vaccine: Cross-season and cross-lineage protection with unchanged vaccine. J. Infect. Dis. 2014, 210, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Ohmit, S.E.; Thompson, M.G.; Petrie, J.G.; Thaker, S.N.; Jackson, M.L.; Belongia, E.A.; Zimmerman, R.K.; Gaglani, M.; Lamerato, L.; Spencer, S.M.; et al. Influenza vaccine effectiveness in the 2011–2012 season: Protection against each circulating virus and the effect of prior vaccination on estimates. Clin. Infect. Dis. 2013, 58, 319–327. [Google Scholar] [CrossRef] [PubMed]
- McLean, H.Q.; Thompson, M.G.; Sundaram, M.E.; Kieke, B.A.; Gaglani, M.; Murthy, K.; Piedra, P.A.; Zimmerman, R.K.; Nowalk, M.P.; Raviotta, J.M.; et al. Influenza vaccine effectiveness in the United States during 2012–2013: Variable protection by age and virus type. J. Infect. Dis. 2014, 211, 1529–1540. [Google Scholar] [CrossRef] [PubMed]
- Gaglani, M.; Pruszynski, J.; Murthy, K.; Clipper, L.; Robertson, A.; Reis, M.; Chung, J.R.; Piedra, P.A.; Avadhanula, V.; Nowalk, M.P.; et al. Influenza vaccine effectiveness against 2009 pandemic influenza A (H1N1) virus differed by vaccine type during 2013–2014 in the United States. J. Infect. Dis. 2016, 213, 1546–1556. [Google Scholar] [CrossRef] [PubMed]
- Ohmit, S.E.; Petrie, J.G.; Malosh, R.E.; Johnson, E.; Truscon, R.; Aaron, B.; Martens, C.; Cheng, C.; Fry, A.M.; Monto, A.S. Substantial influenza vaccine effectiveness in households with children during the 2013–2014 influenza season, when 2009 pandemic influenza A (H1N1) virus predominated. J. Infect. Dis. 2015, 213, 1229–1236. [Google Scholar] [CrossRef] [PubMed]
- Skowronski, D.M.; Chambers, C.; Sabaiduc, S.; De Serres, G.; Winter, A.L.; Dickinson, J.A.; Gubbay, J.; Fonseca, K.; Charest, H.; Krajden, M.; et al. Integrated sentinel surveillance linking genetic, antigenic, and epidemiologic monitoring of influenza vaccine-virus relatedness and effectiveness during the 2013–2014 influenza season. J. Infect. Dis. 2015, 212, 726–739. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, R.K.; Nowalk, M.P.; Chung, J.; Jackson, M.L.; Jackson, L.A.; Petrie, J.G.; Monto, A.S.; McLean, H.Q.; Belongia, E.A.; Gaglani, M.; et al. 2014–2015 influenza vaccine effectiveness in the United States by vaccine type. Clin. Infect. Dis. 2016, ciw635. [Google Scholar] [CrossRef] [PubMed]
- Petrie, J.G.; Ohmit, S.E.; Cheng, C.K.; Martin, E.T.; Malosh, R.E.; Lauring, A.S.; Lamerato, L.E.; Reyes, K.C.; Flannery, B.; Ferdinands, J.M.; et al. Influenza vaccine effectiveness against antigenically drifted influenza higher than expected in hospitalized adults: 2014–2015. Clin. Infect. Dis. 2016, 63, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lewnard, J.A.; Cobey, S. Immune History and Influenza Vaccine Effectiveness. Vaccines 2018, 6, 28. https://doi.org/10.3390/vaccines6020028
Lewnard JA, Cobey S. Immune History and Influenza Vaccine Effectiveness. Vaccines. 2018; 6(2):28. https://doi.org/10.3390/vaccines6020028
Chicago/Turabian StyleLewnard, Joseph A., and Sarah Cobey. 2018. "Immune History and Influenza Vaccine Effectiveness" Vaccines 6, no. 2: 28. https://doi.org/10.3390/vaccines6020028
APA StyleLewnard, J. A., & Cobey, S. (2018). Immune History and Influenza Vaccine Effectiveness. Vaccines, 6(2), 28. https://doi.org/10.3390/vaccines6020028





