Factors that Influence the Immunological Adjuvant Effect of Lactobacillus fermentum PC1 on Specific Immune Responses in Mice to Orally Administered Antigens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Cultures
2.2. Mice
2.3. Preparation of ST Cell Lysate (STCL)
2.4. In Vivo Experimental Protocol
2.5. Antigen-Induced Lymphocyte Assay
2.6. Immunoglobulin Quantification
2.7. Statistical Analysis
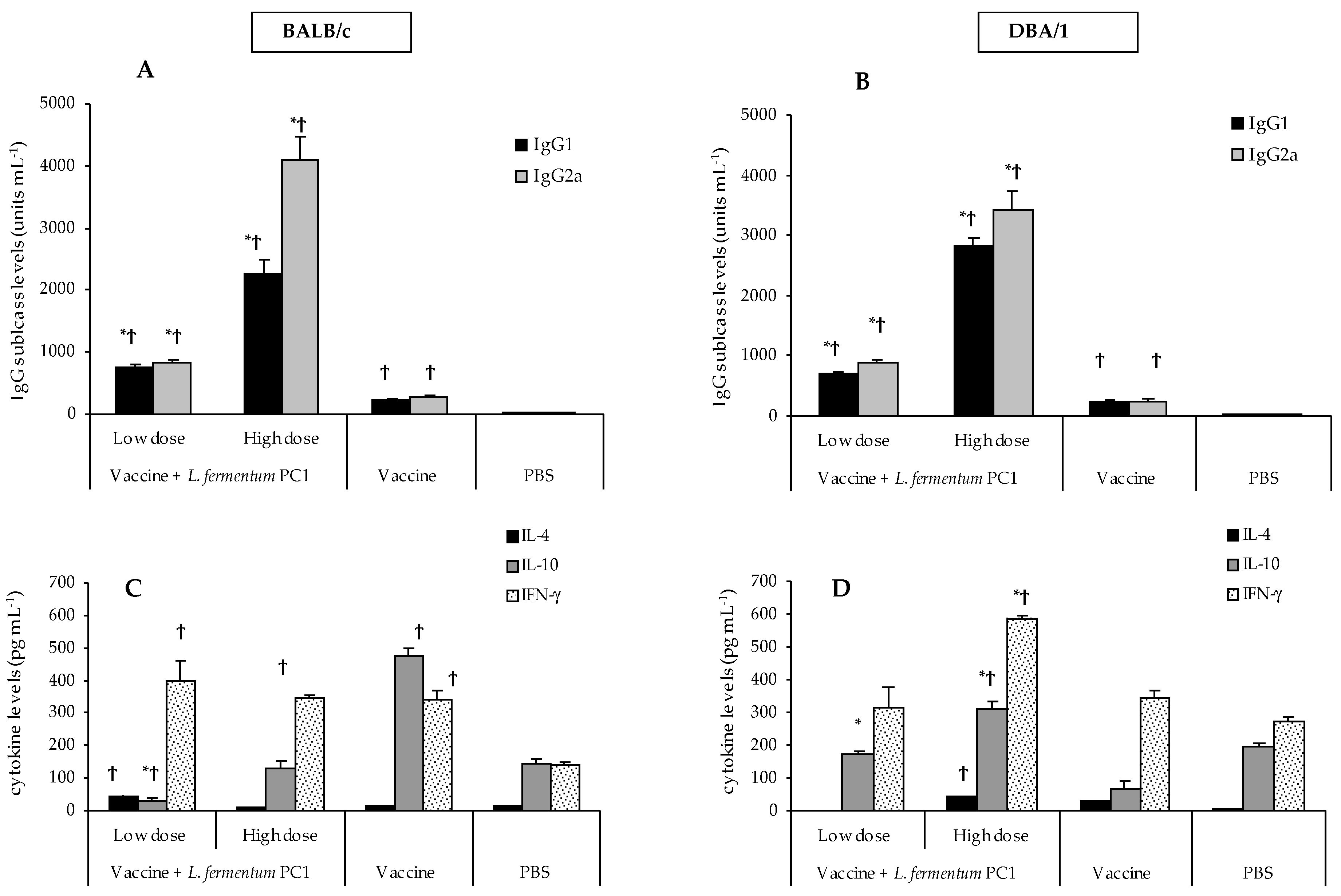
3. Results
3.1. STCL-Specific sIgA in Intestinal Fluid
3.2. STCL-Specific Antibodies in Sera
3.3. OVA-Specific sIgA in Intestinal Fluid
3.4. OVA-Specific Antibodies in Sera
3.5. Differential Immune Responses to STCL and OVA
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pasetti, M.F.; Simon, J.K.; Sztein, M.B.; Levine, M.M. Immunology of gut mucosal vaccines. Immunol. Rev. 2011, 239, 125–148. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.F.; Mathias-Santos, C.; Sbrogio-Almeida, M.E.; Amorim, J.H.; Cabrera-Crespo, J.; Balan, A.; Ferreira, L.C. Functional diversity of heat-labile toxins (lt) produced by enterotoxigenic escherichia coli: Differential enzymatic and immunological activities of lt1 (hlt) and lt4 (plt). J. Biol. Chem. 2011, 286, 5222–5233. [Google Scholar] [CrossRef] [PubMed]
- Marinaro, M.; Staats, H.F.; Hiroi, T.; Jackson, R.J.; Coste, M.; Boyaka, P.N.; Okahashi, N.; Yamamoto, M.; Kiyono, H.; Bluethmann, H.; et al. Mucosal adjuvant effect of cholera toxin in mice results from induction of t helper 2 (Th2) cells and il-4. J. Immunol. 1995, 155, 4621–4629. [Google Scholar] [PubMed]
- Savelkoul, H.F.; Ferro, V.A.; Strioga, M.M.; Schijns, V.E. Choice and design of adjuvants for parenteral and mucosal vaccines. Vaccines 2015, 3, 148–171. [Google Scholar] [CrossRef] [PubMed]
- Seabrook, T.J.; Iglesias, M.; Bloom, J.K.; Spooner, E.T.; Lemere, C.A. Differences in the immune response to long term abeta vaccination in c57bl/6 and b6d2f1 mice. Vaccine 2004, 22, 4075–4083. [Google Scholar] [CrossRef] [PubMed]
- Boyaka, P.N.; Tafaro, A.; Fischer, R.; Leppla, S.H.; Fujihashi, K.; McGhee, J.R. Effective mucosal immunity to anthrax: Neutralizing antibodies and th cell responses following nasal immunization with protective antigen. J. Immunol. 2003, 170, 5636–5643. [Google Scholar] [CrossRef] [PubMed]
- Power, C.A.; Wei, G.; Bretscher, P.A. Mycobacterial dose defines the Th1/Th2 nature of the immune response independently of whether immunization is administered by the intravenous, subcutaneous, or intradermal route. Infect. Immun. 1998, 66, 5743–5750. [Google Scholar] [PubMed]
- Marinaro, M.; Boyaka, P.N.; Jackson, R.J.; Finkelman, F.D.; Kiyono, H.; McGhee, J.R. Interleukin-12 alters helper t-cell subsets and antibody profiles induced by the mucosal adjuvant cholera toxin. Ann. N. Y. Acad. Sci. 1996, 795, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Link-Amster, H.; Rochat, F.; Saudan, K.Y.; Mignot, O.; Aeschlimann, J.M. Modulation of a specific humoral immune response and changes in intestinal flora mediated through fermented milk intake. FEMS Immunol. Med. Microbiol. 1994, 10, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Donnet-Hughes, A.; Rochat, F.; Serrant, P.; Aeschlimann, J.M.; Schiffrin, E.J. Modulation of nonspecific mechanisms of defense by lactic acid bacteria: Effective dose. J. Dairy Sci. 1999, 82, 863–869. [Google Scholar] [CrossRef]
- Plant, L.J.; Conway, P.L. Adjuvant properties and colonization potential of adhering and non-adhering lactobacillus spp following oral administration to mice. FEMS Immunol. Med. Microbiol. 2002, 34, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Ivory, K.; Chambers, S.J.; Pin, C.; Prieto, E.; Arques, J.L.; Nicoletti, C. Oral delivery of lactobacillus casei shirota modifies allergen-induced immune responses in allergic rhinitis. Clin. Exp. Allergy 2008, 38, 1282–1289. [Google Scholar] [CrossRef] [PubMed]
- Segawa, S.; Nakakita, Y.; Takata, Y.; Wakita, Y.; Kaneko, T.; Kaneda, H.; Watari, J.; Yasui, H. Effect of oral administration of heat-killed lactobacillus brevis sbc8803 on total and ovalbumin-specific immunoglobulin e production through the improvement of Th1/Th2 balance. Int. J. Food Microbiol. 2008, 121, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Maassen, C.B.; van Holten-Neelen, C.; Balk, F.; den Bak-Glashouwer, M.J.; Leer, R.J.; Laman, J.D.; Boersma, W.J.; Claassen, E. Strain-dependent induction of cytokine profiles in the gut by orally administered lactobacillus strains. Vaccine 2000, 18, 2613–2623. [Google Scholar] [CrossRef]
- Cross, M.L.; Mortensen, R.R.; Kudsk, J.; Gill, H.S. Dietary intake of lactobacillus rhamnosus hnoo1 enhances production of both Th1 and Th2 cytokines in antigen-primed mice. Med. Microbiol. Immunol. 2002, 191, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Esvaran, M.; Conway, P.L. Strain dependent protection conferred by lactobacillus spp. Administered orally with a Salmonella Typhimurium vaccine in a murine challenge model. Vaccine 2012, 30, 2654–2661. [Google Scholar] [CrossRef] [PubMed]
- Mols-Vorstermans, T.; Hemphill, A.; Monney, T.; Schaap, D.; Boerhout, E. Differential effects on survival, humoral immune responses and brain lesions in inbred BALB/c, CBA/ca, and C57bl/6 mice experimentally infected with neospora caninum tachyzoites. ISRN Parasitol. 2013. [Google Scholar] [CrossRef] [PubMed]
- Wilson-Welder, J.H.; Torres, M.P.; Kipper, M.J.; Mallapragada, S.K.; Wannemuehler, M.J.; Narasimhan, B. Vaccine adjuvants: Current challenges and future approaches. J. Pharm. Sci. 2009, 98, 1278–1316. [Google Scholar] [CrossRef] [PubMed]
- Alignani, D.; Maletto, B.; Liscovsky, M.; Ropolo, A.; Moron, G.; Pistoresi-Palencia, M.C. Orally administered OVA/CpG-ODN induces specific mucosal and systemic immune response in young and aged mice. J. Leukoc. Biol. 2005, 77, 898–905. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Fujihashi, K.; Kato, R.; Yuki, Y.; McGhee, J.R. Oral tolerance revisited: Prior oral tolerization abrogates cholera toxin-induced mucosal iga responses. J. Immunol. 2001, 166, 3114–3121. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, I.; Matsushita, K.; Nisizawa, T.; Okahashi, N.; Russell, M.W.; Suzuki, Y.; Munekata, E.; Koga, T. Genetic control of immune responses in mice to synthetic peptides of a streptococcus mutans surface protein antigen. Infect. Immun. 1992, 60, 623–629. [Google Scholar] [PubMed]
- Scott, D.E.; Agranovich, I.; Inman, J.; Gober, M.; Golding, B. Inhibition of primary and recall allergen-specific t helper cell type 2-mediated responses by a t helper cell type 1 stimulus. J. Immunol. 1997, 159, 107–116. [Google Scholar] [PubMed]
- Sbrogio-Almeida, M.E.; Mosca, T.; Massis, L.M.; Abrahamsohn, I.A.; Ferreira, L.C. Host and bacterial factors affecting induction of immune responses to flagellin expressed by attenuated salmonella vaccine strains. Infect. Immun. 2004, 72, 2546–2555. [Google Scholar] [CrossRef] [PubMed]
- Fransen, F.; Zagato, E.; Mazzini, E.; Fosso, B.; Manzari, C.; El Aidy, S.; Chiavelli, A.; D’Erchia, A.M.; Sethi, M.K.; Pabst, O.; et al. Balb/c and c57bl/6 mice differ in polyreactive iga abundance, which impacts the generation of antigen-specific iga and microbiota diversity. Immunity 2015, 43, 527–540. [Google Scholar] [CrossRef] [PubMed]
- Wen, K.; Tin, C.; Wang, H.; Yang, X.; Li, G.; Giri-Rachman, E.; Kocher, J.; Bui, T.; Clark-Deener, S.; Yuan, L. Probiotic lactobacillus rhamnosus gg enhanced Th1 cellular immunity but did not affect antibody responses in a human gut microbiota transplanted neonatal gnotobiotic pig model. PLoS ONE 2014, 9, e94504. [Google Scholar] [CrossRef] [PubMed]
- Wen, K.; Li, G.; Bui, T.; Liu, F.; Li, Y.; Kocher, J.; Lin, L.; Yang, X.; Yuan, L. High dose and low dose lactobacillus acidophilus exerted differential immune modulating effects on t cell immune responses induced by an oral human rotavirus vaccine in gnotobiotic pigs. Vaccine 2012, 30, 1198–1207. [Google Scholar] [CrossRef] [PubMed]
- Ibnou-Zekri, N.; Blum, S.; Schiffrin, E.J.; von der Weid, T. Divergent patterns of colonization and immune response elicited from two intestinal lactobacillus strains that display similar properties in vitro. Infect. Immun. 2003, 71, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Boge, T.; Remigy, M.; Vaudaine, S.; Tanguy, J.; Bourdet-Sicard, R.; van der Werf, S. A probiotic fermented dairy drink improves antibody response to influenza vaccination in the elderly in two randomised controlled trials. Vaccine 2009, 27, 5677–5684. [Google Scholar] [CrossRef] [PubMed]
- Rizzardini, G.; Eskesen, D.; Calder, P.C.; Capetti, A.; Jespersen, L.; Clerici, M. Evaluation of the immune benefits of two probiotic strains bifidobacterium animalis ssp. Lactis, BB-12® and lactobacillus paracasei ssp. Paracasei, l. Casei 431® in an influenza vaccination model: A randomised, double-blind, placebo-controlled study. Br. J. Nutr. 2012, 107, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Paineau, D.; Carcano, D.; Leyer, G.; Darquy, S.; Alyanakian, M.A.; Simoneau, G.; Bergmann, J.F.; Brassart, D.; Bornet, F.; Ouwehand, A.C. Effects of seven potential probiotic strains on specific immune responses in healthy adults: A double-blind, randomized, controlled trial. FEMS Immunol. Med. Microbiol. 2008, 53, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Taverniti, V.; Guglielmetti, S. The immunomodulatory properties of probiotic microorganisms beyond their viability (ghost probiotics: Proposal of paraprobiotic concept). Genes Nutr. 2011, 6, 261–274. [Google Scholar] [CrossRef] [PubMed]
- Arulanandam, B.P.; Mittler, J.N.; Lee, W.T.; O’Toole, M.; Metzger, D.W. Neonatal administration of il-12 enhances the protective efficacy of antiviral vaccines. J. Immunol. 2000, 164, 3698–3704. [Google Scholar] [CrossRef] [PubMed]
- Barrios, C.; Brawand, P.; Berney, M.; Brandt, C.; Lambert, P.H.; Siegrist, C.A. Neonatal and early life immune responses to various forms of vaccine antigens qualitatively differ from adult responses: Predominance of a Th2-biased pattern which persists after adult boosting. Eur. J. Immunol. 1996, 26, 1489–1496. [Google Scholar] [CrossRef] [PubMed]

| GROUPS | BALB/c | DBA/1 |
|---|---|---|
| L. fermentum PC1—Low dose | 3320.26 ± 458.48 *,† | 2563.58 ± 563.58 *,† |
| L. fermentum PC1—High dose | 3558.48 ± 598.49 *,† | 2687.71 ± 472.94 *,† |
| Vaccine alone | 847.15 ± 158.97 † | 1245.84 ± 256.59 † |
| PBS control | 156.35 ± 35.56 | 163.95 ± 49.47 |
| GROUPS | BALB/c | DBA/1 |
|---|---|---|
| L. fermentum PC1—Low dose | 2587.48 ± 742.63 *,† | 1985.47 ± 596.81 *,† |
| L. fermentum PC1—High dose | 2758.41 ± 563.49 *,† | 1987.56 ± 695.32 *,† |
| Vaccine alone | 685.71 ± 217.32 † | 658.14 ± 147.29 † |
| PBS control | 98.47 ± 45.69 | 49.36 ± 34.83 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esvaran, M.; Conway, P.L. Factors that Influence the Immunological Adjuvant Effect of Lactobacillus fermentum PC1 on Specific Immune Responses in Mice to Orally Administered Antigens. Vaccines 2016, 4, 24. https://doi.org/10.3390/vaccines4030024
Esvaran M, Conway PL. Factors that Influence the Immunological Adjuvant Effect of Lactobacillus fermentum PC1 on Specific Immune Responses in Mice to Orally Administered Antigens. Vaccines. 2016; 4(3):24. https://doi.org/10.3390/vaccines4030024
Chicago/Turabian StyleEsvaran, Meera, and Patricia L. Conway. 2016. "Factors that Influence the Immunological Adjuvant Effect of Lactobacillus fermentum PC1 on Specific Immune Responses in Mice to Orally Administered Antigens" Vaccines 4, no. 3: 24. https://doi.org/10.3390/vaccines4030024
APA StyleEsvaran, M., & Conway, P. L. (2016). Factors that Influence the Immunological Adjuvant Effect of Lactobacillus fermentum PC1 on Specific Immune Responses in Mice to Orally Administered Antigens. Vaccines, 4(3), 24. https://doi.org/10.3390/vaccines4030024






