Multilevel Analysis of Zero-Dose Children in Sub-Saharan Africa: A Three Delays Model Study
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Setting
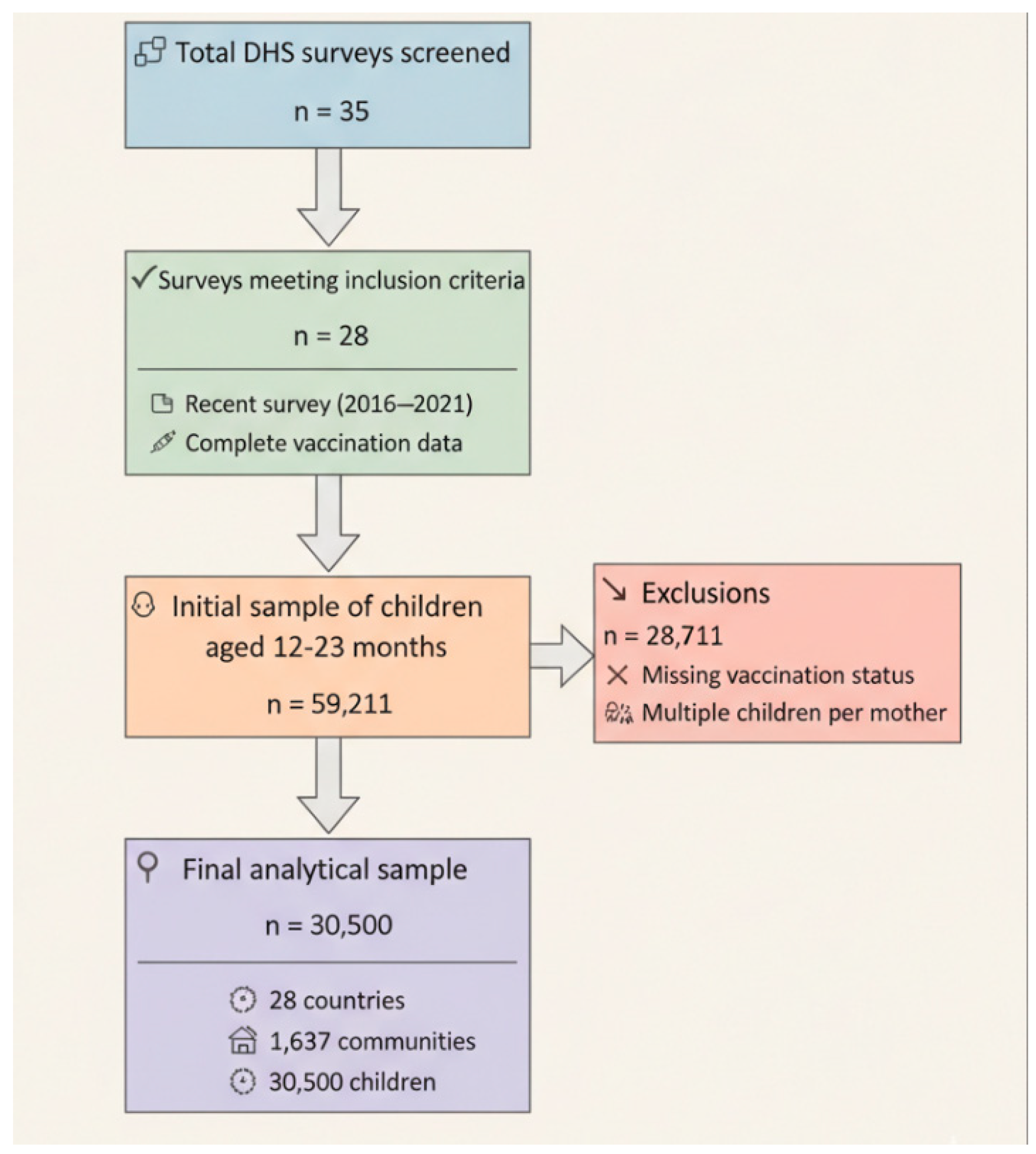
2.3. Participants
2.4. Data Sources and Measurement
2.5. Sampling Technique
2.6. Community and Neighborhood Definitions
2.7. Outcome Variable
2.8. Explanatory Variables
2.9. Individual-Level Variables
2.10. Community-Level Variables
2.11. Country-Level Variables
2.12. Bias
2.13. Study Size
2.14. Quantitative Variables
2.15. Statistical Methods
Descriptive Statistics
2.16. Modeling Approaches
2.17. Fixed Effects (Measures of Association)
2.18. Random Effects (Measures of Variation)
2.19. Model Fit and Specifications
2.20. Ethical Considerations
3. Results
3.1. Sample Characteristics
3.2. Variation in Zero-Dose Prevalence Across Sub-Saharan African Countries
3.3. Measures of Association (Fixed Effects Model)
3.4. Measures of Variations (Random Effects)
4. Discussion
4.1. Main Findings
4.2. Comparison with Previous Studies
4.3. Implications for Policy and Future Research
4.4. Study Strengths and Limitations
4.5. Generalisability of Findings
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Variable | Definition | Type of Delay | Hypothesis/Justification |
|---|---|---|---|
| Individual-Level Variables | |||
| Age | Maternal age in years | Delay 1 | Younger women may lack experience and knowledge about postnatal care importance |
| Education level | No education, primary, secondary, higher | Delay 1 | Education enhances health literacy and awareness of postnatal care benefits |
| Wealth index | Poorest to richest quintiles | Delays 1 & 2 | Wealth affects both decision-making capacity and ability to afford/access care |
| Partner’s education | No education, primary, secondary, higher | Delay 1 | Partner’s education influences household health decisions and support for care-seeking |
| Parity | Total children ever born (0–10+) | Delay 1 | Higher parity may reduce perceived need for care due to experience or resource constraints |
| Employment status | Working vs. not working | Delays 1 & 2 | Employment provides economic resources and may increase autonomy in health decisions |
| Decision-making power | Participates in household decisions vs. not | Delay 1 | Autonomy in decision-making directly affects ability to decide to seek care |
| Media exposure | Access to newspapers/radio/TV vs. not | Delay 1 | Media exposure increases awareness of health services and danger signs |
| Maternal health-seeking behavior index | Composite index (0–4) based on: (1) possession of health card/vaccination record, (2) receipt of antenatal care, (3) facility delivery, and (4) tetanus vaccination during pregnancy. Categorized as poor/no health-seeking (0–2 behaviors) vs. adequate (3–4 behaviors) | Delay 1 | Maternal engagement with health services during pregnancy and delivery reflects health knowledge, attitudes toward healthcare, and established patterns of service utilization. Women who demonstrate consistent health-seeking behaviors are more likely to value and pursue preventive services for their children, including vaccination. This composite measure captures the “pathway to care” effect, where previous positive healthcare experiences facilitate future service utilization. |
| Money problems accessing care | Problem vs. no problem | Delays 1 & 2 | Financial barriers affect both decision to seek care and ability to reach facilities |
| Distance problems accessing care | Problem vs. no problem | Delay 2 | Geographic barriers directly impact ability to reach health facilities |
| Place of delivery | Hospital vs. home | Delays 2 & 3 | Facility delivery indicates successful navigation of access barriers and system engagement |
| Health insurance | Covered vs. not covered | Delays 1 & 2 | Insurance coverage reduces financial barriers and facilitates access |
| Household size | Number of household members (1–10+) | Delay 1 | Larger households may have competing resource demands affecting care prioritization |
| Community-Level Variables | |||
| Place of residence | Urban vs. rural | Delay 2 | Rural areas typically have greater distance to health facilities and transport challenges |
| Community poverty rate | Proportion of poor households in community | Delays 1 & 2 | Community poverty affects collective resources and health infrastructure availability |
| Community illiteracy rate | Proportion of illiterate individuals in community | Delay 1 | Community education levels influence social norms and health-seeking behaviors |
| Community unemployment rate | Proportion of unemployed individuals in community | Delays 1 & 2 | Community economic status affects local resources and transport infrastructure |
| Country-Level Variables | |||
| Region | Geographic regions (5 categories) | All delays | Regional variations capture cultural, economic, and health system differences |
| Health expenditure (% GDP) | Government health spending as percentage of GDP | Delay 3 | Higher health spending improves health system capacity and service quality |
| Urbanization rate | Percentage of population in urban areas | Delay 2 | Urbanization affects distribution of health facilities and accessibility |
| Physician density | Number of physicians per 10,000 population | Delay 3 | Provider availability directly affects ability to receive adequate care |
| Human Development Index | Composite measure of development | All delays | Overall development affects health system strength and population health literacy |
| Gender Development Index | Gender-specific human development measure | Delay 1 | Gender equality affects women’s autonomy and health decision-making power |
| Gender Inequality Index | Measure of gender-based disadvantage | Delay 1 | Gender inequality constrains women’s ability to make independent health decisions |
| Survey year | Year of data collection | Control | Controls for temporal trends in health service utilization |
| Birth year | Year of child’s birth | Control | Controls for cohort effects and changing health service availability |
| Item No. | Recommendation | Page | |
|---|---|---|---|
| Title and abstract | 1 | (a) Indicate the study’s design with a commonly used term in the title or the abstract | 1 |
| (b) Provide in the abstract an informative and balanced summary of what was done and what was found | 1 | ||
| Introduction | |||
| Background/rationale | 2 | Explain the scientific background and rationale for the investigation being reported | 2–3 |
| Objectives | 3 | State specific objectives, including any prespecified hypotheses | 3 |
| Methods | |||
| Study design | 4 | Present key elements of study design early in the paper | 3 |
| Setting | 5 | Describe the setting, locations, and relevant dates, including periods of recruitment, exposure, follow-up, and data collection | 3 |
| Participants | 6 | (a) Give the eligibility criteria, and the sources and methods of selection of participants | 3–4 |
| Variables | 7 | Clearly define all outcomes, exposures, predictors, potential confounders, and effect modifiers. Give diagnostic criteria, if applicable | 4–6 |
| Data sources/measurement | 8 | For each variable of interest, give sources of data and details of methods of assessment (measurement). Describe comparability of assessment methods if there is more than one group | 4 |
| Bias | 9 | Describe any efforts to address potential sources of bias | 6 |
| Study size | 10 | Explain how the study size was arrived at | 6–7 |
| Quantitative variables | 11 | Explain how quantitative variables were handled in the analyses. If applicable, describe which groupings were chosen and why | 7 |
| Statistical methods | 12 | (a) Describe all statistical methods, including those used to control for confounding | 7–9 |
| (b) Describe any methods used to examine subgroups and interactions | 7–9 | ||
| (c) Explain how missing data were addressed | 7–9 | ||
| (d) If applicable, describe analytical methods taking account of sampling strategy | 7–9 | ||
| (e) Describe any sensitivity analyses | 7–9 | ||
| Results | |||
| Participants | 13 | (a) Report numbers of individuals at each stage of study—e.g., numbers potentially eligible, examined for eligibility, confirmed eligible, included in the study, completing follow-up, and analysed | 9–12 |
| (b) Give reasons for non-participation at each stage | 9–12 | ||
| (c) Consider use of a flow diagram | 9–12 | ||
| Descriptive data | 14 | (a) Give characteristics of study participants (e.g., demographic, clinical, social) and information on exposures and potential confounders | 12 |
| (b) Indicate number of participants with missing data for each variable of interest | 12 | ||
| Outcome data | 15 | Report numbers of outcome events or summary measures | 12–14 |
| Main results | 16 | (a) Give unadjusted estimates and, if applicable, confounder-adjusted estimates and their precision (e.g., 95% confidence interval). Make clear which confounders were adjusted for and why they were included | 14–16 |
| (b) Report category boundaries when continuous variables were categorized | 14–16 | ||
| (c) If relevant, consider translating estimates of relative risk into absolute risk for a meaningful time period | 14–16 | ||
| Other analyses | 17 | Report other analyses done—e.g., analyses of subgroups and interactions, and sensitivity analyses | 14–16 |
| Discussion | |||
| Key results | 18 | Summarise key results with reference to study objectives | 16 |
| Limitations | 19 | Discuss limitations of the study, taking into account sources of potential bias or imprecision. Discuss both direction and magnitude of any potential bias | 18–19 |
| Interpretation | 20 | Give a cautious overall interpretation of results considering objectives, limitations, multiplicity of analyses, results from similar studies, and other relevant evidence | 16–18 |
| Generalisability | 21 | Discuss the generalisability (external validity) of the study results | 19 |
| Other information | |||
| Funding | 22 | Give the source of funding and the role of the funders for the present study and, if applicable, for the original study on which the present article is based | 23 |
References
- Shattock, A.J.; Johnson, H.C.; Sim, S.Y.; Carter, A.; Lambach, P.; Hutubessy, R.C.W.; Thompson, K.M.; Badizadegan, K.; Lambert, B.; Ferrari, M.J.; et al. Contribution of vaccination to improved survival and health: Modelling 50 years of the Expanded Programme on Immunization. Lancet 2024, 403, 2307–2316. [Google Scholar] [CrossRef]
- Gavi Alliance. Progress and Challenges with Achieving Universal Immunization Coverage: 2019 Estimates of Immunization Coverage; Gavi: Geneva, Switzerland, 2020. [Google Scholar]
- World Health Organization. Immunization Agenda 2030: A Global Strategy to Leave no One Behind; WHO Press: Geneva, Switzerland, 2020. [Google Scholar]
- UNICEF. The State of the World’s Children 2023: For Every Child, Vaccination; UNICEF: New York, NY, USA, 2023. [Google Scholar]
- Causey, K.; Fullman, N.; Sorensen, R.J.D.; Galles, N.C.; Zheng, P.; Aravkin, A.; Danovaro-Holliday, M.C.; Martinez-Piedra, R.; Sodha, S.V.; Velandia-Gonzalez, M.P.; et al. Estimating global and regional disruptions to routine childhood vaccine coverage during the COVID-19 pandemic in 2020: A modelling study. Lancet 2021, 398, 522–534. [Google Scholar] [CrossRef] [PubMed]
- Antai, D. Faith and child survival: The role of religion in childhood immunization in Nigeria. J. Biosoc. Sci. 2009, 41, 57–76. [Google Scholar] [CrossRef] [PubMed]
- Closser, S.; Cox, K.; Parris, T.M.; Landis, R.M.; Justice, J.; Gopinath, R.; Maes, K.; Banteyerga Amaha, H.; Mohammed, I.Z.; Dukku, A.M.; et al. The impact of polio eradication on routine immunization and primary health care: A mixed-methods study. J. Infect. Dis. 2014, 210 (Suppl. 1), S504–S513. [Google Scholar] [CrossRef]
- Patel, M.K.; Goodson, J.L.; Alexander, J.P.; Kretsinger, K., Jr.; Sodha, S.V.; Steulet, C.; Gacic-Dobo, M.; Rota, P.A.; McFarland, J.; Menning, L.; et al. Progress Toward Regional Measles Elimination—Worldwide, 2000–2019. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1700–1705. [Google Scholar] [CrossRef]
- Thaddeus, S.; Maine, D. Too far to walk: Maternal mortality in context. Soc. Sci. Med. 1994, 38, 1091–1110. [Google Scholar] [CrossRef] [PubMed]
- Masters, S.H.; Burstein, R.; Amofah, G.; Abaogye, P.; Kumar, S.; Hanlon, M. Travel time to maternity care and its effect on utilization in rural Ghana: A multilevel analysis. Soc. Sci. Med. 2013, 93, 147–154. [Google Scholar] [CrossRef]
- Gabrysch, S.; Campbell, O.M. Still too far to walk: Literature review of the determinants of delivery service use. BMC Pregnancy Childbirth 2009, 9, 34. [Google Scholar] [CrossRef]
- Diez-Roux, A.V. Multilevel analysis in public health research. Annu. Rev. Public Health 2000, 21, 171–192. [Google Scholar] [CrossRef]
- Subramanian, S.V.; Jones, K.; Kaddour, A.; Krieger, N. Revisiting Robinson: The perils of individualistic and ecologic fallacy. Int. J. Epidemiol. 2009, 38, 342–360. [Google Scholar] [CrossRef]
- Restrepo-Mendez, M.C.; Barros, A.J.; Wong, K.L.; Johnson, H.L.; Pariyo, G.; Franca, G.V.; Wehrmeister, F.C.; Victora, C.G. Inequalities in full immunization coverage: Trends in low- and middle-income countries. Bull. World Health Organ. 2016, 94, 794–805B. [Google Scholar] [CrossRef]
- Phillips, D.E.; Dieleman, J.L.; Lim, S.S.; Shearer, J. Determinants of effective vaccine coverage in low and middle-income countries: A systematic review and interpretive synthesis. BMC Health Serv. Res. 2017, 17, 681. [Google Scholar] [CrossRef]
- Corsi, D.J.; Neuman, M.; Finlay, J.E.; Subramanian, S.V. Demographic and health surveys: A profile. Int. J. Epidemiol. 2012, 41, 1602–1613. [Google Scholar] [CrossRef]
- Aliaga, A.; Ren, R. Optimal Sample Sizes for Two-Stage Cluster Sampling in Demographic and Health Surveys; ORC Macro: Calverton, MD, USA, 2006. [Google Scholar]
- Kravdal, Ø. A simulation-based assessment of the bias produced when using averages from small DHS clusters as contextual variables in multilevel models. Demogr. Res. 2006, 15, 1–20. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for reporting observational studies. Int. J. Surg. 2014, 12, 1495–1499. [Google Scholar] [CrossRef]
- Agimas, M.C.; Alemayehu, M.A.; Tesfie, T.K.; Tilahun, W.M.; Asferie, W.N.; Aweke, M.N.; Abebe, M.T.; Yalew, A.K. Individual and community level maternal factors for zero-dose children in Ethiopia using mini-EDHS 2019: A mixed effects model. BMJ Open 2025, 15, e085235. [Google Scholar] [CrossRef] [PubMed]
- Muchie, K.F.; Azene, A.G.; Bogale, K.A.; Asmamaw, D.B.; Negash, W.D.; Belachew, T.B.; Tarekegn, B.T.; Terefe, B.; Bantie, G.M.; Eshetu, H.B.; et al. Spatial distribution and associated factors of zero-dose immunization among 12–23 month-old children in Ethiopia: Spatial and survey regression analysis. BMC Pediatr. 2025, 25, 552. [Google Scholar] [CrossRef]
- Asnake, A.A.; Seifu, B.L.; Gebrehana, A.K. Prediction of zero-dose children using supervised machine learning algorithm in Tanzania: Evidence from the recent 2022 Tanzania Demographic and Health Survey. BMJ Open 2025, 15, e097395. [Google Scholar] [CrossRef] [PubMed]
- Cata-Preta, B.O.; Santos, T.M.; Mengistu, T.; Hogan, D.R.; Barros, A.J.D.; Victora, C.G. Zero-dose children and the immunisation cascade: Understanding immunisation pathways in low and middle-income countries. Vaccine 2021, 39, 4564–4570. [Google Scholar] [CrossRef] [PubMed]
- Johri, M.; Rajpal, S.; Subramanian, S.V. Progress in reaching unvaccinated (zero-dose) children in India, 1992–2016: A multilevel, geospatial analysis of repeated cross-sectional surveys. Lancet Glob. Health 2021, 9, e1697–e1706. [Google Scholar] [CrossRef]
- Avila-Aguero, M.L.; Brenes-Chacon, H.; Melgar, M.; Becerra-Posada, F.; Chacon-Cruz, E.; Gentile, A.; Ospina, M.; Sandoval, N.; Sanwogou, J.; Urena, A.; et al. Zero-dose children in Latin America: Analysis of the problem and possible solutions. F1000Res 2024, 13, 1060. [Google Scholar] [CrossRef]
- Gichuki, J.; Ngoye, B.; Mategula, D. Mapping zero-dose children in Kenya—A spatial analysis and examination of the socio-demographic and media exposure determinants. PLoS ONE 2025, 20, e0321652. [Google Scholar] [CrossRef] [PubMed]
- Bogale, B.; Tiruneh, G.T.; Belete, N.; Hunegnaw, B.M.; Fesseha, N.; Zergaw, T.S.; Tadesse, H.; Yeshiwas, T.; Meseret, H.; Emaway, D. Risk factors associated with zero-dose and under-immunized children, and the number of vaccination doses received by children in Ethiopia: A negative binomial regression analysis. BMC Public Health 2025, 25, 1693. [Google Scholar] [CrossRef]
- Mohamoud, S.A.; Ali-Salad, M.A.; Bile, A.S.; Singh, N.S.; Mahmud, A.J.; Nor, B. Determinants and prevalence of zero-dose children in Somalia: Analysis of the 2020 Health Demographic Survey data. PLoS Glob. Public Health 2024, 4, e0002612. [Google Scholar] [CrossRef] [PubMed]
- Santos, T.M.; Cata-Preta, B.O.; Wendt, A.; Arroyave, L.; Blumenberg, C.; Mengistu, T.; Hogan, D.R.; Victora, C.G.; Barros, A.J.D. Exploring the “Urban Advantage” in Access to Immunization Services: A Comparison of Zero-Dose Prevalence Between Rural, and Poor and Non-poor Urban Households Across 97 Low- and Middle-Income Countries. J. Urban. Health 2024, 101, 638–647. [Google Scholar] [CrossRef]
- Bangura, J.B.; Xiao, S.; Qiu, D.; Ouyang, F.; Chen, L. Barriers to childhood immunization in sub-Saharan Africa: A systematic review. BMC Public Health 2020, 20, 1108. [Google Scholar] [CrossRef]
- Budu, E.; Ahinkorah, B.O.; Aboagye, R.G.; Armah-Ansah, E.K.; Seidu, A.A.; Adu, C.; Ameyaw, E.K.; Yaya, S. Maternal healthcare utilsation and complete childhood vaccination in sub-Saharan Africa: A cross-sectional study of 29 nationally representative surveys. BMJ Open 2021, 11, e045992. [Google Scholar] [CrossRef]
- Tekelab, T.; Chojenta, C.; Smith, R.; Loxton, D. The impact of antenatal care on neonatal mortality in sub-Saharan Africa: A systematic review and meta-analysis. PLoS ONE 2019, 14, e0222566. [Google Scholar] [CrossRef] [PubMed]
- Ekholuenetale, M.; Ochagu, V.A.; Ilesanmi, O.S.; Badejo, O.; Arora, A. Childhood Vaccinations and Associated Factors in 35 Sub-Saharan African Countries: Secondary Analysis of Demographic and Health Surveys Data from 358 949 Under-5 Children. Glob. Pediatr. Health 2024, 11, 2333794X241310487. [Google Scholar] [CrossRef]
- Engelbrecht, M.C.; Kigozi, N.G.; Heunis, J.C. Factors Associated with Limited Vaccine Literacy: Lessons Learnt from COVID-19. Vaccines 2022, 10, 865. [Google Scholar] [CrossRef]
- Jain, M.; Shisler, S.; Lane, C.; Bagai, A.; Brown, E.; Engelbert, M. Use of community engagement interventions to improve child immunisation in low-income and middle-income countries: A systematic review and meta-analysis. BMJ Open 2022, 12, e061568. [Google Scholar] [CrossRef] [PubMed]
- Ogutu, E.; Ellis, A.S.; Hester, K.A.; Rodriguez, K.; Sakas, Z.; Jaishwal, C.; Yang, C.; Dixit, S.; Bose, A.S.; Sarr, M.; et al. Success in vaccination programming through community health workers: A case study of Nepal, Senegal, and Zambia. medRxiv 2023, medRxiv:2023-05. [Google Scholar] [CrossRef]
- Bendera, A.; Nakamura, K.; Tran, X.M.T.; Kapologwe, N.A.; Bendera, E.; Mahamba, D.; Meshi, E.B. Persistent socioeconomic disparities in childhood vaccination coverage in Tanzania: Insights from multiple rounds of demographic and health surveys. Vaccine 2025, 52, 126904. [Google Scholar] [CrossRef]
- Ekezie, W.; Igein, B.; Varughese, J.; Butt, A.; Ukoha-Kalu, B.O.; Ikhile, I.; Bosah, G. Vaccination Communication Strategies and Uptake in Africa: A Systematic Review. Vaccines 2024, 12, 1333. [Google Scholar] [CrossRef]
- Fousseni, S.; Ngangue, P.; Barro, A.; Ramde, S.W.; Bihina, L.T.; Ngoufack, M.N.; Bayoulou, S.; Kiki, G.M.; Salfo, O. Navigating the Road to Immunization Equity: Systematic Review of Challenges in Introducing New Vaccines in Sub-Saharan Africa’s Routine Programs. Vaccines 2025, 13, 269. [Google Scholar] [CrossRef] [PubMed]
- Nalwanga, R.; Natukunda, A.; Zirimenya, L.; Chi, P.; Luzze, H.; Elliott, A.M.; Kaleebu, P.; Trotter, C.L.; Webb, E.L. Mapping Community Vulnerability to reduced Vaccine Impact in Uganda and Kenya: A spatial Data-driven Approach. NIHR Open Res. 2025, 5, 24. [Google Scholar] [CrossRef]
- Idris, I.O.; Ouma, L.; Tapkigen, J.; Ayomoh, F.I.; Ayeni, G.O. Is health expenditure on immunisation associated with immunisation coverage in sub-Saharan Africa? A multicountry analysis, 2013–2017. BMJ Open 2024, 14, e073789. [Google Scholar] [CrossRef]
- Petu, A.; Masresha, B.; Wiysonge, C.S.; Mwenda, J.; Nyarko, K.; Bwaka, A.; Wanyoike, S.; Mboussou, F.; Impouma, B.; Usman, A.; et al. Reflections on 50 years of immunisation programmes in the WHO African region: An impetus to build on the progress and address the unfinished immunisation business. BMJ Glob. Health 2025, 10, e017982. [Google Scholar] [CrossRef]
- Dimitrova, A.; Carrasco-Escobar, G.; Richardson, R.; Benmarhnia, T. Essential childhood immunization in 43 low- and middle-income countries: Analysis of spatial trends and socioeconomic inequalities in vaccine coverage. PLoS Med. 2023, 20, e1004166. [Google Scholar] [CrossRef]
- Diress, F.; Negesse, Y.; Worede, D.T.; Bekele Ketema, D.; Geitaneh, W.; Temesgen, H. Multilevel and geographically weighted regression analysis of factors associated with full immunization among children aged 12–23 months in Ethiopia. Sci. Rep. 2024, 14, 22743. [Google Scholar] [CrossRef]
- Sarder, M.A.; Lee, K.Y.; Keramat, S.A.; Hashmi, R.; Ahammed, B. A multilevel analysis of individual and community-level factors associated with childhood immunisation in Bangladesh: Evidence from a pooled cross-sectional survey. Vaccine X 2023, 14, 100285. [Google Scholar] [CrossRef]
- Francis, M.R.; Nuorti, J.P.; Lumme-Sandt, K.; Kompithra, R.Z.; Balraj, V.; Kang, G.; Mohan, V.R. Vaccination coverage and the factors influencing routine childhood vaccination uptake among communities experiencing disadvantage in Vellore, southern India: A mixed-methods study. BMC Public Health 2021, 21, 1807. [Google Scholar] [CrossRef]
- Muluneh, M.D.; Abebe, S.; Ayele, M.; Mesfin, N.; Abrar, M.; Stulz, V.; Berhan, M. Vaccination Coverage and Predictors of Vaccination Among Children Aged 12–23 Months in the Pastoralist Communities of Ethiopia: A Mixed Methods Design. Int. J. Environ. Res. Public Health 2024, 21, 1112. [Google Scholar] [CrossRef]
- Lyons, C.; Nambiar, D.; Johns, N.E.; Allorant, A.; Bergen, N.; Hosseinpoor, A.R. Inequality in Childhood Immunization Coverage: A Scoping Review of Data Sources, Analyses, and Reporting Methods. Vaccines 2024, 12, 850. [Google Scholar] [CrossRef] [PubMed]
- Harrison, M.S.; Yarinbab, T.; Liyew, T.; Kirub, E.; Teshome, B.; Muldrow, M. Twenty-five years after its publication, innovative solutions to the three delays model are essential. Int. J. Gynaecol. Obstet. 2020, 148, 123–124. [Google Scholar] [CrossRef]
- Leyland, A.H.; Groenewegen, P.P. What Is Multilevel Modelling? In Multilevel Modelling for Public Health and Health Services Research: Health in Context, 1st ed.; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Savitz, D.A.; Wellenius, G.A. Can Cross-Sectional Studies Contribute to Causal Inference? It Depends. Am. J. Epidemiol. 2023, 192, 514–516. [Google Scholar] [CrossRef]
- Porth, J.M.; Wagner, A.L.; Tefera, Y.A.; Boulton, M.L. Childhood Immunization in Ethiopia: Accuracy of Maternal Recall Compared to Vaccination Cards. Vaccines 2019, 7, 48. [Google Scholar] [CrossRef]
- Gilano, G.; Sako, S.; Molla, B.; Dekker, A.; Fijten, R. The effect of mHealth on childhood vaccination in Africa: A systematic review and meta-analysis. PLoS ONE 2024, 19, e0294442. [Google Scholar] [CrossRef] [PubMed]
- Onigbogi, O.; Ojo, O.Y.; Kinnunen, U.M.; Saranto, K. Mobile health interventions on vaccination coverage among children under 5 years of age in Low and Middle-Income countries; a scoping review. Front. Public Health 2025, 13, 1392709. [Google Scholar] [CrossRef] [PubMed]

| Zero Dose | |||
|---|---|---|---|
| No | Yes | Total | |
| N | 26,834 (88.0%) | 3655 (12.0%) | 30,489 (100.0%) |
| Survey year | 2019 (2015 2024) | 2019 (2015 2024) | 2019 (2015 2024) |
| Birth year | 2017 (2014 2023) | 2017 (2014 2023) | 2017 (2014 2023) |
| Maternal age | |||
| Young Adult | 10,734 (40.0%) | 1634 (44.7%) | 12,369 (40.6%) |
| Adult | 14,297 (53.3%) | 1722 (47.1%) | 16,019 (52.5%) |
| Middle-Aged/Older Adult | 1802 (6.7%) | 299 (8.2%) | 2101 (6.9%) |
| Maternal education | |||
| no education | 6605 (24.6%) | 2054 (56.2%) | 8659 (28.4%) |
| primary | 8951 (33.4%) | 882 (24.1%) | 9833 (32.2%) |
| secondary | 9388 (35.0%) | 630 (17.2%) | 10,018 (32.9%) |
| higher | 1890 (7.0%) | 89 (2.4%) | 1979 (6.5%) |
| Wealth index | |||
| poorest | 4623 (17.2%) | 1132 (31.0%) | 5755 (18.9%) |
| poorer | 5114 (19.1%) | 931 (25.5%) | 6045 (19.8%) |
| middle | 5409 (20.2%) | 707 (19.3%) | 6116 (20.1%) |
| richer | 5746 (21.4%) | 524 (14.3%) | 6271 (20.6%) |
| richest | 5941 (22.1%) | 361 (9.9%) | 6303 (20.7%) |
| husband/partner’s education level | |||
| no education | 5228 (25.3%) | 1616 (55.3%) | 6845 (29.0%) |
| primary | 6063 (29.3%) | 598 (20.4%) | 6661 (28.2%) |
| secondary | 6999 (33.8%) | 588 (20.1%) | 7587 (32.1%) |
| higher | 2390 (11.6%) | 122 (4.2%) | 2512 (10.6%) |
| Antenatal visits | |||
| no visit | 1421 (5.5%) | 1238 (35.7%) | 2659 (9.1%) |
| lessthan4 | 6707 (26.1%) | 996 (28.7%) | 7703 (26.4%) |
| 4 or more | 17,563 (68.4%) | 1235 (35.6%) | 18,798 (64.5%) |
| Parity | |||
| 1 | 11,426 (42.6%) | 1502 (41.1%) | 12,928 (42.4%) |
| 2 | 4448 (16.6%) | 495 (13.5%) | 4943 (16.2%) |
| 3 | 3476 (13.0%) | 417 (11.4%) | 3893 (12.8%) |
| 4 | 2613 (9.7%) | 319 (8.7%) | 2933 (9.6%) |
| 5 | 1865 (7.0%) | 316 (8.6%) | 2181 (7.2%) |
| 6 | 1288 (4.8%) | 195 (5.3%) | 1482 (4.9%) |
| 7 | 806 (3.0%) | 160 (4.4%) | 966 (3.2%) |
| 8 | 467 (1.7%) | 102 (2.8%) | 569 (1.9%) |
| 9 | 258 (1.0%) | 62 (1.7%) | 320 (1.0%) |
| 10 | 186 (0.7%) | 87 (2.4%) | 273 (0.9%) |
| Not working | |||
| 0 | 16,198 (60.4%) | 1936 (53.0%) | 18,134 (59.5%) |
| 1 | 10,636 (39.6%) | 1719 (47.0%) | 12,355 (40.5%) |
| No decision-making power | |||
| 0 | 16,797 (62.6%) | 1881 (51.5%) | 18,677 (61.3%) |
| 1 | 10,037 (37.4%) | 1775 (48.5%) | 11,812 (38.7%) |
| No media access | |||
| 0 | 18,828 (70.2%) | 1679 (45.9%) | 20,506 (67.3%) |
| 1 | 8006 (29.8%) | 1976 (54.1%) | 9982 (32.7%) |
| Household size | |||
| 1 | 69 (0.3%) | 8 (0.2%) | 77 (0.3%) |
| 2 | 723 (2.7%) | 116 (3.2%) | 839 (2.8%) |
| 3 | 5192 (19.3%) | 680 (18.6%) | 5872 (19.3%) |
| 4 | 4167 (15.5%) | 496 (13.6%) | 4663 (15.3%) |
| 5 | 4016 (15.0%) | 541 (14.8%) | 4557 (14.9%) |
| 6 | 3411 (12.7%) | 385 (10.5%) | 3796 (12.5%) |
| 7 | 2578 (9.6%) | 379 (10.4%) | 2957 (9.7%) |
| 8 | 1861 (6.9%) | 271 (7.4%) | 2132 (7.0%) |
| 9 | 1247 (4.6%) | 187 (5.1%) | 1434 (4.7%) |
| 10 | 3570 (13.3%) | 591 (16.2%) | 4161 (13.6%) |
| Money problem accessing care | |||
| No | 14,700 (54.8%) | 1639 (44.8%) | 16,339 (53.6%) |
| Yes | 12,133 (45.2%) | 2017 (55.2%) | 14,150 (46.4%) |
| Distance problem accessing care | |||
| No | 18,321 (68.3%) | 2038 (55.8%) | 20,359 (66.8%) |
| Yes | 8513 (31.7%) | 1617 (44.2%) | 10,130 (33.2%) |
| Health insurance | |||
| No | 6055 (22.6%) | 492 (13.5%) | 6546 (21.5%) |
| Yes | 20,779 (77.4%) | 3163 (86.5%) | 23,942 (78.5%) |
| Place of resident | |||
| Urban | 10,533 (39.3%) | 967 (26.4%) | 11,499 (37.7%) |
| Rural | 16,301 (60.7%) | 2689 (73.6%) | 18,990 (62.3%) |
| Community poverty rate | 18.2 (24.9) | 28.9 (30.8) | 19.5 (25.9) |
| Community illiteracy rate | 27.8 (29.7) | 54.7 (34.1) | 31.0 (31.5) |
| Community unemployment rate | 31.0 (26.1) | 31.3 (30.3) | 31.0 (26.6) |
| Gross domestic product | 4075.6 (3270.2) | 4525.8 (3384.7) | 4129.6 (3287.4) |
| Percentage health expenditure | 5.1 (2.0) | 4.4 (1.4) | 5.1 (1.9) |
| Human development index | 0.5 (0.1) | 0.5 (0.1) | 0.5 (0.1) |
| Gender Development Index | 0.9 (0.0) | 0.9 (0.0) | 0.9 (0.0) |
| Gender Inequality Index | 0.6 (0.1) | 0.6 (0.1) | 0.6 (0.1) |
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | |
|---|---|---|---|---|---|
| OR (95% CrI) | OR (95% CrI) | OR (95% CrI) | OR (95% CrI) | OR (95% CrI) | |
| Measures of associations (Fixed Effects Model) | |||||
| Individual-level factors | |||||
| Survey year | 1.02 (1.01–1.02) | 1.03 (1.03–1.03) | |||
| Birth year | 0.91 (0.91–0.91) | 0.92 (0.91–0.92) | |||
| Maternal age | |||||
| Young adult | 1.40 (1.08–1.82) | 1.35 (1.04–1.69) | |||
| Adult | 1.00 (0.82–1.22) | 0.99 (0.80–1.20) | |||
| Middle-Age/Older Adult | |||||
| Education | |||||
| No education | 1.92 (1.32–2.75) | 1.51 (0.97–2.17) | |||
| Primary | 1.32 (0.90–1.89) | 1.24 (0.81–1.72) | |||
| Secondary | 1.13 (0.79–1.61) | 1.11 (0.73–1.54) | |||
| Tertiary | |||||
| Wealth | |||||
| Poorest | 1.29 (1.01–1.54) | 1.22 (0.94–1.55) | |||
| Poorer | 1.15 (0.92–1.38) | 1.13 (0.90–1.39) | |||
| Middle | 1.11 (0.90–1.33) | 1.09 (0.89–1.33) | |||
| Richer | 0.95 (0.77–1.13) | 0.94 (0.77–1.15) | |||
| Richest | |||||
| Partner Education | |||||
| No education | 1.66 (1.31–2.05) | 1.52 (1.20–1.96) | |||
| Primary | 1.22 (0.96–1.52) | 1.26 (0.98–1.65) | |||
| Secondary | 1.12 (0.90–1.38) | 1.17 (0.92–1.51) | |||
| Tertiary | |||||
| Parity | 1.01 (0.98–1.04) | 1.02 (0.98–1.04) | |||
| Not working | 1.20 (1.06–1.34) | 1.09 (0.97–1.23) | |||
| No decision-making power | 1.27 (1.13–1.41) | 1.23 (1.08–1.39) | |||
| No media access | 1.34 (1.19–1.49) | 1.32 (1.18–1.48) | |||
| Household size | 1.00 (0.98–1.02) | 1.00 (0.98–1.02) | |||
| Money problem accessing care | 0.95 (0.84–1.07) | 0.96 (0.84–1.08) | |||
| Distance problem accessing care | 1.16 (1.02–1.30) | 1.14 (1.00–1.29) | |||
| Money problem accessing care | 2.05 (1.83–2.31) | 1.98 (1.77–2.22) | |||
| No health insurance | 1.10 (0.87–1.36) | 1.13 (0.92–1.41) | |||
| Poor/No maternal health seeking | 12.62 (10.37–15.29) | 12.00 (9.78–14.55) | |||
| Community -level factors | |||||
| Rural resident | 1.27 (1.12–1.46) | 0.93 (0.80–1.07) | |||
| Community poverty rate | 1.07 (1.04–1.09) | 1.00 (0.98–1.03) | |||
| Community illiteracy rate | 1.30 (1.27–1.33) | 1.08 (1.05–1.11) | |||
| Community unemployment rate | 1.10 (1.07–1.12) | 1.05 (1.02–1.08) | |||
| Societal -level factors | |||||
| Gross domestic product | 0.74 (0.22–1.61) | 0.63 (0.26–1.29) | |||
| Percentage health expenditure | 1.12 (0.54–2.17) | 2.29 (1.31–3.96) | |||
| Human development index | 0.70 (0.34–1.66) | 1.51 (0.64–2.57) | |||
| Gender Development Index | 6.83 (3.28–12.75) | 1.52 (0.89–2.38) | |||
| Gender Inequality Index | 0.81 (0.30–1.60) | 1.22 (0.68–2.21) | |||
| Measures of variations (random effects) | |||||
| Country-level | |||||
| Variance (95% CrI) | 2.65 (1.52–4.63) | 1.15 (0.64–2.00) | 1.86 (1.05–3.27) | 1.91 (1.03–3.46) | 0.77 (0.41–1.39) |
| VPC (%) | 31.8 (22.0–43.2) | 25.9 (16.6–37.8) | 28.1 (18.8–39.5) | 25.0–15.9–36.0) | 18.7 (11.0–29.3) |
| MOR (95% CrI) | 4.72 (3.24–7.79) | 2.78 (2.14–3.85) | 3.68 (2.66–5.62) | 3.74 (2.63–5.89) | 2.30 (1.84–3.08) |
| Explained variance (%) | reference | 56.6 (56.8–57.9) | 29.7 (29.3–30.7) | 27.8 (25.3–32.5) | 71.1 (70.0–73.1) |
| Community-level | |||||
| Variance (95% CrI) | 2.40 (2.10–2.79) | 0.00 (0.00–0.00) | 1.47 (1.25–1.72) | 2.45 (2.12–2.85) | 0.03 (0.01–0.06) |
| VPC (%) | 60.5 (52.4–69.3) | 25.9 (16.6–37.8) | 50.3 (−41.2–60.2) | 57.0 (48.9–65.7) | 19.5 (11.4–30.6) |
| MOR (95% CrI) | 4.38 (3.98–4.92) | 1.04 (1.03–1.05) | 3.18 (2.91–3.49) | 4.45 (4.02–5.00) | 1.19 (1.12–1.27) |
| Explained variance (%) | reference | 99.9 (99.9–99.9) | 38.6 (38.5–40.3) | −1.9 (−2.2–−1.1) | 98.7 (97.8–99.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiysonge, C.S.; Uthman, M.M.B.; Ndwandwe, D.; Uthman, O.A. Multilevel Analysis of Zero-Dose Children in Sub-Saharan Africa: A Three Delays Model Study. Vaccines 2025, 13, 987. https://doi.org/10.3390/vaccines13090987
Wiysonge CS, Uthman MMB, Ndwandwe D, Uthman OA. Multilevel Analysis of Zero-Dose Children in Sub-Saharan Africa: A Three Delays Model Study. Vaccines. 2025; 13(9):987. https://doi.org/10.3390/vaccines13090987
Chicago/Turabian StyleWiysonge, Charles S., Muhammed M. B. Uthman, Duduzile Ndwandwe, and Olalekan A. Uthman. 2025. "Multilevel Analysis of Zero-Dose Children in Sub-Saharan Africa: A Three Delays Model Study" Vaccines 13, no. 9: 987. https://doi.org/10.3390/vaccines13090987
APA StyleWiysonge, C. S., Uthman, M. M. B., Ndwandwe, D., & Uthman, O. A. (2025). Multilevel Analysis of Zero-Dose Children in Sub-Saharan Africa: A Three Delays Model Study. Vaccines, 13(9), 987. https://doi.org/10.3390/vaccines13090987







