Cord Blood RSV-Neutralizing Antibodies and Risk of Hospitalization for RSV-Associated Acute Respiratory Infection in Vietnamese Children: A Case–Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Participants
2.3. RSV-ARI Hospitalization Surveillance and Case Definition
2.4. Data Collection
2.5. Virological Testing
2.6. Bacteriological Testing
2.7. Neutralizing Antibody Testing
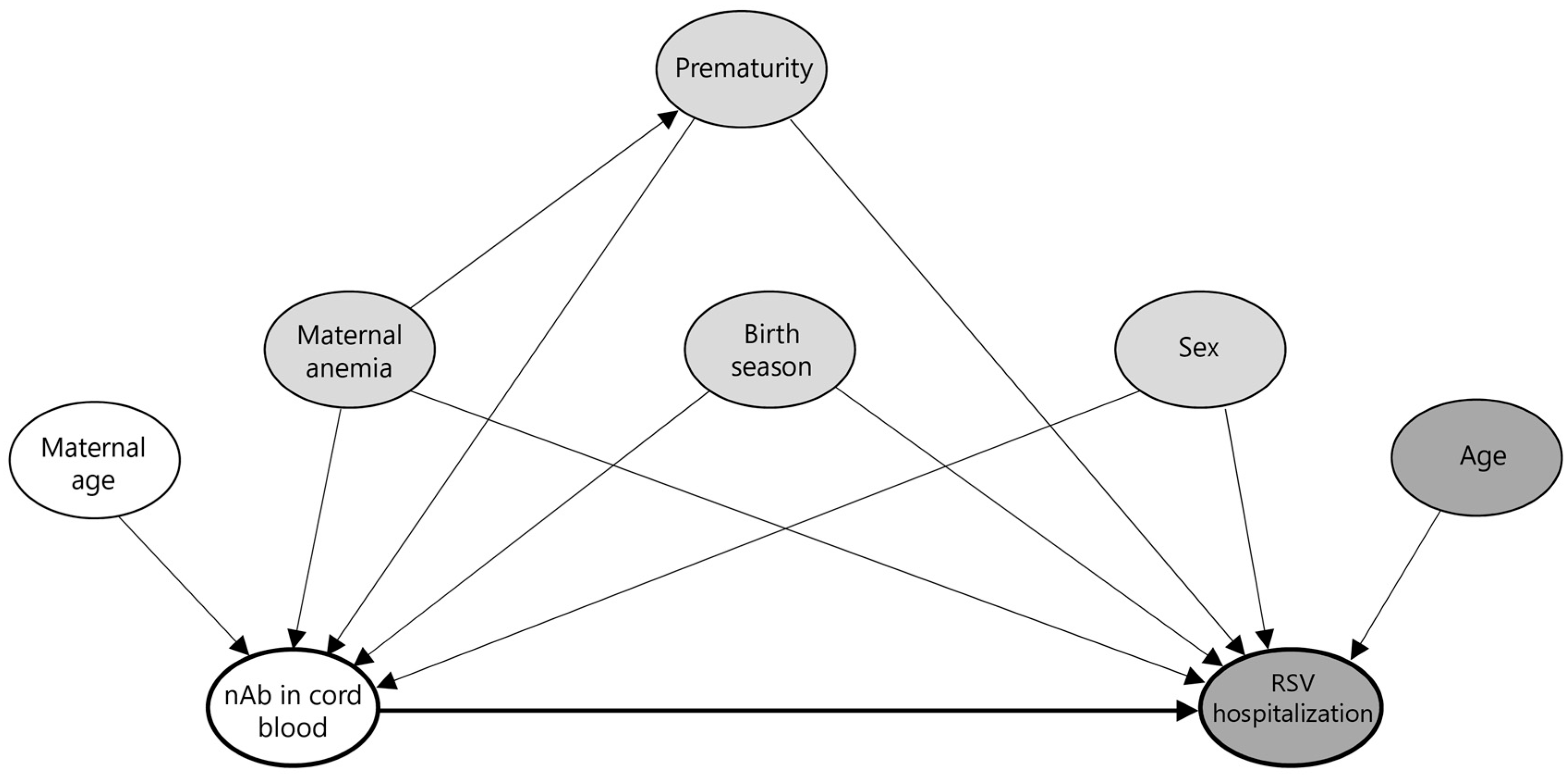
2.8. Statistical Analysis
3. Results
3.1. Study Population
3.2. Incidence of RSV-ARI Hospitalization
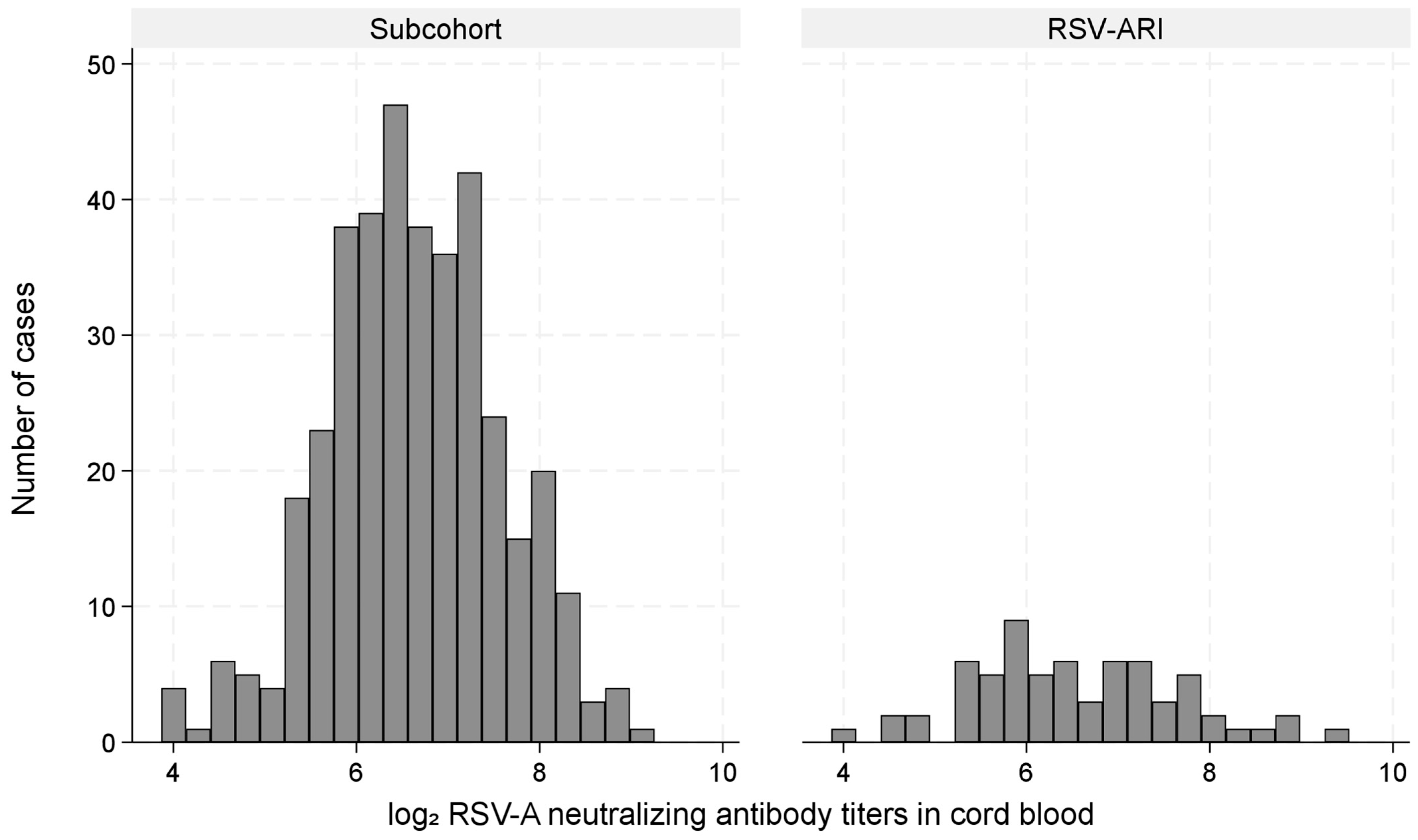
3.3. Cord Blood Antibodies and RSV-ARI Hospitalization Risk (Primary Analysis)
3.4. Additional Analysis: 12-Month Follow-Up
3.5. Subgroup, Interaction, and Sensitivity Analyses
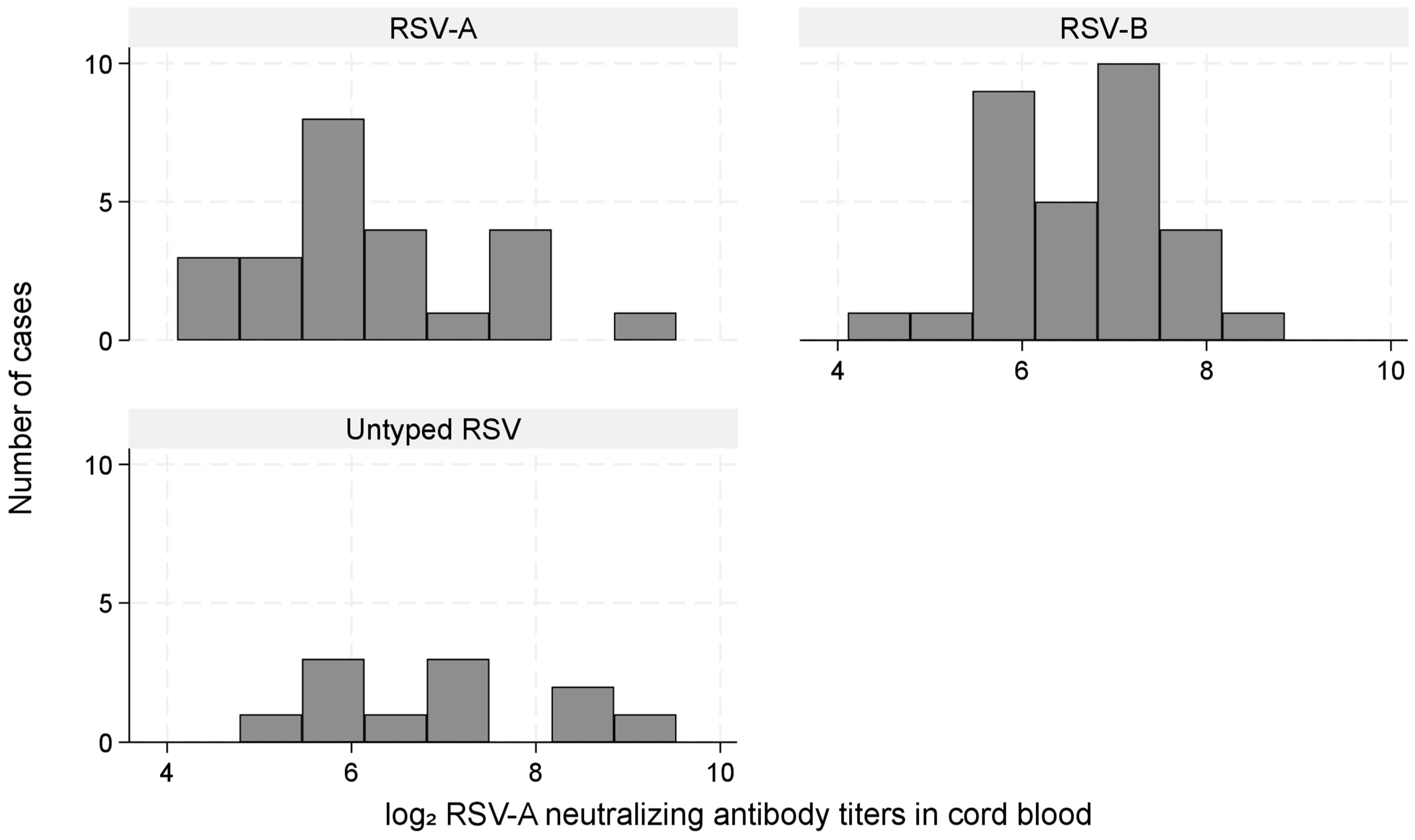
3.6. RSV-Neutralizing Antibody Titer by Infecting RSV Type
3.7. Representativeness and Clinical Features
4. Discussion
4.1. High Incidence of RSV-ARI Hospitalization in Infancy
4.2. Cord Blood RSV-nAb Titers and Risk of RSV-ARI Hospitalization
4.3. Maternal and Perinatal Risk Factors
4.4. Strengths
4.5. Limitations
4.6. Public Health Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| aHR | Adjusted hazard ratio |
| IC50 | 50% inhibitory concentration |
| KHGH | Khanh Hoa General Hospital |
| LRI | Lower respiratory infection |
| nAb | Neutralizing antibody |
| RT-PCR | Reverse transcription polymerase chain reaction |
| RSV | Respiratory syncytial virus |
| RSV-ARI | Respiratory syncytial virus-associated acute respiratory infection |
References
- Shi, T.; McAllister, D.A.; O’Brien, K.L.; Simoes, E.A.F.; Madhi, S.A.; Gessner, B.D.; Polack, F.P.; Balsells, E.; Acacio, S.; Aguayo, C.; et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: A systematic review and modelling study. Lancet 2017, 390, 946–958. [Google Scholar] [CrossRef]
- Toizumi, M.; Suzuki, M.; Nguyen, H.A.T.; Le, M.N.; Ariyoshi, K.; Moriuchi, H.; Hashizume, M.; Dang, D.A.; Yoshida, L.M. Viral Acute Respiratory Illnesses in Young Infants Increase the Risk of Respiratory Readmission. Pediatr. Infect. Dis. J. 2018, 37, 1217–1222. [Google Scholar] [CrossRef]
- Kampmann, B.; Madhi, S.A.; Munjal, I.; Simões, E.A.F.; Pahud, B.A.; Llapur, C.; Baker, J.; Marc, G.P.; Radley, D.; Shittu, E.; et al. Bivalent Prefusion F Vaccine in Pregnancy to Prevent RSV Illness in Infants. N. Engl. J. Med. 2023, 388, 1451–1464. [Google Scholar] [CrossRef]
- Hammitt, L.L.; Dagan, R.; Yuan, Y.; Cots, M.B.; Bosheva, M.; Madhi, S.A.; Muller, W.J.; Zar, H.J.; Brooks, D.; Grenham, A.; et al. Nirsevimab for Prevention of RSV in Healthy Late-Preterm and Term Infants. N. Engl. J. Med. 2022, 386, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Lamprecht, C.L.; Krause, H.E.; Mufson, M.A. Role of Maternal Antibody in Pneumonia and Bronchiolitis Due to Respiratory Syncytial Virus. J. Infect. Dis. 1976, 134, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Ogilvie, M.M.; Santhire Vathenen, A.; Radford, M.; Codd, J.; Key, S. Maternal antibody and respiratory syncytial virus infection in infancy. J. Med. Virol. 1981, 7, 263–271. [Google Scholar] [CrossRef]
- Glezen, W.P.; Paredes, A.; Allison, J.E.; Taber, L.H.; Frank, A.L. Risk of respiratory syncytial virus infection for infants from low-income families in relationship to age, sex, ethnic group, and maternal antibody level. J. Pediatr. 1981, 98, 708–715. [Google Scholar] [CrossRef]
- Roca, A.; Abacassamo, F.; Loscertales, M.P.; Quintó, L.; Gómez-Olivé, X.; Fenwick, F.; Saiz, J.C.; Toms, G.; Alonso, P.L. Prevalence of respiratory syncytial virus IgG antibodies in infants living in a rural area of Mozambique. J. Med. Virol. 2002, 67, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Stensballe, L.G.; Ravn, H.; Kristensen, K.; Meakins, T.; Aaby, P.; Simoes, E.A.F. Seasonal Variation of Maternally Derived Respiratory Syncytial Virus Antibodies and Association with Infant Hospitalizations for Respiratory Syncytial Virus. J. Pediatr. 2009, 154, 296–298.e291. [Google Scholar] [CrossRef]
- Buchwald, A.G.; Graham, B.S.; Traore, A.; Haidara, F.C.; Chen, M.; Morabito, K.; Lin, B.C.; Sow, S.O.; Levine, M.M.; Pasetti, M.F.; et al. Respiratory Syncytial Virus (RSV) Neutralizing Antibodies at Birth Predict Protection from RSV Illness in Infants in the First 3 Months of Life. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2021, 73, e4421–e4427. [Google Scholar] [CrossRef]
- Holberg, C.J.; Wright, A.L.; Martinez, F.D.; Ray, C.G.; Taussig, L.M.; Lebowitz, M.D. Risk factors for respiratory syncytial virus-associated lower respiratory illnesses in the first year of life. Am. J. Epidemiol. 1991, 133, 1135–1151. [Google Scholar] [CrossRef]
- Bulkow, L.R.; Singleton, R.J.; Karron, R.A.; Harrison, L.H. Risk factors for severe respiratory syncytial virus infection among Alaska native children. Pediatrics 2002, 109, 210–216. [Google Scholar] [CrossRef]
- Chu, H.Y.; Tielsch, J.; Katz, J.; Magaret, A.S.; Khatry, S.; LeClerq, S.C.; Shrestha, L.; Kuypers, J.; Steinhoff, M.C.; Englund, J.A. Transplacental transfer of maternal respiratory syncytial virus (RSV) antibody and protection against RSV disease in infants in rural Nepal. J. Clin. Virol. Off. Publ. Pan Am. Soc. Clin. Virol. 2017, 95, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Nyiro, J.U.; Sande, C.J.; Mutunga, M.; Kiyuka, P.K.; Munywoki, P.K.; Scott, J.A.G.; Nokes, D.J. Absence of Association between Cord Specific Antibody Levels and Severe Respiratory Syncytial Virus (RSV) Disease in Early Infants: A Case Control Study from Coastal Kenya. PLoS ONE 2016, 11, e0166706. [Google Scholar] [CrossRef]
- Toizumi, M.; Tanaka, S.; Moriuchi, M.; Nguyen, H.A.T.; Takegata, M.; Iwasaki, C.; Kitamura, N.; Do, H.T.; Dang, D.A.; Yoshida, L.M.; et al. Rubella seroprevalence among mothers and incidence of congenital rubella three years after rubella vaccine introduction in Vietnam. Hum. Vaccines Immunother. 2021, 17, 3156–3161. [Google Scholar] [CrossRef]
- Yoshida, L.M.; Suzuki, M.; Yamamoto, T.; Nguyen, H.A.; Nguyen, C.D.; Nguyen, A.T.; Oishi, K.; Vu, T.D.; Le, T.H.; Le, M.Q.; et al. Viral pathogens associated with acute respiratory infections in central vietnamese children. Pediatr. Infect. Dis. J. 2010, 29, 75–77. [Google Scholar] [CrossRef]
- World Health Organization. Integrated Management of Childhood Illness—Chart booklet; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Sato, M.; Saito, R.; Sakai, T.; Sano, Y.; Nishikawa, M.; Sasaki, A.; Shobugawa, Y.; Gejyo, F.; Suzuki, H. Molecular epidemiology of respiratory syncytial virus infections among children with acute respiratory symptoms in a community over three seasons. J. Clin. Microbiol. 2005, 43, 36–40. [Google Scholar] [CrossRef]
- Nguyen, H.A.T.; Fujii, H.; Vu, H.T.T.; Parry, C.M.; Dang, A.D.; Ariyoshi, K.; Yoshida, L.M. An alarmingly high nasal carriage rate of Streptococcus pneumoniae serotype 19F non-susceptible to multiple beta-lactam antimicrobials among Vietnamese children. BMC Infect. Dis. 2019, 19, 241. [Google Scholar] [CrossRef]
- Yamagata, Y.; Toizumi, M.; Eleouet, J.-F.; Rameix-Welti, M.-A.; Takeda, M.; Yoshida, L.-M. Improved RSV Neutralization Assay Using Recombinant RSV Expressing Reporter Fluorescent Protein. Methods Protoc. 2025, 8, 60. [Google Scholar] [CrossRef] [PubMed]
- Emukule, G.O.; Khagayi, S.; McMorrow, M.L.; Ochola, R.; Otieno, N.; Widdowson, M.-A.; Ochieng, M.; Feikin, D.R.; Katz, M.A.; Mott, J.A. The Burden of Influenza and RSV among Inpatients and Outpatients in Rural Western Kenya, 2009–2012. PLoS ONE 2014, 9, e105543. [Google Scholar] [CrossRef] [PubMed]
- Zar, H.J.; Nduru, P.; Stadler, J.A.M.; Gray, D.; Barnett, W.; Lesosky, M.; Myer, L.; Nicol, M.P. Early-life respiratory syncytial virus lower respiratory tract infection in a South African birth cohort: Epidemiology and effect on lung health. Lancet Glob. Health 2020, 8, e1316–e1325. [Google Scholar] [CrossRef]
- Tam, C.; Yeo, K.T.; Tee, N.; Lin, R.; Mak, T.M.; Thoon, K.C.; Jit, M.; Yung, C.F. Burden and Cost of Hospitalization for Respiratory Syncytial Virus in Young Children, Singapore. Emerg. Infect. Dis. J. 2020, 26, 1489. [Google Scholar] [CrossRef]
- Fischer Langley, G.; McCracken, J.; Arvelo, W.; Estevez, A.; Villarruel, G.; Prill, M.; Iwane, M.; Gray, J.; Moscoso, F.; Reyes, L.; et al. The Epidemiology and Clinical Characteristics of Young Children Hospitalized With Respiratory Syncytial Virus Infections in Guatemala (2007–2010). Pediatr. Infect. Dis. J. 2013, 32, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Kubale, J.; Kuan, G.; Gresh, L.; Ojeda, S.; Azziz-Baumgartner, E.; Sanchez, N.; Lopez, R.; Harris, E.; Balmaseda, A.; Gordon, A. Assessing the Incidence of Symptomatic Respiratory Syncytial Virus Illness Within a Prospective Birth Cohort in Managua, Nicaragua. Clin. Infect. Dis. 2019, 70, 2029–2035. [Google Scholar] [CrossRef] [PubMed]
- Fong, Y.; Huang, Y.; Borate, B.; van der Laan, L.W.P.; Zhang, W.; Carpp, L.N.; Cho, I.; Glenn, G.; Fries, L.; Gottardo, R.; et al. Antibody Correlates of Protection From Severe Respiratory Syncytial Virus Disease in a Vaccine Efficacy Trial. Open Forum Infect. Dis. 2023, 10, ofac693. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, M.; Wang, Y.; Yu, H. 916. The Transfer of Maternal Antibodies and Dynamics of Maternal and Natural Infection-induced Antibodies against RSV in Children: A Longitudinal Cohort Study. Open Forum Infect. Dis. 2023, 10 (Suppl S2), ofad500.961. [Google Scholar] [CrossRef]
- Ochola, R.; Sande, C.; Fegan, G.; Scott, P.D.; Medley, G.F.; Caine, P.A.; Nokes, D.J. The Level and Duration of RSV-Specific Maternal IgG in Infants in Kilifi Kenya. PLoS ONE 2009, 4, e8088. [Google Scholar] [CrossRef]
- Hacimustafaoglu, M.; Celebi, S.; Aynaci, E.; Sinirtas, M.; Koksal, N.; Kucukerdogan, A.; Ercan, I.; Goral, G.; Ildirim, I. The progression of maternal RSV antibodies in the offspring. Arch. Dis. Child. 2004, 89, 52–53. [Google Scholar] [CrossRef]
- Alcalay, I.; Wainstock, T.; Sheiner, E. Maternal anemia and long-term respiratory morbidity of the offspring—Results of a population-based cohort. Arch. Gynecol. Obstet. 2023, 308, 1189–1195. [Google Scholar] [CrossRef]
- Anika Jane, T.; Gil, G.; Eyal, S.; Tamar, W. Maternal Anemia and Long-Term Offspring Infectious Morbidity. Am. J. Perinatol. 2022, 41, e968–e973. [Google Scholar] [CrossRef]
- Wu, G.; Imhoff-Kunsch, B.; Girard, A.W. Biological mechanisms for nutritional regulation of maternal health and fetal development. Paediatr. Perinat. Epidemiol. 2012, 26, 4–26. [Google Scholar] [CrossRef]
- Beckert, R.H.; Baer, R.J.; Anderson, J.G.; Jelliffe-Pawlowski, L.L.; Rogers, E.E. Maternal anemia and pregnancy outcomes: A population-based study. J. Perinatol. Off. J. Calif. Perinat. Assoc. 2019, 39, 911–919. [Google Scholar] [CrossRef]
- Khezri, R.; Rezaei, F.; Jahanfar, S.; Ebrahimi, K. Association between maternal anemia during pregnancy with low birth weight their infants. Sci. Rep. 2025, 15, 6446. [Google Scholar] [CrossRef]
- Kumer, P.; Rouf, M.A.; Bhuiyan, M.M.; Taleb, M.A.; Nesa, V. Association of iron deficiency anemia with acute bronchiolitis in children below 2 years of age. Int. J. Contemp. Pediatr. 2023, 11, 20–27. [Google Scholar] [CrossRef]
- Ragab, S.A.; Razik, A.A.; Sharaby, R.E.; Elmeazawy, R. Iron status and anemia as predictors for acute bronchiolitis severity. Egypt. J. Bronchol. 2024, 18, 62. [Google Scholar] [CrossRef]
- Miller, R.A.J.; Williams, A.P.; Kovats, S. Sex chromosome complement and sex steroid signaling underlie sex differences in immunity to respiratory virus infection. Front. Pharmacol. 2023, 14, 1150282. [Google Scholar] [CrossRef] [PubMed]
- Chamekh, M.; Deny, M.; Romano, M.; Lefèvre, N.; Corazza, F.; Duchateau, J.; Casimir, G. Differential Susceptibility to Infectious Respiratory Diseases between Males and Females Linked to Sex-Specific Innate Immune Inflammatory Response. Front. Immunol. 2017, 8, 1806. [Google Scholar] [CrossRef] [PubMed]
- Kadel, S.; Kovats, S. Sex Hormones Regulate Innate Immune Cells and Promote Sex Differences in Respiratory Virus Infection. Front. Immunol. 2018, 9, 1653. [Google Scholar] [CrossRef]
- Gantenberg, J.R.; van Aalst, R.; Bhuma, M.R.; Limone, B.; Diakun, D.; Smith, D.M.; Nelson, C.B.; Bengtson, A.M.; Chaves, S.S.; La Via, W.V.; et al. Risk Analysis of Respiratory Syncytial Virus Among Infants in the United States by Birth Month. J. Pediatr. Infect. Dis. Soc. 2024, 13, 317–327. [Google Scholar] [CrossRef]
- Li, T.; Fang, H.; Liu, X.; Deng, Y.; Zang, N.; Xie, J.; Xie, X.; Luo, Z.; Luo, J.; Liu, Y.; et al. Defining RSV epidemic season in southwest China and assessing the relationship between birth month and RSV infection: A 10-year retrospective study from June 2009 to May 2019. J. Med. Virol. 2023, 95, e28928. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Shi, T.; Bont, L.J.; Chu, H.Y.; Zar, H.J.; Wahi-Singh, B.; Ma, Y.; Cong, B.; Sharland, E.; et al. Global disease burden of and risk factors for acute lower respiratory infections caused by respiratory syncytial virus in preterm infants and young children in 2019: A systematic review and meta-analysis of aggregated and individual participant data. Lancet 2024, 403, 1241–1253. [Google Scholar] [CrossRef] [PubMed]
- Packnett, E.R.; Winer, I.H.; Larkin, H.; Oladapo, A.; Gonzales, T.; Wojdyla, M.; Goldstein, M.; Smith, V.C. RSV-related hospitalization and outpatient palivizumab use in very preterm (born at <29 wGA) infants: 2003-2020. Hum. Vaccin. Immunother. 2022, 18, 2140533. [Google Scholar] [CrossRef] [PubMed]
- Cox, F.; Saeland, E.; Thoma, A.; van den Hoogen, W.; Tettero, L.; Drijver, J.; Vaneman, C.; van Polanen, Y.; Ritschel, T.; Bastian, A.R.; et al. RSV A2-Based Prefusion F Vaccine Candidates Induce RSV A and RSV B Cross Binding and Neutralizing Antibodies and Provide Protection against RSV A and RSV B Challenge in Preclinical Models. Vaccines 2023, 11, 672. [Google Scholar] [CrossRef] [PubMed]


| Characteristic | Subcohort (n = 392), N (%) | RSV-ARI Hospitalization Cases (n = 66), N (%) | Unadjusted HR (95% CI) | Adjusted HR (95% CI) |
|---|---|---|---|---|
| Child | ||||
| Sex | ||||
| Boys | 215 (54.9) | 43 (65.2) | Reference | Reference |
| Girls | 177 (45.2) | 23 (34.9) | 0.64 (0.38–1.10) | 0.56 (0.32–0.996) |
| Birth weight | ||||
| <2500 g | 9 (2.3) | 0 (0.0) | 1.65 × 10−15 (8.16 × 10−16–3.33 × 10−15) | |
| ≥2500 g | 383 (97.7) | 66 (100.0) | Reference | |
| Gestational age | ||||
| <37 weeks | 17 (4.3) | 4 (6.1) | 1.37 (0.46–4.11) | 0.94 (0.25–3.45) |
| ≥37 weeks | 375 (95.7) | 62 (93.9) | Reference | Reference |
| Birth season | ||||
| January–March | 42 (10.7) | 9 (13.6) | Reference | Reference |
| April–June | 93 (23.7) | 23 (34.9) | 1.15 (0.50–2.67) | 1.40 (0.56–3.46) |
| July–September | 172 (43.9) | 25 (37.9) | 0.64 (0.28–1.47) | 0.69 (0.29–1.66) |
| October–December | 85 (21.7) | 9 (13.6) | 0.46 (0.17–1.23) | 0.60 (0.21–1.72) |
| RSV antibody titer | ||||
| Low | 98 (25.0) | 23 (34.9) | 1.84 (1.01–3.38) | 1.80 (0.93–3.50) |
| Middle | 196 (50.0) | 26 (39.4) | Reference | Reference |
| High | 98 (25.0) | 17 (25.8) | 1.28 (0.67–2.44) | 1.32 (0.68–2.58) |
| Mother at childbirth | ||||
| Mode of delivery | ||||
| Vaginal | 246 (62.8) | 36 (54.6) | Reference | |
| Cesarean section | 146 (37.2) | 30 (45.5) | 1.43 (0.85–2.41) | |
| Maternal age (years) | ||||
| ≤24 | 91 (23.2) | 12 (18.2) | Reference | |
| 25–29 | 144 (36.7) | 29 (43.9) | 1.49 (0.73–3.04) | |
| 30–34 | 110 (28.1) | 17 (25.8) | 1.16 (0.53–2.52) | |
| ≥35 | 47 (12.0) | 8 (12.1) | 1.33 (0.51–3.44) | |
| Maternal education | ||||
| No school/primary | 31 (7.9) | 1 (1.5) | 0.17 (0.02–1.28) | |
| Secondary | 107 (27.3) | 18 (27.3) | 0.90 (0.48–1.68) | |
| High school | 90 (23.0) | 16 (24.2) | 0.92 (0.48–1.76) | |
| College/university | 164 (41.8) | 31 (47.0) | Reference | |
| Parity | ||||
| Primipara | 153 (39.0) | 28 (42.4) | 1.20 (0.71–2.02) | |
| Multipara | 239 (61.0) | 38 (57.6) | Reference | |
| Maternal anemia (n = 387) | ||||
| Yes | 87 (22.5) | 35 (53.0) | 3.99 (2.35–6.78) | 4.10 (2.30–7.28) |
| No | 300 (77.5) | 31 (47.0) | Reference | Reference |
| Residential area | ||||
| Urban | 235 (60.0) | 40 (60.6) | 0.99 (0.59–1.68) | |
| Rural | 157 (40.1) | 26 (39.4) | Reference |
| Subcohort (n = 392) | RSV-ARI Hospitalization Cases < 12 m (n = 48) | Unadjusted HR (95% CI) | Adjusted HR (95% CI) | |
|---|---|---|---|---|
| Child | ||||
| Sex | ||||
| Boys | 215 (54.9%) | 31 (64.6%) | Reference | Reference |
| Girls | 177 (45.2%) | 17 (35.4%) | 0.66 (0.36–1.23) | 0.57 (0.29–1.12) |
| Birth weight | ||||
| <2500 g | 9 (2.3%) | 0 (0.0%) | 1.65 × 10−15 (8.04 × 10−16–3.38 × 10−15) | |
| ≥2500 g | 383 (97.7%) | 48 (100%) | Reference | |
| Gestational age | ||||
| <37 weeks | 17 (4.3%) | 3 (6.3%) | 1.42 (0.41–4.93) | 0.81 (0.17–3.85) |
| ≥37 weeks | 375 (95.7%) | 45 (93.8%) | Reference | Reference |
| Birth season | ||||
| January–March | 42 (10.7%) | 7 (14.6%) | Reference | Reference |
| April–June | 93 (23.7%) | 19 (39.6%) | 1.21 (0.48–3.07) | 1.51 (0.55–4.15) |
| July–September | 172 (43.9%) | 17 (35.4%) | 0.56 (0.22–1.43) | 0.61 (0.23–1.67) |
| October–December | 85 (21.7%) | 5 (10.4%) | 0.33 (0.10–1.09) | 0.47 (0.13–1.66) |
| RSV antibody titer | ||||
| Low | 98 (25.0%) | 20 (41.7%) | 2.44 (1.23–4.84) | 2.37 (1.09–5.14) |
| Middle | 196 (50.0%) | 17 (35.4%) | Reference | Reference |
| High | 98 (25.0%) | 11 (22.9%) | 1.26 (0.58–2.78) | 1.36 (0.61–3.06) |
| Mother at childbirth | ||||
| Mode of delivery | ||||
| Vaginal | 246 (62.8%) | 24 (50.0%) | Reference | |
| Cesarean section | 146 (37.2%) | 24 (50.0%) | 1.71 (0.94–3.11) | |
| Maternal age (years) | ||||
| ≤24 | 91 (23.2%) | 8 (16.7%) | Reference | |
| 25–29 | 144 (36.7%) | 22 (45.8%) | 1.69 (0.73–3.94) | |
| 30–34 | 110 (28.1%) | 11 (22.9%) | 1.12 (0.44–2.89) | |
| ≥35 | 47 (12.0%) | 7 (14.6%) | 1.74 (0.60–5.04) | |
| Maternal education | ||||
| No school/primary | 31 (7.9%) | 1 (2.1) | 0.23 (0.03–1.75) | |
| Secondary | 107 (27.3%) | 13 (27.1) | 0.87 (0.43–1.78) | |
| High school | 90 (23.0%) | 11 (22.9) | 0.85 (0.40–1.82) | |
| College/university | 164 (41.8%) | 23 (47.9) | Reference | |
| Parity | ||||
| Primipara | 153 (39.0%) | 20 (41.7%) | 1.15 (0.63–2.10) | |
| Multipara | 239 (61.0%) | 28 (58.3%) | Reference | |
| Maternal anemia (n = 387) | ||||
| Yes | 87 (22.5%) | 29 (60.4%) | 5.35 (2.89–9.92) | 5.45 (2.80–10.60) |
| No | 300 (77.5%) | 19 (39.6%) | Reference | Reference |
| Residential area | ||||
| Urban | 235 (60.0%) | 30 (62.5%) | 1.08 (0.58–1.99) | |
| Rural | 157 (40.1%) | 18 (37.5%) | Reference |
| (A) Interaction with age band. | ||||
| Age Band (Months) | Titer Group | aHR | 95% CI | p-Value |
| 0–5 | Low | 4.05 | 1.51–10.89 | 0.006 |
| High | 1.17 | 0.33–4.07 | 0.811 | |
| 6–11 | Low | 1.23 | 0.43–3.58 | 0.698 |
| High | 1.42 | 0.52–3.87 | 0.492 | |
| 12–17 | Low | 0.40 | 0.04–3.54 | 0.409 |
| High | 2.39 | 0.71–8.11 | 0.161 | |
| 18–23 | Low | 1.02 | 0.18–5.72 | 0.986 |
| High | 7.31 × 10−10 | 2.09 × 10−10–2.55 × 10−9 | <0.001 | |
| (B) Interaction with maternal anemia. | ||||
| Maternal Anemia Status | Titer Group | aHR | 95% CI | p-Value |
| No anemia | Low | 2.74 | 1.12–6.69 | 0.027 |
| High | 1.68 | 0.65–4.35 | 0.286 | |
| Anemia present | Low | 1.25 | 0.46–3.41 | 0.662 |
| High | 1.09 | 0.40–2.96 | 0.865 | |
| Antibody Titer Group | aHR for RSV-A (95% CI) | aHR for RSV-B (95% CI) |
|---|---|---|
| Low | 2.80 (1.00–7.86) (n = 3) | 1.76 × 1010 (2.02 × 109–1.54 × 1011) (n = 6) |
| High | 1.46 (0.46–4.63) (n = 1) | NE (n = 0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toizumi, M.; Yamagata, Y.; Nguyen, H.A.T.; Otomaru, H.; Le, H.H.; Moriuchi, H.; Eleouet, J.-F.; Rameix-Welti, M.-A.; Takeda, M.; Do, H.T.; et al. Cord Blood RSV-Neutralizing Antibodies and Risk of Hospitalization for RSV-Associated Acute Respiratory Infection in Vietnamese Children: A Case–Cohort Study. Vaccines 2025, 13, 963. https://doi.org/10.3390/vaccines13090963
Toizumi M, Yamagata Y, Nguyen HAT, Otomaru H, Le HH, Moriuchi H, Eleouet J-F, Rameix-Welti M-A, Takeda M, Do HT, et al. Cord Blood RSV-Neutralizing Antibodies and Risk of Hospitalization for RSV-Associated Acute Respiratory Infection in Vietnamese Children: A Case–Cohort Study. Vaccines. 2025; 13(9):963. https://doi.org/10.3390/vaccines13090963
Chicago/Turabian StyleToizumi, Michiko, Yutaro Yamagata, Hien Anh Thi Nguyen, Hirono Otomaru, Hoang Huy Le, Hiroyuki Moriuchi, Jean-Francois Eleouet, Marie-Anne Rameix-Welti, Makoto Takeda, Hung Thai Do, and et al. 2025. "Cord Blood RSV-Neutralizing Antibodies and Risk of Hospitalization for RSV-Associated Acute Respiratory Infection in Vietnamese Children: A Case–Cohort Study" Vaccines 13, no. 9: 963. https://doi.org/10.3390/vaccines13090963
APA StyleToizumi, M., Yamagata, Y., Nguyen, H. A. T., Otomaru, H., Le, H. H., Moriuchi, H., Eleouet, J.-F., Rameix-Welti, M.-A., Takeda, M., Do, H. T., & Yoshida, L.-M. (2025). Cord Blood RSV-Neutralizing Antibodies and Risk of Hospitalization for RSV-Associated Acute Respiratory Infection in Vietnamese Children: A Case–Cohort Study. Vaccines, 13(9), 963. https://doi.org/10.3390/vaccines13090963








