Case Report: A Multi-Peptide Vaccine Targeting Individual Somatic Mutations Induces Tumor Infiltration of Neoantigen-Specific T Cells in a Patient with Metastatic Colorectal Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Vaccine Design and Vaccination
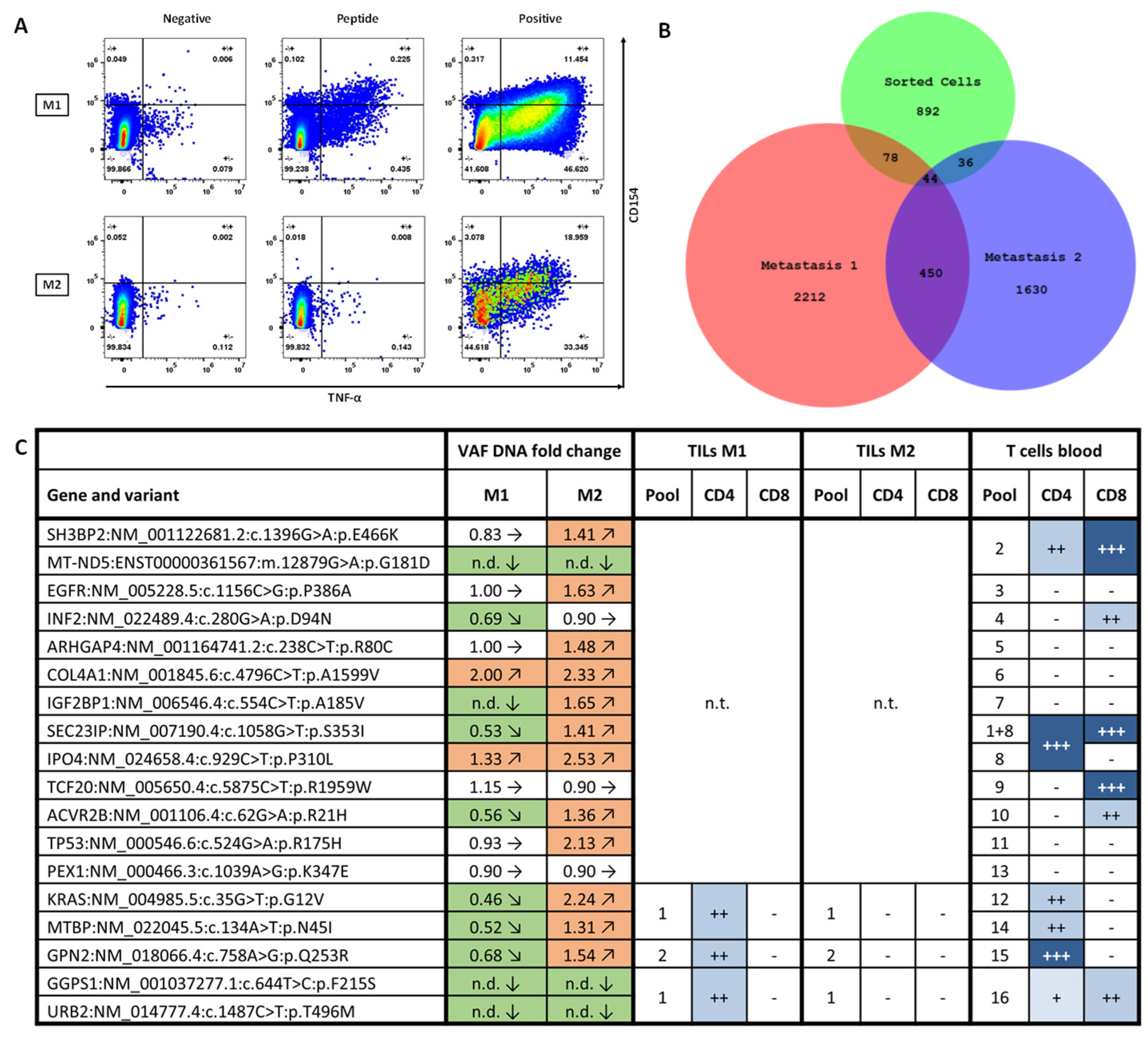
2.2. Immunomonitoring
2.3. TIL Isolation
2.4. Fluorescence-Activated Cell Sorting
2.5. DNA Isolation
2.6. TCRβ CDR3 Sequencing
3. Results
3.1. Case Presentation
3.2. Vaccine Immunogenicity and Safety
3.3. Tumor Infiltration by Vaccine-Induced T Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| mCRC | Metastatic colorectal cancer |
| TIL | Tumor-infiltrating lymphocytes |
| ICS | Intracellular cytokine staining |
| TCRβ | T cell receptor beta chain |
| CRC | Colorectal cancer |
| VEGF | Vascular endothelial growth factor |
| MSS | Microsatellite-stable |
| pMMR | Proficient mismatch repair |
| CPI | Checkpoint inhibition |
| MSI | Microsatellite-instable |
| MMRd | Mismatch repair deficient |
| EGFR | Epidermal growth factor receptor |
| RFA | Radiofrequency ablation |
| HIPEC | Hyperthermic intraperitoneal chemotherapy |
| TME | Tumor microenvironment |
| OS | Overall survival |
| MRI | Magnetic resonance imaging |
| TMB | Tumor mutational burden |
| GM-CSF | Granulocyte/Macrophage-colony stimulating factor |
| PBMC | Peripheral blood mononuclear cells |
| HLA | Human leukocyte antigen |
| SPPS | Solid phase peptide synthesis |
| MHC | Major histocompatibility complex |
| DMSO | Dimethylsulfoxide |
| NGS | Next generation sequencing |
| SD | Stable disease |
| PD | Progressive disease |
| SI | Stimulation index |
| VAF | Variant allele frequency |
References
- Eng, C.; Yoshino, T.; Ruíz-García, E.; Mostafa, N.; Cann, C.G.; O’BRian, B.; Benny, A.; O Perez, R.; Cremolini, C. Colorectal cancer. Lancet 2024, 404, 294–310. [Google Scholar] [CrossRef] [PubMed]
- Morgan, E.; Arnold, M.; Gini, A.; Lorenzoni, V.; Cabasag, C.J.; Laversanne, M.; Vignat, J.; Ferlay, J.; Murphy, N.; Bray, F. Global burden of colorectal cancer in 2020 and 2040: Incidence and mortality estimates from GLOBOCAN. Gut 2023, 72, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Morris, V.K.; Kennedy, E.B.; Baxter, N.N.; Benson, A.B.; Cercek, A.; Cho, M.; Ciombor, K.K.; Cremolini, C.; Davis, A.; Deming, D.A.; et al. Treatment of Metastatic Colorectal Cancer: ASCO Guideline. J. Clin. Oncol. 2023, 41, 678–700. [Google Scholar] [CrossRef] [PubMed]
- Ott, P.A.; Hu, Z.; Keskin, D.B.; Shukla, S.A.; Sun, J.; Bozym, D.J.; Zhang, W.; Luoma, A.; Giobbie-Hurder, A.; Peter, L.; et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature 2017, 547, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Hilf, N.; Kuttruff-Coqui, S.; Frenzel, K.; Bukur, V.; Stevanović, S.; Gouttefangeas, C.; Platten, M.; Tabatabai, G.; Dutoit, V.; Van Der Burg, S.H.; et al. Actively personalized vaccination trial for newly diagnosed glioblastoma. Nature 2019, 565, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Puig-Saus, C.; Sennino, B.; Peng, S.; Wang, C.L.; Pan, Z.; Yuen, B.; Purandare, B.; An, D.; Quach, B.B.; Nguyen, D.; et al. Neoantigen-targeted CD8+ T cell responses with PD-1 blockade therapy. Nature 2023, 615, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Latzer, P.; Zelba, H.; Battke, F.; Reinhardt, A.; Shao, B.; Bartsch, O.; Rabsteyn, A.; Harter, J.; Schulze, M.; Okech, T.; et al. A real-world observation of patients with glioblastoma treated with a personalized peptide vaccine. Nat. Commun. 2024, 15, 6870. [Google Scholar] [CrossRef] [PubMed]
- Zelba, H.; Shao, B.; Rabsteyn, A.; Reinhardt, A.; Greve, C.; Oenning, L.; Kayser, S.; Kyzirakos, C.; Latzer, P.; Riedlinger, T.; et al. In-depth characterization of vaccine-induced neoantigen-specific T cells in patients with IDH1-mutant glioma undergoing personalized peptide vaccination. J. Immuno-Ther. Cancer 2025, 13, e011070. [Google Scholar] [CrossRef] [PubMed]
- Schreiber Robert, D.; Old Lloyd, J.; Smyth Mark, J. Cancer immunoediting: Integrating immunity’s roles in cancer suppression and promotion. Science 2011, 331, 1565–1570. [Google Scholar] [CrossRef]
- Blumendeller, C.; Boehme, J.; Frick, M.; Schulze, M.; Rinckleb, A.; Kyzirakos, C.; Kayser, S.; Kopp, M.; Kelkenberg, S.; Pieper, N.; et al. Use of plasma ctDNA as a potential biomarker for longitudinal monitoring of a patient with metastatic high-risk upper tract urothelial carcinoma receiving pembrolizumab and personalized neoepitope-derived multipeptide vaccinations: A case report. J. Immunother. Cancer 2021, 9, e001406. [Google Scholar] [CrossRef] [PubMed]
- Blass, E.; Keskin, D.B.; Tu, C.R.; Forman, C.; Vanasse, A.; Sax, H.E.; Shim, B.; Chea, V.; Kim, N.; Carulli, I.; et al. A multi-adjuvant personal neoantigen vaccine generates potent immunity in melanoma. Cell 2025. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Braun, D.A.; Moranzoni, G.; Chea, V.; McGregor, B.A.; Blass, E.; Tu, C.R.; Vanasse, A.P.; Forman, C.; Forman, J.; Afeyan, A.B.; et al. A neoantigen vaccine generates antitumour immunity in renal cell carcinoma. Nature 2025, 639, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Parkhurst, M.R.; Robbins, P.F.; Tran, E.; Prickett, T.D.; Gartner, J.J.; Jia, L.; Ivey, G.; Li, Y.F.; El-Gamil, M.; Lalani, A.; et al. Unique Neoantigens Arise from Somatic Mutations in Patients with Gastrointestinal Cancers. Cancer Discov. 2019, 9, 1022–1035. [Google Scholar] [CrossRef] [PubMed]
- Reeves, E.; James, E. Antigen processing and immune regulation in the response to tumours. Immunology 2017, 150, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef] [PubMed]
- Parkhurst, M.; Goff, S.L.; Lowery, F.J.; Beyer, R.K.; Halas, H.; Robbins, P.F.; Prickett, T.D.; Gartner, J.J.; Sindiri, S.; Krishna, S.; et al. Adoptive transfer of personalized neoantigen-reactive TCR-transduced T cells in metastatic colorectal cancer: Phase 2 trial interim results. Nat. Med. 2024, 30, 2586–2595. [Google Scholar] [CrossRef] [PubMed]
- Mlecnik, B.; Tosolini, M.; Kirilovsky, A.; Berger, A.; Bindea, G.; Meatchi, T.; Bruneval, P.; Trajanoski, Z.; Fridman, W.-H.; Pagès, F.; et al. Histopathologic-based prognostic factors of colorectal cancers are associated with the state of the local immune reaction. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2011, 29, 610–618. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rabsteyn, A.; Zelba, H.; Shao, B.; Oenning, L.; Kyzirakos, C.; Kayser, S.; Riedlinger, T.; Harter, J.; Feldhahn, M.; Hadaschik, D.; et al. Case Report: A Multi-Peptide Vaccine Targeting Individual Somatic Mutations Induces Tumor Infiltration of Neoantigen-Specific T Cells in a Patient with Metastatic Colorectal Cancer. Vaccines 2025, 13, 960. https://doi.org/10.3390/vaccines13090960
Rabsteyn A, Zelba H, Shao B, Oenning L, Kyzirakos C, Kayser S, Riedlinger T, Harter J, Feldhahn M, Hadaschik D, et al. Case Report: A Multi-Peptide Vaccine Targeting Individual Somatic Mutations Induces Tumor Infiltration of Neoantigen-Specific T Cells in a Patient with Metastatic Colorectal Cancer. Vaccines. 2025; 13(9):960. https://doi.org/10.3390/vaccines13090960
Chicago/Turabian StyleRabsteyn, Armin, Henning Zelba, Borong Shao, Lisa Oenning, Christina Kyzirakos, Simone Kayser, Tabea Riedlinger, Johannes Harter, Magdalena Feldhahn, Dirk Hadaschik, and et al. 2025. "Case Report: A Multi-Peptide Vaccine Targeting Individual Somatic Mutations Induces Tumor Infiltration of Neoantigen-Specific T Cells in a Patient with Metastatic Colorectal Cancer" Vaccines 13, no. 9: 960. https://doi.org/10.3390/vaccines13090960
APA StyleRabsteyn, A., Zelba, H., Shao, B., Oenning, L., Kyzirakos, C., Kayser, S., Riedlinger, T., Harter, J., Feldhahn, M., Hadaschik, D., Battke, F., Scheble, V., Königsrainer, A., & Biskup, S. (2025). Case Report: A Multi-Peptide Vaccine Targeting Individual Somatic Mutations Induces Tumor Infiltration of Neoantigen-Specific T Cells in a Patient with Metastatic Colorectal Cancer. Vaccines, 13(9), 960. https://doi.org/10.3390/vaccines13090960






