Emerging Therapeutic Strategies for Lung Cancer: The Role of Immunotherapy and HPV-Targeted Cancer Vaccines
Abstract
1. Introduction
2. Epidemiology and Risk Factors of Lung Cancer
Controversies and Limitations of Current Evidence
3. Molecular Classification and Pathogenesis
3.1. SCLC vs. NSCLC Subtypes (LUAD, LUSC, LCC)
3.2. Genetic Mutations and Immune Evasion Mechanisms
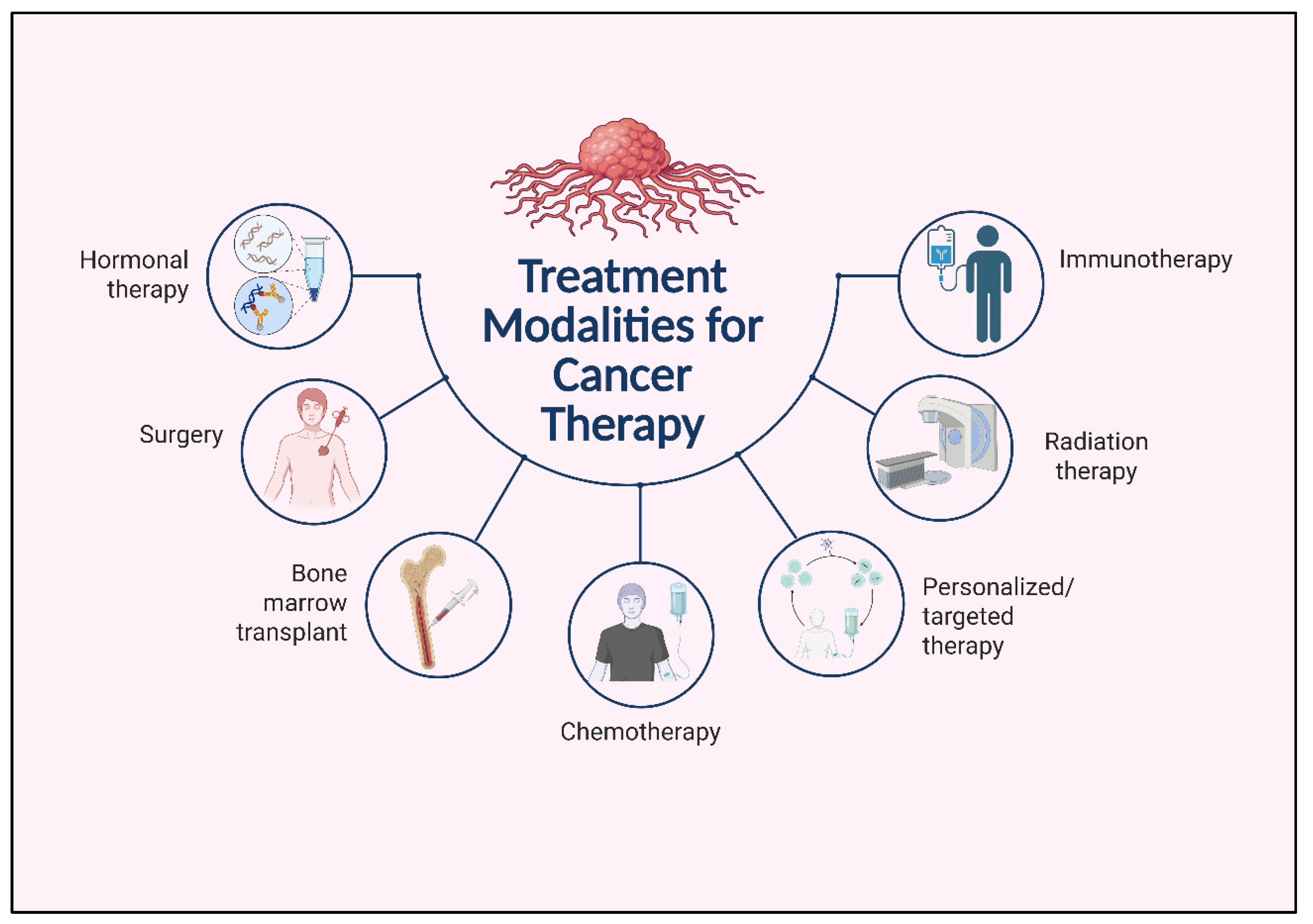
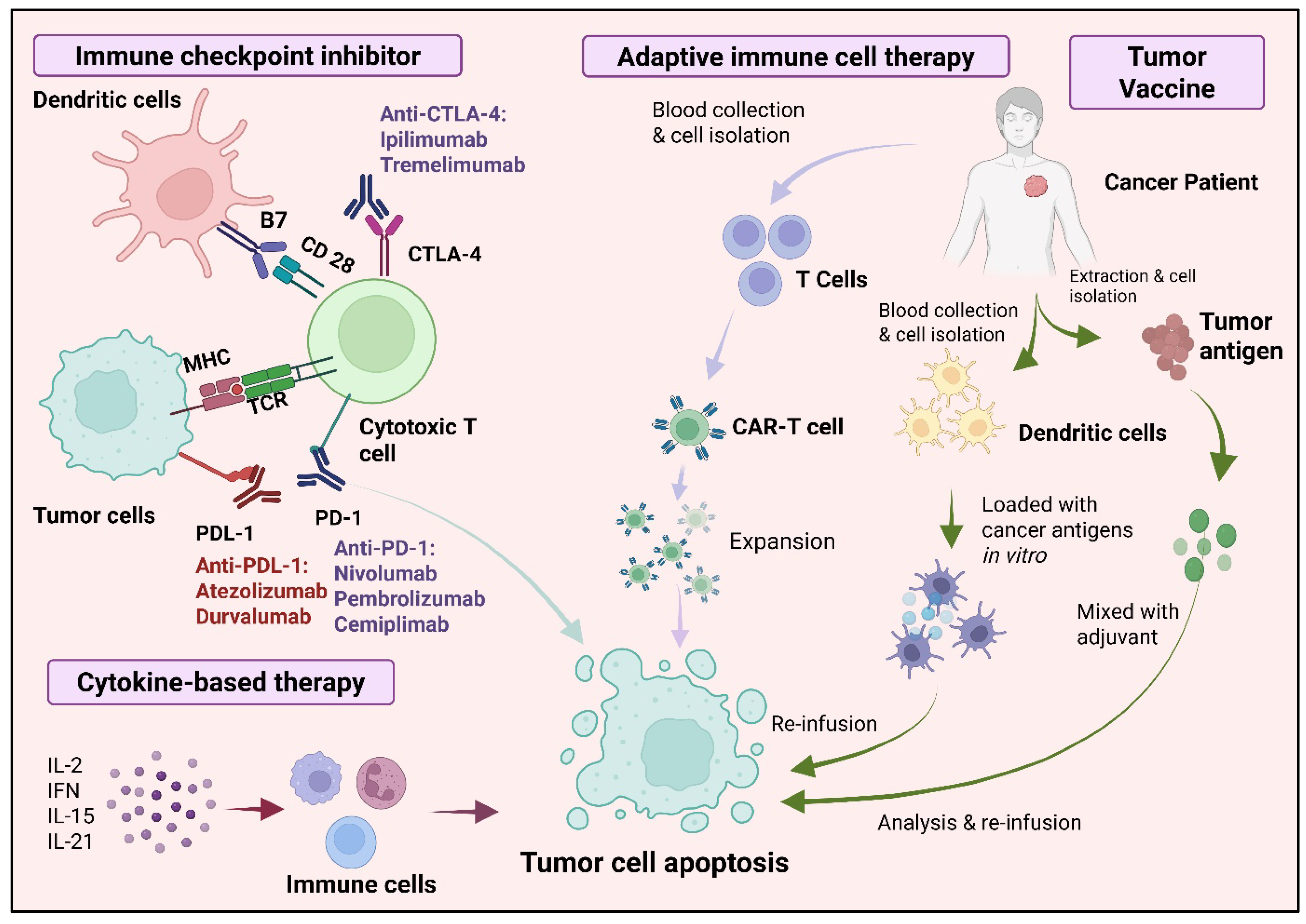
4. Immunotherapy in Lung Cancer
5. Cancer Vaccines in Lung Cancer
5.1. Traditional and Neoantigen-Based Vaccines
5.2. Nucleic Acid Vaccines
5.3. Peptide-Based Cancer Vaccines
5.4. Bacterial Ghost-Based Cancer Vaccine
6. Vaccine Adjuvants for Enhancing Cancer Immunity
6.1. Traditional Adjuvants: TLR Agonists, Aluminum Salts, Poly-ICLC
6.2. Emerging Adjuvants: STING Agonists, CD40, Cytokines, and Inorganic Nanoparticles
6.3. Clinical and Preclinical Findings
7. Imidazoquinolines (IMDs) as Cancer Immunomodulators
7.1. Mechanisms: TLR7/8 Activation
7.2. FDA-Approved Agents: Imiquimod and Resiquimod
7.3. Applications as Vaccine Adjuvants and Topical Immunotherapies
7.4. Future Directions and Clinical Integration
8. Future Perspectives and Research Directions
8.1. Personalized Neoantigen Vaccines and Immunotherapy
8.2. Multi-Modal Immunotherapy Combinations
8.3. Integrating Virology and Oncology
8.4. Predictive Biomarkers and Precision Approaches
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Lei, S.; Ding, L.; Xu, Y.; Wu, X.; Wang, H.; Zhang, Z.; Gao, T.; Zhang, Y.; Li, L. Global burden and trends of lung cancer incidence and mortality. Chin. Med. J. 2023, 136, 1583–1590. [Google Scholar] [CrossRef] [PubMed]
- Abbas, T.; Dutta, A. p21 in cancer: Intricate networks and multiple activities. Nat. Rev. Cancer 2009, 9, 400–414. [Google Scholar] [CrossRef] [PubMed]
- Zappa, C.; Mousa, S.A. Non-small cell lung cancer: Current treatment and future advances. Transl. Lung Cancer Res. 2016, 5, 288–300. [Google Scholar] [CrossRef]
- Chen, K.-C.; Tsai, S.-W.; Shie, R.-H.; Zeng, C.; Yang, H.-Y. Indoor air pollution increases the risk of lung cancer. Int. J. Environ. Res. Public Health 2022, 19, 1164. [Google Scholar] [CrossRef]
- Lancaster, H.L.; Heuvelmans, M.A.; Oudkerk, M. Low-dose computed tomography lung cancer screening: Clinical evidence and implementation research. J. Intern. Med. 2022, 292, 68–80. [Google Scholar] [CrossRef]
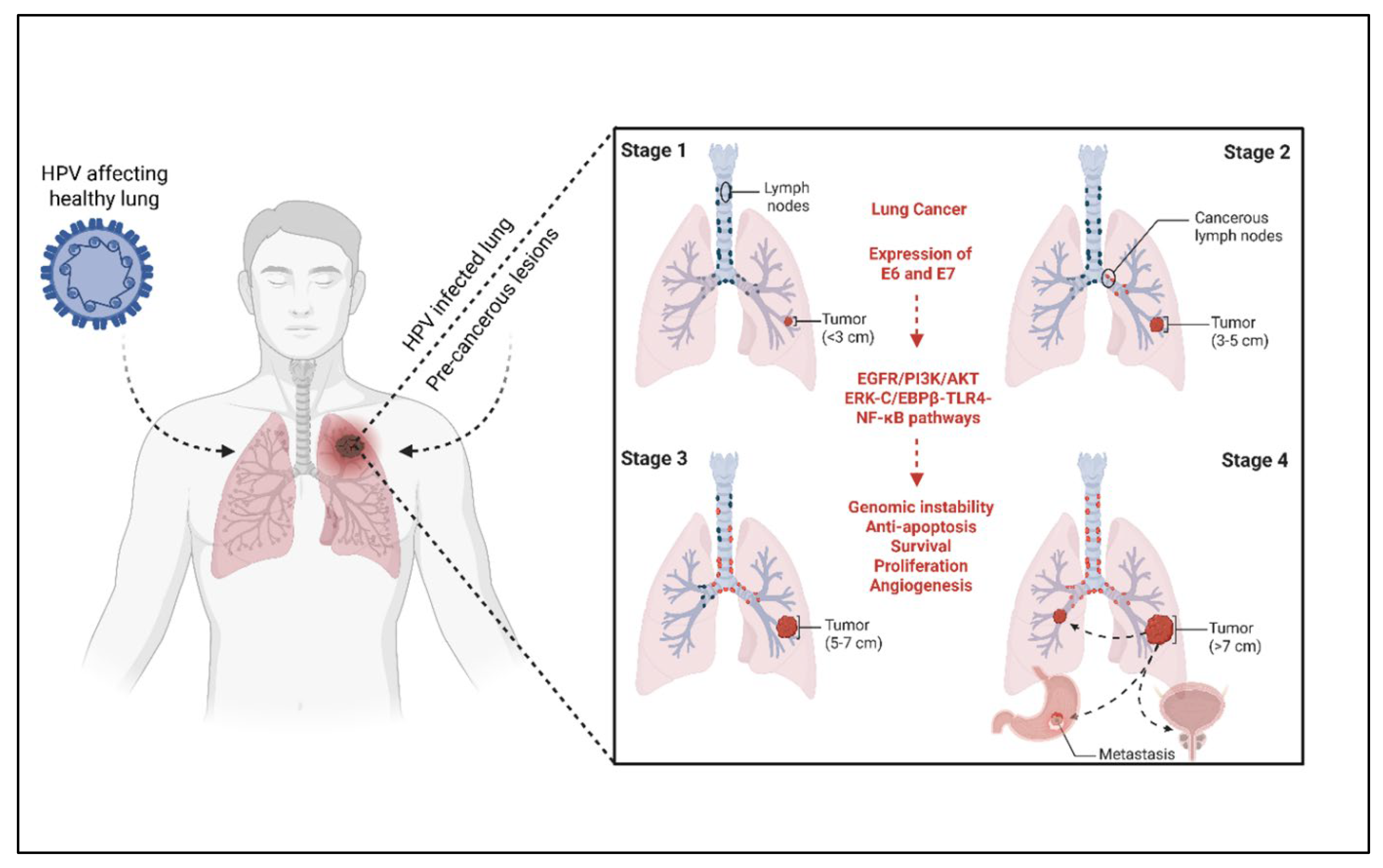
- Han, Z.; Aizezi, A.; Ma, L.; Su, Y.; Fan, L.; Liu, J. The association between human papillomavirus and lung cancer: A Mendelian randomization study. Infect. Genet. Evol. 2024, 123, 105646. [Google Scholar] [CrossRef]
- Osorio, J.C.; Candia-Escobar, F.; Corvalan, A.H.; Calaf, G.M.; Aguayo, F. High-risk human papillomavirus infection in lung cancer: Mechanisms and perspectives. Biology 2022, 11, 1691. [Google Scholar] [CrossRef]
- Xiong, W.-M.; Xu, Q.-P.; Li, X.; Xiao, R.-D.; Cai, L.; He, F. The association between human papillomavirus infection and lung cancer: A system review and meta-analysis. Oncotarget 2017, 8, 96419. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- American Cancer Society. Global Cancer Facts & Figures, 5th ed.; American Cancer Society: Atlanta, GA, USA, 2024; Available online: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/global-cancer-facts-and-figures/global-cancer-facts-and-figures-2024.pdf (accessed on 1 September 2025).
- Thun, M.J.; Carter, B.D.; Feskanich, D.; Freedman, N.D.; Prentice, R.; Lopez, A.D.; Hartge, P.; Gapstur, S.M. 50-year trends in smoking-related mortality in the United States. N. Engl. J. Med. 2013, 368, 351–364. [Google Scholar] [CrossRef]
- World Health Organization. WHO Report on the Global Tobacco Epidemic, 2025: Warning About the Dangers of Tobacco. 2025. Available online: https://www.who.int/publications/i/item/9789240112063 (accessed on 1 September 2025).
- Aredo, J.V.; Luo, S.J.; Gardner, R.M.; Sanyal, N.; Choi, E.; Hickey, T.P.; Riley, T.L.; Huang, W.-Y.; Kurian, A.W.; Leung, A.N. Tobacco smoking and risk of second primary lung cancer. J. Thorac. Oncol. 2021, 16, 968–979. [Google Scholar] [CrossRef]
- Samet, J.M.; Avila-Tang, E.; Boffetta, P.; Hannan, L.M.; Olivo-Marston, S.; Thun, M.J.; Rudin, C.M. Lung cancer in never smokers: Clinical epidemiology and environmental risk factors. Clin. Cancer Res. 2009, 15, 5626–5645. [Google Scholar] [CrossRef]
- Travis, R.C.; Allen, N.E.; Appleby, P.N.; Spencer, E.A.; Roddam, A.W.; Key, T.J. A prospective study of vegetarianism and isoflavone intake in relation to breast cancer risk in British women. Int. J. Cancer 2008, 122, 705–710. [Google Scholar] [CrossRef] [PubMed]
- Klaunig, J.E. Chemical carcinogenesis. In Casarett and Doull’s Toxicology: The Basic Science of Poisons, 8th ed.; McGraw-Hill Education: Columbus, OH, USA, 2014; p. 259. Available online: https://accesspharmacy.mhmedical.com/Content.aspx?bookid=958§ionid=53483729 (accessed on 1 September 2025).
- O’Keeffe, L.M.; Taylor, G.; Huxley, R.R.; Mitchell, P.; Woodward, M.; Peters, S.A. Smoking as a risk factor for lung cancer in women and men: A systematic review and meta-analysis. BMJ Open 2018, 8, e021611. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.J.; Brauer, M.; Burnett, R.; Anderson, H.R.; Frostad, J.; Estep, K.; Balakrishnan, K.; Brunekreef, B.; Dandona, L.; Dandona, R. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: An analysis of data from the Global Burden of Diseases Study 2015. Lancet 2017, 389, 1907–1918. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chen, R.; Sera, F.; Vicedo-Cabrera, A.M.; Guo, Y.; Tong, S.; Coelho, M.S.; Saldiva, P.H.; Lavigne, E.; Matus, P. Ambient particulate air pollution and daily mortality in 652 cities. N. Engl. J. Med. 2019, 381, 705–715. [Google Scholar] [CrossRef]
- Lin, S.-H.; Lee, H.-Y.; Chang, Y.-Y.; Jang, Y.; Chen, P.-C.; Wang, J.-D. Increased mortality risk for workers with a compensated, permanent occupational disability of the upper or lower extremities: A 21-year follow-up study. Am. J. Epidemiol. 2010, 171, 917–923. [Google Scholar] [CrossRef]
- Stayner, L.; Welch, L.S.; Lemen, R. The worldwide pandemic of asbestos-related diseases. Annu. Rev. Public Health 2013, 34, 205–216. [Google Scholar] [CrossRef]
- Rigamonti, A.; Viatore, M.; Polidori, R.; Rahal, D.; Erreni, M.; Fumagalli, M.R.; Zanini, D.; Doni, A.; Putignano, A.R.; Bossi, P. Integrating AI-Powered digital pathology and imaging mass cytometry identifies key classifiers of tumor cells, stroma, and immune cells in Non–Small cell lung Cancer. Cancer Res. 2024, 84, 1165–1177. [Google Scholar] [CrossRef]
- Sequeira, T.; Pinto, R.; Cardoso, C.; Almeida, C.; Aragão, R.; Almodovar, T.; Bicho, M.; Bicho, M.C.; Bárbara, C. HPV and Lung Cancer: A Systematic Review. Cancers 2024, 16, 3325. [Google Scholar] [CrossRef]
- Taniguchi, Y.; Matsuda, Y.; Mori, M.; Ito, M.; Ikari, T.; Tokoro, A.; Aiki, S.; Hoshino, S.; Kiuchi, D.; Suzuki, K. Effectiveness and safety of opioids for dyspnea in patients with lung cancer: Secondary analysis of multicenter prospective observational study. Transl. Lung Cancer Res. 2022, 11, 2395–2402. [Google Scholar] [CrossRef]
- Fois, S.S.; Paliogiannis, P.; Zinellu, A.; Fois, A.G.; Cossu, A.; Palmieri, G. Molecular epidemiology of the main druggable genetic alterations in non-small cell lung cancer. Int. J. Mol. Sci. 2021, 22, 612. [Google Scholar] [CrossRef]
- Reagan, M. Causes of cancer: Genetic, epigenetic, viral, microenvironmental, and environmental contributions to cancer. In Cancer: Prevention, Early Detection, Treatment and Recovery; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2019; pp. 53–74. [Google Scholar] [CrossRef]
- Travis, W.D.; Brambilla, E.; Nicholson, A.G.; Yatabe, Y.; Austin, J.H.; Beasley, M.B.; Chirieac, L.R.; Dacic, S.; Duhig, E.; Flieder, D.B. The 2015 World Health Organization classification of lung tumors: Impact of genetic, clinical and radiologic advances since the 2004 classification. J. Thorac. Oncol. 2015, 10, 1243–1260. [Google Scholar] [CrossRef] [PubMed]
- George, J.; Lim, J.S.; Jang, S.J.; Cun, Y.; Ozretić, L.; Kong, G.; Leenders, F.; Lu, X.; Fernández-Cuesta, L.; Bosco, G. Comprehensive genomic profiles of small cell lung cancer. Nature 2015, 524, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Gay, C.M.; Stewart, C.A.; Park, E.M.; Diao, L.; Groves, S.M.; Heeke, S.; Nabet, B.Y.; Fujimoto, J.; Solis, L.M.; Lu, W. Patterns of transcription factor programs and immune pathway activation define four major subtypes of SCLC with distinct therapeutic vulnerabilities. Cancer Cell 2021, 39, 346–360.e7. [Google Scholar] [CrossRef]
- Pan, X.-B.; Chen, K.-H.; Huang, S.-T.; Jiang, Y.-M.; Ma, J.-L.; Liang, Z.-G.; Qu, S.; Li, L.; Chen, L.; Zhu, X.-D. Comparison of the efficacy between intensity-modulated radiotherapy and two-dimensional conventional radiotherapy in stage II nasopharyngeal carcinoma. Oncotarget 2017, 8, 78096–78104. [Google Scholar] [CrossRef][Green Version]
- Skoulidis, F.; Goldberg, M.E.; Greenawalt, D.M.; Hellmann, M.D.; Awad, M.M.; Gainor, J.F.; Schrock, A.B.; Hartmaier, R.J.; Trabucco, S.E.; Gay, L. STK11/LKB1 mutations and PD-1 inhibitor resistance in KRAS-mutant lung adenocarcinoma. Cancer Discov. 2018, 8, 822–835. [Google Scholar] [CrossRef]
- Binnewies, M.; Roberts, E.W.; Kersten, K.; Chan, V.; Fearon, D.F.; Merad, M.; Coussens, L.M.; Gabrilovich, D.I.; Ostrand-Rosenberg, S.; Hedrick, C.C. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 2018, 24, 541–550. [Google Scholar] [CrossRef]
- Kamali, A.N.; Bautista, J.M.; Eisenhut, M.; Hamedifar, H. Immune checkpoints and cancer immunotherapies: Insights into newly potential receptors and ligands. Ther. Adv. Vaccines Immunother. 2023, 11, 25151355231192043. [Google Scholar] [CrossRef]
- Buchbinder, E.I.; Desai, A. CTLA-4 and PD-1 pathways: Similarities, differences, and implications of their inhibition. Am. J. Clin. Oncol. 2016, 39, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Shiravand, Y.; Khodadadi, F.; Kashani, S.M.A.; Hosseini-Fard, S.R.; Hosseini, S.; Sadeghirad, H.; Ladwa, R.; O’Byrne, K.; Kulasinghe, A. Immune checkpoint inhibitors in cancer therapy. Curr. Oncol. 2022, 29, 3044–3060. [Google Scholar] [CrossRef] [PubMed]
- Doroshow, D.B.; Sanmamed, M.F.; Hastings, K.; Politi, K.; Rimm, D.L.; Chen, L.; Melero, I.; Schalper, K.A.; Herbst, R.S. Immunotherapy in non–small cell lung cancer: Facts and hopes. Clin. Cancer Res. 2019, 25, 4592–4602. [Google Scholar] [CrossRef] [PubMed]
- Mamdani, H.; Matosevic, S.; Khalid, A.B.; Durm, G.; Jalal, S.I. Immunotherapy in lung cancer: Current landscape and future directions. Front. Immunol. 2022, 13, 823618. [Google Scholar] [CrossRef]
- Tan, S.; Li, D.; Zhu, X. Cancer immunotherapy: Pros, cons and beyond. Biomed. Pharmacother. 2020, 124, 109821. [Google Scholar] [CrossRef]
- Liu, C.; Yang, M.; Zhang, D.; Chen, M.; Zhu, D. Clinical cancer immunotherapy: Current progress and prospects. Front. Immunol. 2022, 13, 961805. [Google Scholar] [CrossRef]
- Acheampong, E.; Abed, A.; Morici, M.; Bowyer, S.; Amanuel, B.; Lin, W.; Millward, M.; S. Gray, E. Tumour PD-L1 expression in small-cell lung cancer: A systematic review and meta-analysis. Cells 2020, 9, 2393. [Google Scholar] [CrossRef]
- Caliman, E.; Fancelli, S.; Petroni, G.; Michelet, M.R.G.; Cosso, F.; Ottanelli, C.; Mazzoni, F.; Voltolini, L.; Pillozzi, S.; Antonuzzo, L. Challenges in the treatment of small cell lung cancer in the era of immunotherapy and molecular classification. Lung Cancer 2023, 175, 88–100. [Google Scholar] [CrossRef]
- National Cancer Institute. Testing the Addition of an Individualized Vaccine to Nab-Paclitaxel, Durvalumab and Tremelimumab and Chemotherapy in Patients with Metastatic Triple Negative Breast Cancer. ClinicalTrials.gov 2021. Available online: https://clinicaltrials.gov/study/NCT03606967 (accessed on 1 September 2025).
- The Lymphoma Academic Research Organisation; National Cancer Institute. Phase Ib Study of Intratumoral Selicrelumab (CD40 Agonist) in Combination with Atezolizumab in Refractory or Relapsed B-Cell Lymphoma. ClinicalTrials.gov 2019. Available online: https://clinicaltrials.gov/study/NCT03892525?cond=%22Hodgkin%27s%20Paragranuloma%22&intr=%22Atezolizumab%22&viewType=Table&rank=3 (accessed on 1 September 2025).
- Lin, A.; Huang, L.; Jiang, A.; Zhu, L.; Mou, W.; Li, Y.; Zhang, C.; Liu, Z.; Zhang, J.; Cheng, Q.; et al. Microbiota boost immuno-therapy? A meta-analysis dives into fecal microbiota transplantation and immune checkpoint inhibitors. BMC Med. 2025, 23, 341. [Google Scholar] [CrossRef]
- Synlogic. Safety and Tolerability of SYNB1891 Injection Alone or in Combination with Atezolizumab in Adult Subjects with Advanced/Metastatic Solid Tumors and Lymphoma. ClinicalTrials.gov 2021. Available online: https://www.mycancergenome.org/content/clinical_trials/NCT04167137/ (accessed on 1 September 2025).
- Grimmett, C.; Heneka, N.; Chambers, S. Psychological interventions prior to cancer surgery: A review of reviews. Curr. An-esthesiol. Rep. 2022, 12, 78–87. [Google Scholar] [CrossRef]
- Belli, C.; Trapani, D.; Viale, G.; D’Amico, P.; Duso, B.A.; Della Vigna, P.; Orsi, F.; Curigliano, G. Targeting the microenvi-ronment in solid tumors. Cancer Treat. Rev. 2018, 65, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Vedenko, A.; Panara, K.; Goldstein, G.; Ramasamy, R.; Arora, H. Tumor microenvironment and nitric oxide: Concepts and mechanisms. In Tumor Microenvironment: Molecular Players–Part B; Springer: Cham, Switzerland, 2020; pp. 143–158. [Google Scholar] [CrossRef]
- Peng, M.; Mo, Y.; Wang, Y.; Wu, P.; Zhang, Y.; Xiong, F.; Guo, C.; Wu, X.; Li, Y.; Li, X. Neoantigen vaccine: An emerging tumor immunotherapy. Mol. Cancer 2019, 18, 128. [Google Scholar] [CrossRef] [PubMed]
- Jou, J.; Harrington, K.J.; Zocca, M.-B.; Ehrnrooth, E.; Cohen, E.E. The changing landscape of therapeutic cancer vaccines—Novel platforms and neoantigen identification. Clin. Cancer Res. 2021, 27, 689–703. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, A.; Champeimont, J.; Mayr, U.B.; Lubitz, W.; Kudela, P. Bacterial ghosts as carriers of protein subunit and DNA-encoded antigens for vaccine applications. Expert Rev. Vaccines 2012, 11, 97–116. [Google Scholar] [CrossRef]
- Anwer, M.; Bhaliya, K.; Munn, A.; Wei, M.Q. Bacterial ghosts: A breakthrough approach to cancer vaccination. Biomed. Pharmacother. 2025, 182, 117766. [Google Scholar] [CrossRef]
- Lawler, S.E.; Speranza, M.-C.; Cho, C.-F.; Chiocca, E.A. Oncolytic Viruses in Cancer Treatment: A Review. JAMA Oncol. 2017, 3, 841–849. [Google Scholar] [CrossRef]
- My Cancer Genome. Trial with BNT111 and Cemiplimab in Combination or as Single Agents in Patients with An-ti-PD1-Refractory/Relapsed, Unresectable Stage III or IV Melanoma. ClinicalTrials.gov 2021. Available online: https://www.mycancergenome.org/content/clinical_trials/NCT04526899/ (accessed on 1 September 2025).
- AbbVie. A Phase 1, Multicenter, Open-Label Study to Determine the Safety, Tolerability, Pharmacokinetics, and Preliminary Efficacy of ABBV-927 with ABBV-368, Budigalimab (ABBV-181), and/or Chemotherapy in Subjects with Locally Advanced or Metastatic Solid Tumors. ClinicalTrials.gov 2019. Available online: https://clinicaltrials.gov/study/NCT03893955 (accessed on 1 September 2025).
- Buonaguro, L.; Tornesello, M.L.; Buonaguro, F.M. Cancer vaccine development: From preclinical models to clinical trials. Vaccines 2020, 8, 402. [Google Scholar] [CrossRef]
- Roswell Park Cancer Institute; National Cancer Institute. FGFR1 Amplification as A Predictor of Efficacy in a Biomarker-Driven Phase II Study of BIBF 1120 in Advanced Squamous Cell Lung Cancer Patients Who Have Failed Up to Two Prior Chemotherapeutic Regimens. ClinicalTrials.gov 2013. Available online: https://www.clinicaltrials.gov/study/NCT01948141 (accessed on 1 September 2025).
- Nemunaitis, J.; Dillman, R.O.; Schwarzenberger, P.O.; Senzer, N.; Cunningham, C.; Cutler, J.; Tong, A.; Kumar, P.; Pappen, B.; Hamilton, C. Phase II study of belagenpumatucel-L, a transforming growth factor beta-2 antisense gene-modified allogeneic tumor cell vaccine in non–small-cell lung cancer. J. Clin. Oncol. 2006, 24, 4721–4730. [Google Scholar] [CrossRef]
- Valanparambil, R.M.; Carlisle, J.; Linderman, S.L.; Akthar, A.; Millett, R.L.; Lai, L.; Chang, A.; McCook, A.A.; Switchenko, J.; Nasti, T.H. Antibody response to SARS-CoV-2 mRNA vaccine in lung cancer patients: Reactivity to vaccine antigen and variants of concern. MedRxiv 2022, 40, 3808–3816. [Google Scholar] [CrossRef]
- Kreiter, S.; Selmi, A.; Diken, M.; Koslowski, M.; Britten, C.M.; Huber, C.; Türeci, Ö.; Sahin, U. Intranodal vaccination with naked antigen-encoding RNA elicits potent prophylactic and therapeutic antitumoral immunity. Cancer Res. 2010, 70, 9031–9040. [Google Scholar] [CrossRef]
- Rezaei, T.; Davoudian, E.; Khalili, S.; Amini, M.; Hejazi, M.; de la Guardia, M.; Mokhtarzadeh, A. Strategies in DNA vaccine for melanoma cancer. Pigment. Cell Melanoma Res. 2021, 34, 869–891. [Google Scholar] [CrossRef]
- Hobernik, D.; Bros, M. DNA vaccines—How far from clinical use? Int. J. Mol. Sci. 2018, 19, 3605. [Google Scholar] [CrossRef] [PubMed]
- Gan, Y.; Li, X.; Han, S.; Liang, Q.; Ma, X.; Rong, P.; Wang, W.; Li, W. The cGAS/STING pathway: A novel target for cancer therapy. Front. Immunol. 2022, 12, 795401. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, H.; Ma, Z.; Barber, G.N. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature 2009, 461, 788–792. [Google Scholar] [CrossRef] [PubMed]
- Lopes, A.; Vanvarenberg, K.; Préat, V.; Vandermeulen, G. Codon-optimized P1A-encoding DNA vaccine: Toward a therapeutic vaccination against P815 mastocytoma. Mol. Ther. Nucleic Acids 2017, 8, 404–415. [Google Scholar] [CrossRef]
- Gupta, M.; Wahi, A.; Sharma, P.; Nagpal, R.; Raina, N.; Kaurav, M.; Bhattacharya, J.; Rodrigues Oliveira, S.M.; Dolma, K.G.; Paul, A.K. Recent advances in cancer vaccines: Challenges, achievements, and futuristic prospects. Vaccines 2022, 10, 2011. [Google Scholar] [CrossRef]
- Abd-Aziz, N.; Poh, C.L. Development of peptide-based vaccines for cancer. J. Oncol. 2022, 2022, 9749363. [Google Scholar] [CrossRef]
- Li, W.; Joshi, M.D.; Singhania, S.; Ramsey, K.H.; Murthy, A.K. Peptide vaccine: Progress and challenges. Vaccines 2014, 2, 515–536. [Google Scholar] [CrossRef]
- Anwer, M.; Wei, M.Q. Harnessing the power of probiotic strains in functional foods: Nutritive, therapeutic, and next-generation challenges. Food Sci.Biotechnol. 2024, 33, 2081–2095. [Google Scholar] [CrossRef]
- Paston, S.J.; Brentville, V.A.; Symonds, P.; Durrant, L.G. Cancer vaccines, adjuvants, and delivery systems. Front. Immunol. 2021, 12, 627932. [Google Scholar] [CrossRef]
- Cuzzubbo, S.; Mangsbo, S.; Nagarajan, D.; Habra, K.; Pockley, A.G.; McArdle, S.E. Cancer vaccines: Adjuvant potency, importance of age, lifestyle, and treatments. Front. Immunol. 2021, 11, 615240. [Google Scholar] [CrossRef]
- Luo, M.; Wang, H.; Wang, Z.; Cai, H.; Lu, Z.; Li, Y.; Du, M.; Huang, G.; Wang, C.; Chen, X. A STING-activating nanovaccine for cancer immunotherapy. Nat. Nanotechnol. 2017, 12, 648–654. [Google Scholar] [CrossRef]
- Gulen, M.F.; Koch, U.; Haag, S.M.; Schuler, F.; Apetoh, L.; Villunger, A.; Radtke, F.; Ablasser, A. Signalling strength determines proapoptotic functions of STING. Nat. Commun. 2017, 8, 427. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-J.; Li, W.-H.; Chen, P.-G.; Zhang, B.-D.; Hu, H.-G.; Li, Q.-Q.; Zhao, L.; Chen, Y.-X.; Zhao, Y.-F.; Li, Y.-M. Targeting STING with cyclic di-GMP greatly augmented immune responses of glycopeptide cancer vaccines. Chem. Commun. 2018, 54, 9655–9658. [Google Scholar] [CrossRef]
- Shao, Y.; Sun, Z.-Y.; Wang, Y.; Zhang, B.-D.; Liu, D.; Li, Y.-M. Designable immune therapeutical vaccine system based on DNA supramolecular hydrogels. ACS Appl. Mater. Interfaces 2018, 10, 9310–9314. [Google Scholar] [CrossRef] [PubMed]
- Vonderheide, R.H.; Glennie, M.J. Agonistic CD40 antibodies and cancer therapy. Clin. Cancer Res. 2013, 19, 1035–1043. [Google Scholar] [CrossRef]
- Nimanong, S.; Ostroumov, D.; Wingerath, J.; Knocke, S.; Woller, N.; Gürlevik, E.; Falk, C.S.; Manns, M.P.; Kühnel, F.; Wirth, T.C. CD40 signaling drives potent cellular immune responses in heterologous cancer vaccinations. Cancer Res. 2017, 77, 1918–1926. [Google Scholar] [CrossRef]
- Chandra, D.; Quispe-Tintaya, W.; Jahangir, A.; Asafu-Adjei, D.; Ramos, I.; Sintim, H.O.; Zhou, J.; Hayakawa, Y.; Karaolis, D.K.; Gravekamp, C. STING ligand c-di-GMP improves cancer vaccination against metastatic breast cancer. Cancer Immunol. Res. 2014, 2, 901–910. [Google Scholar] [CrossRef]
- Sun, B.; Xia, T. Nanomaterial-based vaccine adjuvants. J. Mater. Chem. B 2016, 4, 5496–5509. [Google Scholar] [CrossRef]
- Wang, M.; Sharma, A.; Osazuwa-Peters, N.; Simpson, M.C.; Schootman, M.; Piccirillo, J.F.; Huh, W.K.; Boakye, E.A. Risk of subsequent malignant neoplasms after an index potentially-human papillomavirus (HPV)-associated cancers. Cancer Epidemiol. 2020, 64, 101649. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, C.; Guan, Y.; Wei, X.; Sha, M.; Yi, M.; Jing, M.; Lv, M.; Guo, W.; Xu, J. Manganese salts function as potent adjuvants. Cell Mol. Immunol. 2021, 18, 1222–1234. [Google Scholar] [CrossRef]
- Xia, Y.; Xie, Y.; Yu, Z.; Xiao, H.; Jiang, G.; Zhou, X.; Yang, Y.; Li, X.; Zhao, M.; Li, L. The mevalonate pathway is a druggable target for vaccine adjuvant discovery. Cell 2018, 175, 1059–1073.e21. [Google Scholar] [CrossRef]
- Ma, X.; Wang, Y.; Zhao, T.; Li, Y.; Su, L.-C.; Wang, Z.; Huang, G.; Sumer, B.D.; Gao, J. Ultra-pH-sensitive nanoprobe library with broad pH tunability and fluorescence emissions. J. Am. Chem. Soc. 2014, 136, 11085–11092. [Google Scholar] [CrossRef]
- Horning, K.J.; Caito, S.W.; Tipps, K.G.; Bowman, A.B.; Aschner, M. Manganese is essential for neuronal health. Annu. Rev. Nutr. 2015, 35, 71–108. [Google Scholar] [CrossRef]
- Bando, J.K.; Colonna, M. Group 2 innate lymphoid cells induce antibody production in gastric tissue. Trends Immunol. 2020, 41, 643–645. [Google Scholar] [CrossRef]
- Duan, T.; Du, Y.; Xing, C.; Wang, H.Y.; Wang, R.-F. Toll-like receptor signaling and its role in cell-mediated immunity. Front. Immunol. 2022, 13, 812774. [Google Scholar] [CrossRef]
- Asami, J.; Shimizu, T. Structural and functional understanding of the toll-like receptors. Protein Sci. 2021, 30, 761–772. [Google Scholar] [CrossRef]
- Finn, O.J. The dawn of vaccines for cancer prevention. Nat. Rev. Immunol. 2018, 18, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.H.; Lee, H.-J.; Ko, H.-J.; Yoon, B.-I.; Choe, J.; Kim, K.-C.; Hahn, T.-W.; Han, J.A.; Choi, S.S.; Jung, Y.M. The TLR7 agonist imiquimod induces anti-cancer effects via autophagic cell death and enhances anti-tumoral and systemic immunity during radiotherapy for melanoma. Oncotarget 2017, 8, 24932. [Google Scholar] [CrossRef] [PubMed]
- Dajon, M.; Iribarren, K.; Cremer, I. Toll-like receptor stimulation in cancer: A pro-and anti-tumor double-edged sword. Immunobiology 2017, 222, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Dyavar, S.R.; Singh, R.; Emani, R.; Pawar, G.P.; Chaudhari, V.D.; Podany, A.T.; Avedissian, S.N.; Fletcher, C.V.; Salunke, D.B. Role of toll-like receptor 7/8 pathways in regulation of interferon response and inflammatory mediators during SARS-CoV2 infection and potential therapeutic options. Biomed. Pharmacother. 2021, 141, 111794. [Google Scholar] [CrossRef]
- Sartorius, R.; Trovato, M.; Manco, R.; D’Apice, L.; De Berardinis, P. Exploiting viral sensing mediated by Toll-like receptors to design innovative vaccines. npj Vaccines 2021, 6, 127. [Google Scholar] [CrossRef] [PubMed]
- Lacarrubba, F.; Nasca, M.R.; Micali, G. Advances in the use of topical imiquimod to treat dermatologic disorders. Ther. Clin. Risk Manag. 2008, 4, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Bhaliya, K.R.; Anwer, M.; Munn, A.; Wei, M.Q. New horizons in cancer immunotherapy: The evolving role of R848 and R837. Mol. Clin. Oncol. 2024, 22, 4. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chen, X.; Cao, Z.; Li, J.; Long, H.; Wu, Y.; Zhang, Z.; Sun, Y. R848 is involved in the antibacterial immune response of golden pompano (Trachinotus ovatus) through TLR7/8-MyD88-NF-κb-signaling pathway. Front. Immunol. 2021, 11, 617522. [Google Scholar] [CrossRef]
- Bhagchandani, S.; Johnson, J.A.; Irvine, D.J. Evolution of Toll-like receptor 7/8 agonist therapeutics and their delivery approaches: From antiviral formulations to vaccine adjuvants. Adv. Drug Deliv. Rev. 2021, 175, 113803. [Google Scholar] [CrossRef]
- Alam, M.M.; Yang, D.; Trivett, A.; Meyer, T.J.; Oppenheim, J.J. HMGN1 and R848 synergistically activate dendritic cells using multiple signaling pathways. Front. Immunol. 2018, 9, 2982. [Google Scholar] [CrossRef]
- Ott, P.A.; Hu-Lieskovan, S.; Chmielowski, B.; Govindan, R.; Naing, A.; Bhardwaj, N.; Margolin, K.; Awad, M.M.; Hellmann, M.D.; Lin, J.J. A phase Ib trial of personalized neoantigen therapy plus anti-PD-1 in patients with advanced melanoma, non-small cell lung cancer, or bladder cancer. Cell 2020, 183, 347–362.e24. [Google Scholar] [CrossRef]
- Wu, D.-W.; Jia, S.-P.; Xing, S.-J.; Ma, H.-l.; Wang, X.; Tang, Q.-Y.; Li, Z.-W.; Wu, Q.; Bai, M.; Zhang, X.-Y. Personalized neoantigen cancer vaccines: Current progression, challenges and a bright future. Clin. Exp. Med. 2024, 24, 229. [Google Scholar] [CrossRef]
- Weber, J.S.; Carlino, M.S.; Khattak, A.; Meniawy, T.; Ansstas, G.; Taylor, M.H.; Kim, K.B.; McKean, M.; Long, G.V.; Sullivan, R.J. Individualised neoantigen therapy mRNA-4157 (V940) plus pembrolizumab versus pembrolizumab monotherapy in resected melanoma (KEYNOTE-942): A randomised, phase 2b study. Lancet 2024, 403, 632–644. [Google Scholar] [CrossRef]
- Braun, D.A.; Moranzoni, G.; Chea, V.; McGregor, B.A.; Blass, E.; Tu, C.R.; Vanasse, A.P.; Forman, C.; Forman, J.; Afeyan, A.B. A neoantigen vaccine generates antitumour immunity in renal cell carcinoma. Nature 2025, 639, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Al Fayez, N.; Nassar, M.S.; Alshehri, A.A.; Alnefaie, M.K.; Almughem, F.A.; Alshehri, B.Y.; Alawad, A.O.; Tawfik, E.A. Recent advancement in mRNA vaccine development and applications. Pharmaceutics 2023, 15, 1972. [Google Scholar] [CrossRef] [PubMed]
- Bottomly, D.; Long, N.; Schultz, A.R.; Kurtz, S.E.; Tognon, C.E.; Johnson, K.; Abel, M.; Agarwal, A.; Avaylon, S.; Benton, E. Integrative analysis of drug response and clinical outcome in acute myeloid leukemia. Cancer Cell 2022, 40, 850–864.e9. [Google Scholar] [CrossRef] [PubMed]
- Chiocca, E.A.; Rabkin, S.D. Oncolytic viruses and their application to cancer immunotherapy. Cancer Immunol. Res. 2014, 2, 295–300. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, D.; Li, S.; Ke, L. Role of tumor mutational burden in patients with urothelial carcinoma treated with immune checkpoint inhibitors: A systematic review and meta-analysis. Front. Immunol. 2025, 16, 1592761. [Google Scholar] [CrossRef]
- Foy, S.P.; Jacoby, K.; Bota, D.A.; Hunter, T.; Pan, Z.; Stawiski, E.; Ma, Y.; Lu, W.; Peng, S.; Wang, C.L. Non-viral precision T cell receptor replacement for personalized cell therapy. Nature 2023, 615, 687–696. [Google Scholar] [CrossRef]
- Klempner, S.J.; Fabrizio, D.; Bane, S.; Reinhart, M.; Peoples, T.; Ali, S.M.; Sokol, E.S.; Frampton, G.; Schrock, A.B.; Anhorn, R. Tumor mutational burden as a predictive biomarker for response to immune checkpoint inhibitors: A review of current evidence. Oncologist 2020, 25, e147–e159. [Google Scholar] [CrossRef]

| Challenge | Description | Proposed Solutions/Strategies | Ongoing Trials | References |
|---|---|---|---|---|
| Lack of predictive biomarkers | PD-L1 and TMB are inconsistent predictors of ICI response | Composite biomarkers: gene expression, peripheral immune profiling | NCT03606967: multiomic biomarker analysis in NSCLC | [43] |
| Primary resistance | “Cold” tumors lack immune infiltration | Combination with chemo, radiotherapy, or STING agonists to enhance immunogenicity | NCT03892525: STING agonist + PD-1 blockade in solid tumors | [44] |
| Acquired resistance | Tumor escapes after initial ICI response | Dual checkpoint blockade (e.g., PD-1 + LAG-3), epigenetic therapy | NCT03686202: anti–PD-1 + LAG-3 in resistant NSCLC | [45] |
| Immune-related adverse events | Autoimmunity affecting skin, GI, lungs, etc. | IL-6 blockades, corticosteroids, better screening algorithms | NCT04167137: tocilizumab for irAEs | [46] |
| Development Stage | Vaccine Type | Mechanism of Action | Examples/Status | References |
|---|---|---|---|---|
| Preclinical | Bacterial ghost vaccines | Empty bacterial envelopes deliver tumor antigens, enhance innate immunity | Animal models show promise in lung cancer | [52,53] |
| Oncolytic virus-based vaccines | Selectively replicate in tumors, trigger immune response | Adenovirus and HSV-based vaccines under development | [54] | |
| Early-phase clinical trials | mRNA vaccines | Encode neoantigens, induce antigen-specific T cell response | NCT04526899: personalized mRNA vaccines in NSCLC | [55] |
| DNA vaccines | Plasmid DNA delivered by electroporation induces immune response | NCT03893955: DNA vaccine targeting surviving | [56] | |
| Peptide-based vaccines | Tumor-associated antigens presented on MHC molecules | NCT04397900: multi-epitope vaccine trials | [57] | |
| Advanced/approved | Dendritic cell (DC) vaccines | Autologous DCs loaded with tumor antigens and re-infused | NCT01948141: DC vaccine with chemo in NSCLC | [58] |
| Allogeneic whole-cell vaccines | Genetically modified tumor cells as broad antigen source | Belagenpumatucel-L in Phase III trials (not FDA-approved) | [59] |
| Category | Adjuvant/Agent | Mechanism of Action | Clinical Status/Notes | References |
|---|---|---|---|---|
| Traditional adjuvants | TLR agonists (CpG ODN, imiquimod, MPL) | Activate pattern recognition receptors (TLR7, TLR9, TLR4), enhance dendritic cell maturation, type I IFN secretion | Widely used in cancer vaccines; imiquimod FDA-approved for topical use; effective in boosting immune response | [71,72] |
| Aluminum salts | Promote inflammasome activation, support humoral immunity | Common in preventive vaccines; less effective in eliciting cytotoxic T cell responses | [71] | |
| Poly-ICLC | Synthetic dsRNA analog activates TLR3 and MDA5 pathways, stimulates cytokine production | Used in early-stage cancer vaccine trials; boosts T cell activation | [71] | |
| Emerging adjuvants | STING agonists (cGAMP, CDNs) | Stimulate cytosolic DNA sensing pathways, induce strong type I IFN response | Early-phase clinical trials; potent activators of antitumor immunity but require safety optimization | [73,74] |
| CD40 agonists | Activate dendritic cells and B cells, enhance antigen cross-presentation | Promising in combination therapies; clinical use limited by toxicity concerns | [67] | |
| Cytokines (GM-CSF) | Recruit and activate dendritic cells at vaccination sites | Mixed clinical results; potential to expand immunosuppressive cells under some conditions | [76,77] | |
| Inorganic nanoparticles (TiO2, Mn-based) | Enhance antigen cross-presentation, modulate STING pathway | Preclinical stage; promising delivery platforms with immunomodulatory effects | [80,82] | |
| Imidazoquinolines (IMDs) | Imiquimod (R837), resiquimod (R848) | TLR7/8 agonists; induce NF-κB and IRF activation, promote IL-12 and type I IFN production | Imiquimod FDA-approved for topical cancers; resiquimod in clinical trials; limited by systemic toxicity | [95,96] |
| Immunotherapy Strategy | Current Stage | Estimated Clinical Timeline | Notes |
|---|---|---|---|
| Personalized neoantigen vaccines | Early clinical trials | 3–7 years | mRNA and peptide vaccines showing promising immune responses and manageable safety profiles. |
| Combination with immune checkpoint inhibitors | Clinical trials/ongoing use | 3–5 years | Synergistic effects are well documented, rapidly moving toward broader clinical adoption. |
| Viral vector and oncolytic virus platforms | Preclinical/early trials | 5–10 years | High potential but requires optimization for safety and efficacy. |
| STING and CD40 agonists as adjuvants | Preclinical/Phase I trials | 5–10+ years | Promising immune activation, but toxicity and delivery need refinement. |
| Bacterial ghost and nanoparticle-based vaccines | Preclinical | 7–15 years | Innovative delivery systems with encouraging results in animal models; translation pending. |
| Predictive biomarker development | Early clinical/exploratory | 3–7 years | Biomarkers for patient stratification improving vaccine personalization and response tracking. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhaliya, K.; Anwer, M.; Wei, M.Q. Emerging Therapeutic Strategies for Lung Cancer: The Role of Immunotherapy and HPV-Targeted Cancer Vaccines. Vaccines 2025, 13, 957. https://doi.org/10.3390/vaccines13090957
Bhaliya K, Anwer M, Wei MQ. Emerging Therapeutic Strategies for Lung Cancer: The Role of Immunotherapy and HPV-Targeted Cancer Vaccines. Vaccines. 2025; 13(9):957. https://doi.org/10.3390/vaccines13090957
Chicago/Turabian StyleBhaliya, Krupa, Muneera Anwer, and Ming Q. Wei. 2025. "Emerging Therapeutic Strategies for Lung Cancer: The Role of Immunotherapy and HPV-Targeted Cancer Vaccines" Vaccines 13, no. 9: 957. https://doi.org/10.3390/vaccines13090957
APA StyleBhaliya, K., Anwer, M., & Wei, M. Q. (2025). Emerging Therapeutic Strategies for Lung Cancer: The Role of Immunotherapy and HPV-Targeted Cancer Vaccines. Vaccines, 13(9), 957. https://doi.org/10.3390/vaccines13090957





