Evaluation of Cholera Antigen-Specific Gut-Homing β7-Positive Antibody-Secreting Cells in the Systemic Circulation of Oral Cholera Vaccinees Receiving Doses at Different Intervals
Abstract
1. Introduction
2. Materials and Methods
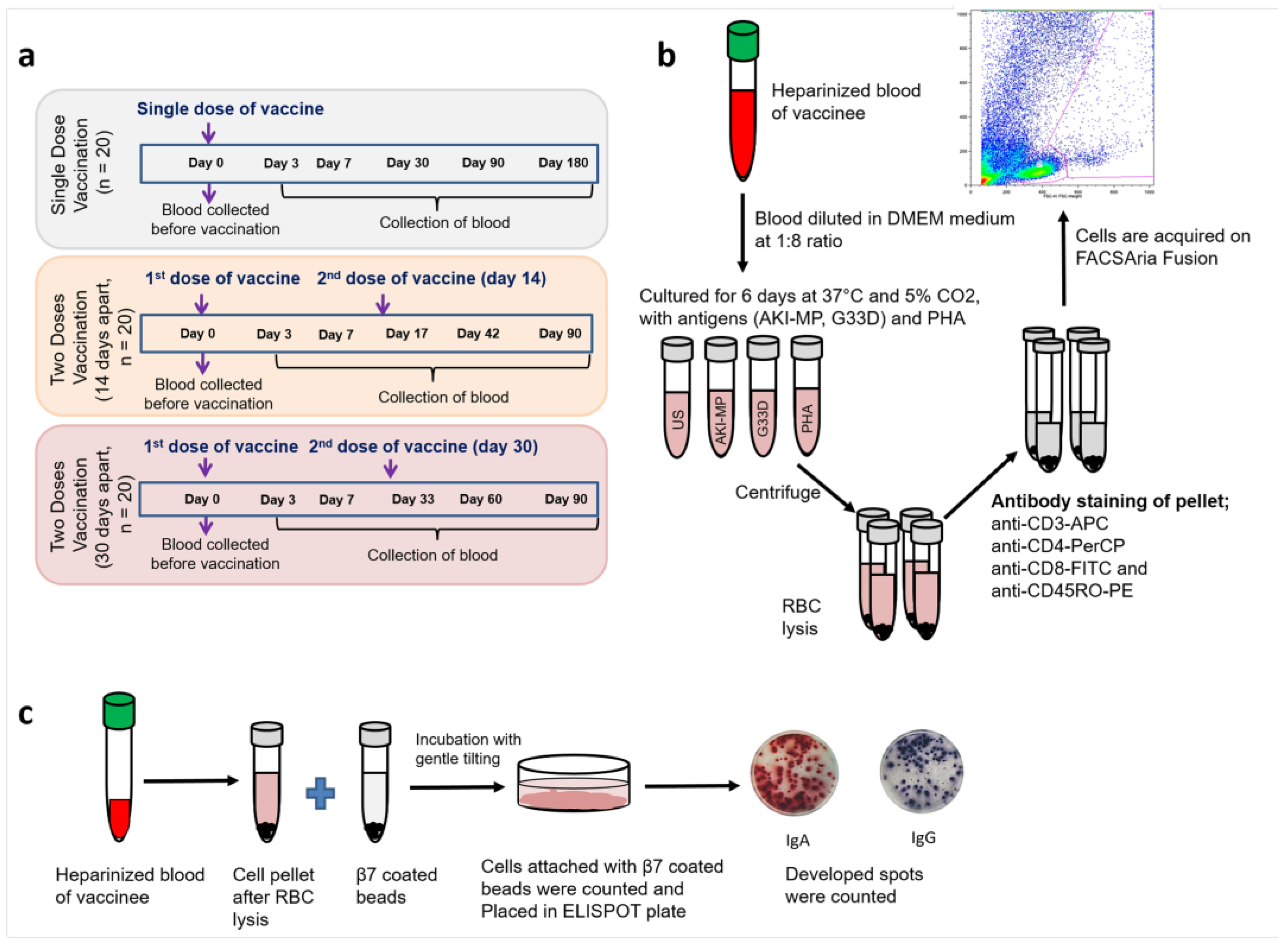
2.1. Study Groups and Recruitment of Participants
2.2. T-Cell Proliferation Analysis
2.3. Gut-Homing Antibody Secreting Cells (ASCs) Assay
2.4. LPS-Specific IgA and IgG Antibody Responses in Plasma
2.5. Statistical Analysis
3. Results
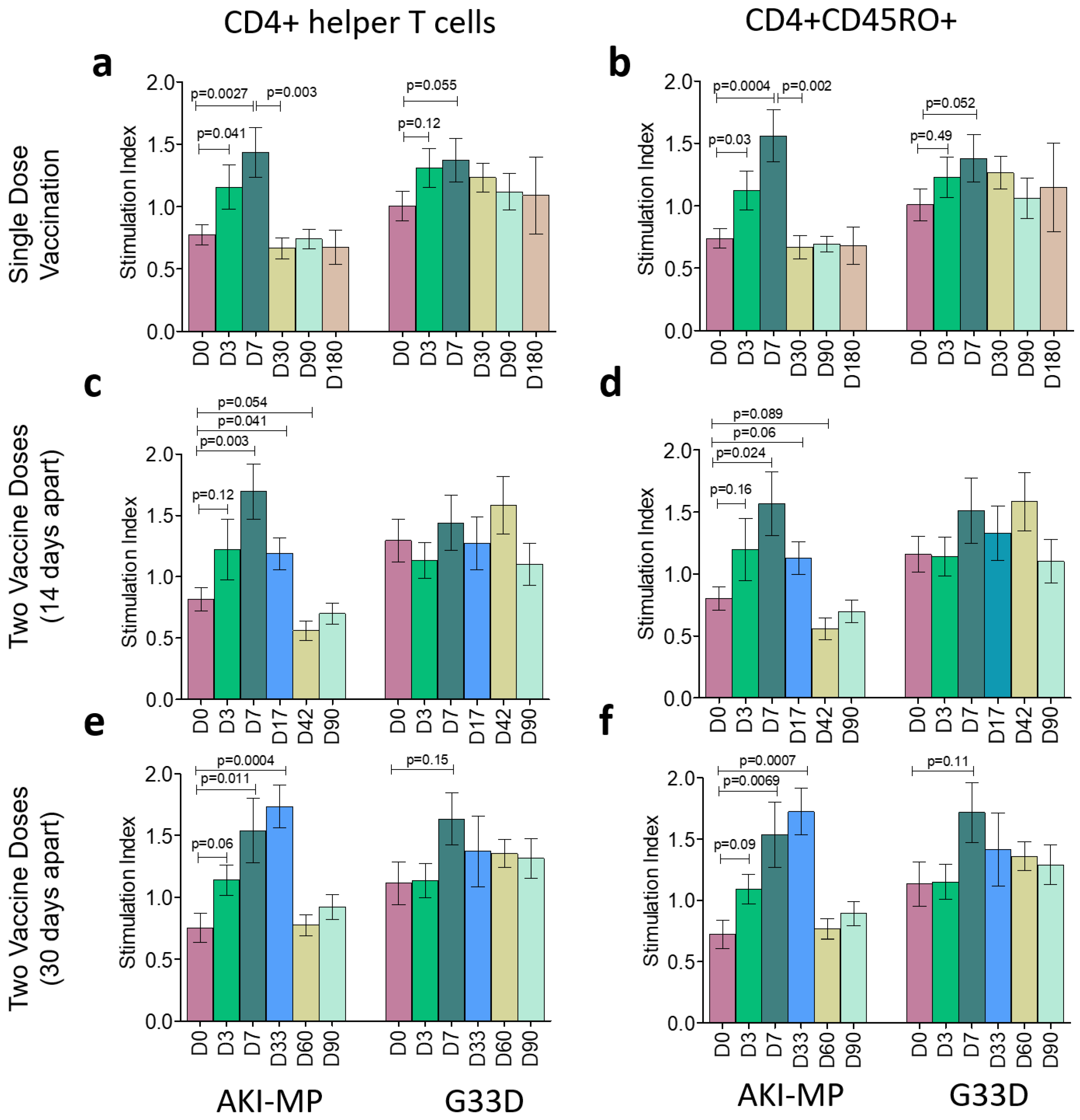
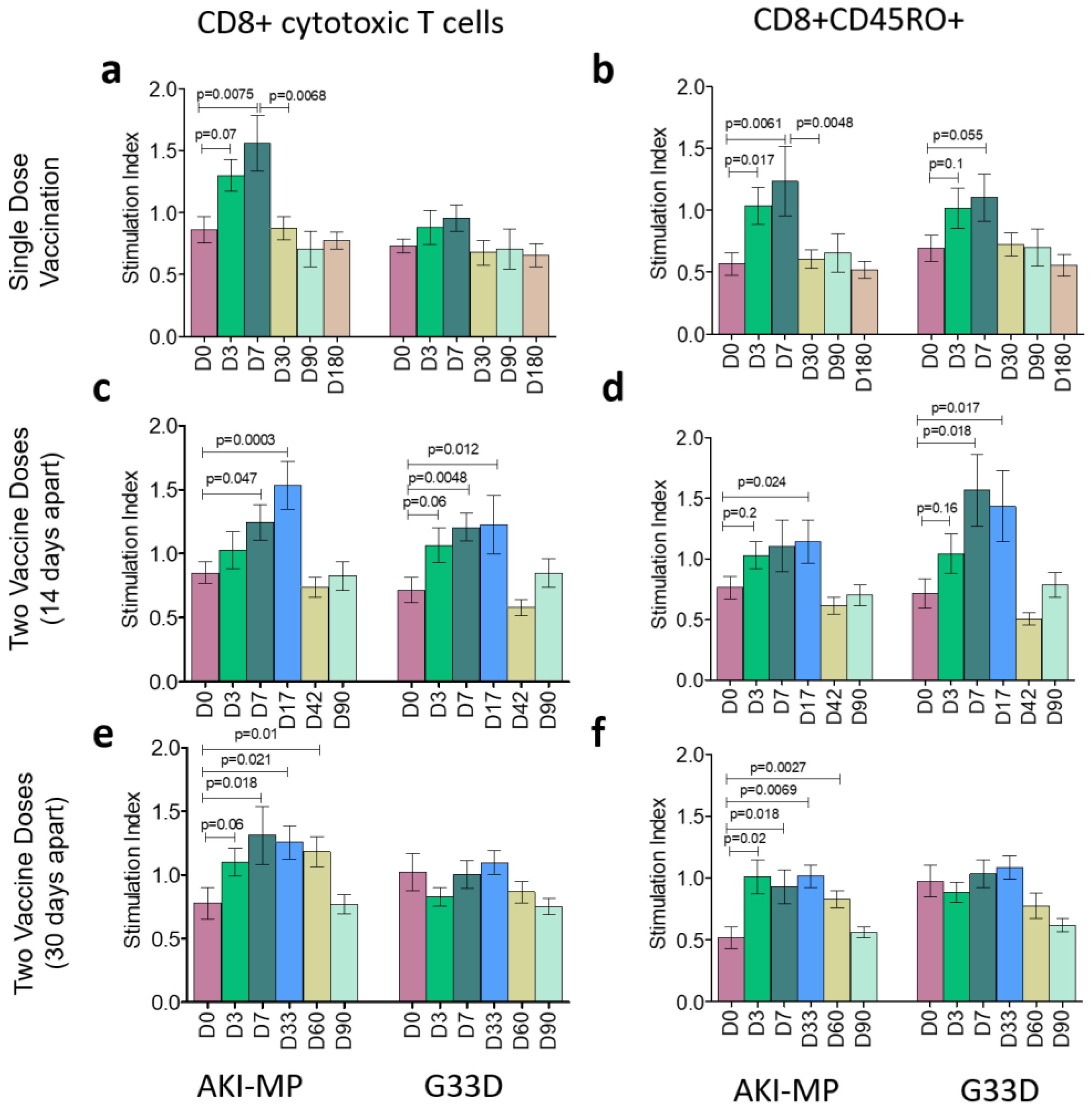
3.1. Frequency of CD4+ and CD8+ Populations with Memory T Cells
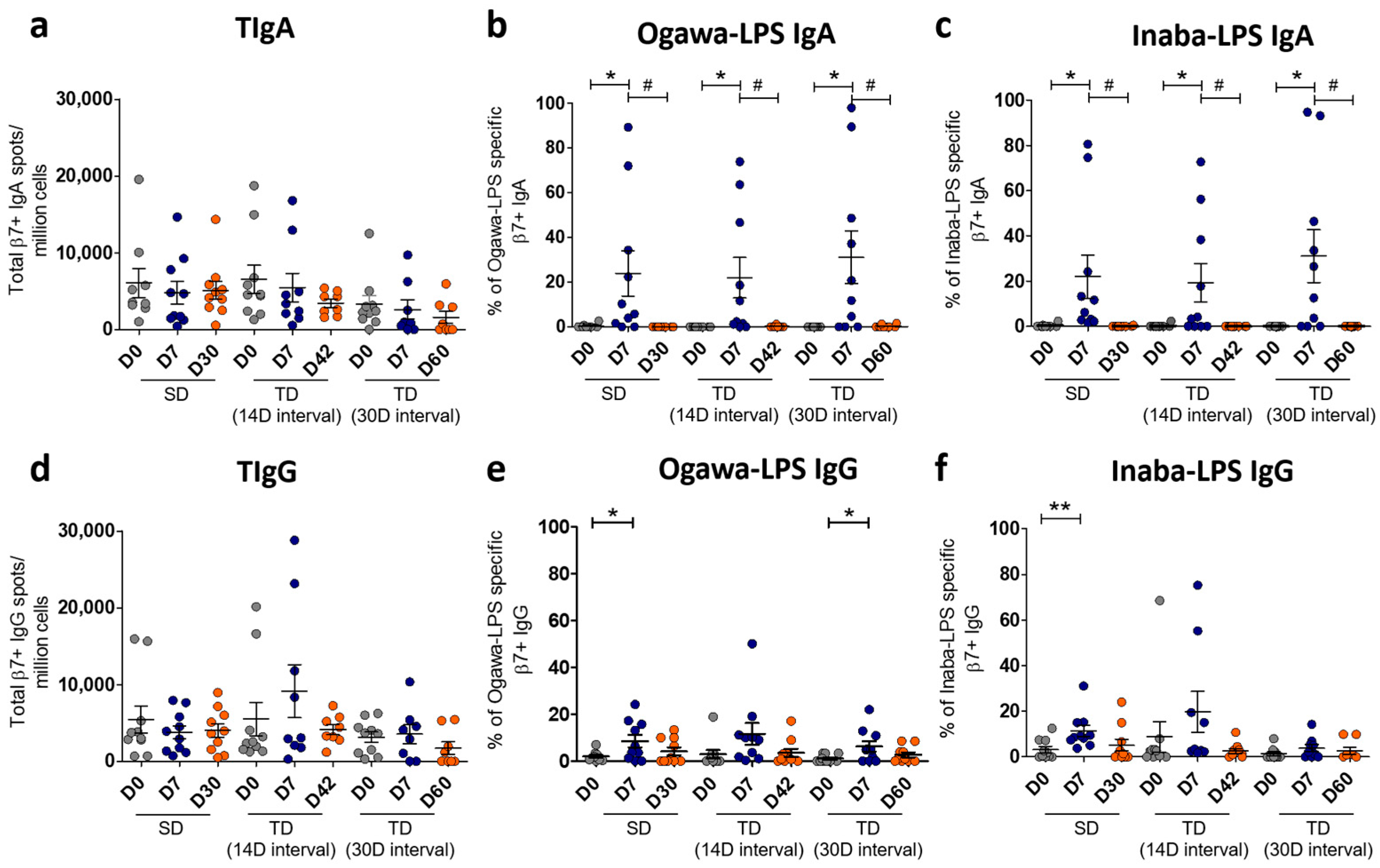
3.2. Total Gut-Homing LPS-Specific Immunoglobulin Responses Among the Vaccine Groups
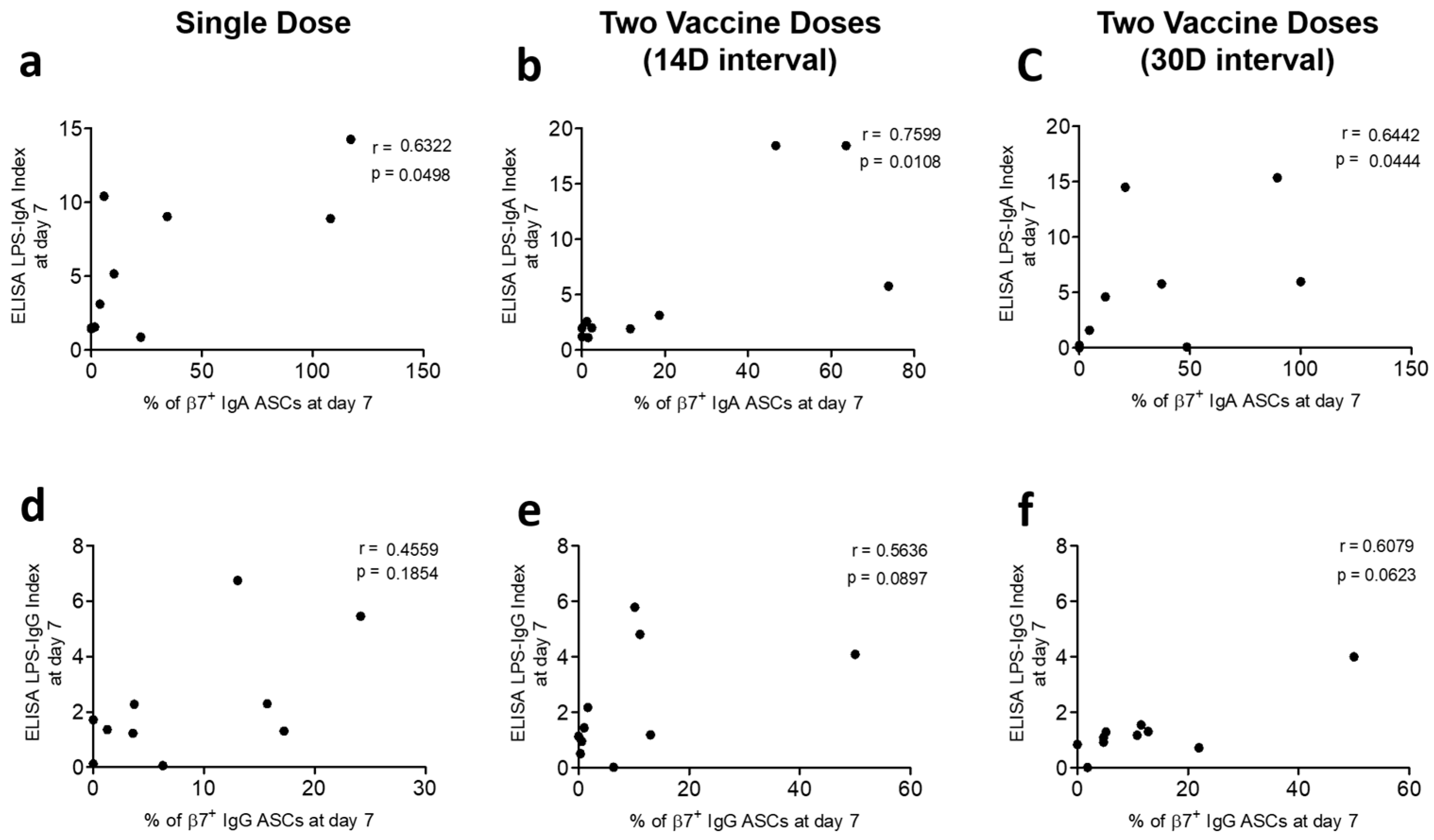
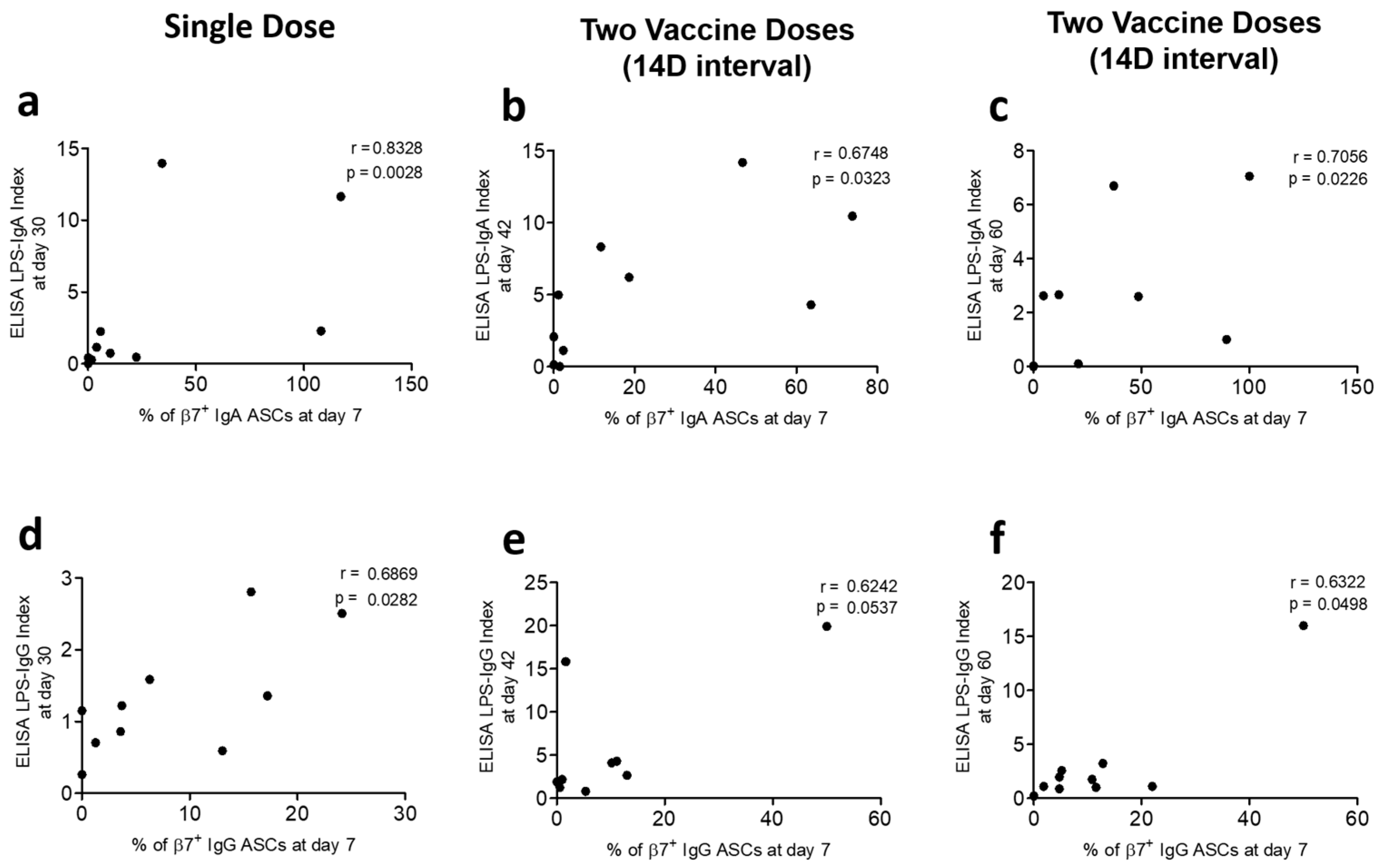
3.3. Correlation of Gut-Homing ASCs and Circulatory Antibody Responses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OCV | Oral Cholera Vaccine |
| LPS | Lipopolysaccharide |
| OSP | O-specific polysaccharide |
| MBC | Memory B cell |
| ASC | Antibody-secretory cell |
| FASCIA | Flow cytometric assay of cell-mediated immune response in activated whole blood |
| DMEM | Dulbecco’s Modified Eagle Medium |
| FACS | Fluorescence-activated cell sorter |
| PHA | Phytohemagglutinin |
| RPMI | Roswell Park Memorial Institute |
| AEC | 3-amino-9-ethylcarbazole |
| BCIP-NBT | 5-bromo-4-chloro-3-indolylphosphate–nitroblue tetrazolium |
| ELISA | Enzyme-linked immunosorbent assay |
| ELISPOT | Enzyme Linked Immunospot |
| TIgA | Total immunoglobulin A |
| TIgG | Total immunoglobulin G |
| TcpA | Toxin-coregulated pilus |
References
- WHO. Cholera; WHO: Geneva, Switzerland, 2024. [Google Scholar]
- Legros, D. Global Cholera Epidemiology: Opportunities to Reduce the Burden of Cholera by 2030. J. Infect. Dis. 2018, 218, S137–S140. [Google Scholar] [CrossRef] [PubMed]
- Chandralekha, C.; Veligandla, G.; Vanaja, R. Emergence of Vibrio cholerae Serotype Hikojima in Northern Tamil Nadu. Indian J. Community Med. 2011, 36, 165–166. [Google Scholar] [CrossRef] [PubMed]
- Aktar, A.; Rahman, M.A.; Afrin, S.; Faruk, M.O.; Uddin, T.; Akter, A.; Sami, M.I.N.; Yasmin, T.; Chowdhury, F.; Khan, A.I.; et al. O-Specific Polysaccharide-Specific Memory B Cell Responses in Young Children, Older Children, and Adults Infected with Vibrio cholerae O1 Ogawa in Bangladesh. Clin. Vaccine Immunol. 2016, 23, 427–435. [Google Scholar] [CrossRef]
- Stroeher, U.H.; Karageorgos, L.E.; Morona, R.; Manning, P.A. Serotype conversion in Vibrio cholerae O1. Proc. Natl. Acad. Sci. USA 1992, 89, 2566–2570. [Google Scholar] [CrossRef] [PubMed]
- Hisatsune, K.; Kondo, S.; Isshiki, Y.; Iguchi, T.; Haishima, Y. Occurrence of 2-O-methyl-N-(3-deoxy-L-glycero-tetronyl)-D-perosamine (4-amino-4,6-dideoxy-D-manno-pyranose) in lipopolysaccharide from Ogawa but not from Inaba O forms of O1 Vibrio cholerae. Biochem. Biophys. Res. Commun. 1993, 190, 302–307. [Google Scholar] [CrossRef]
- Wang, J.; Villeneuve, S.; Zhang, J.; Lei, P.; Miller, C.E.; Lafaye, P.; Nato, F.; Szu, S.C.; Karpas, A.; Bystricky, S.; et al. On the antigenic determinants of the lipopolysaccharides of Vibrio cholerae O:1, serotypes Ogawa and Inaba. J. Biol. Chem. 1998, 273, 2777–2783. [Google Scholar] [CrossRef]
- López-Gigosos, R.M.; Plaza, E.; Díez-Díaz, R.M.; Calvo, M.J. Vaccination strategies to combat an infectious globe: Oral cholera vaccines. J. Glob. Infect. Dis. 2011, 3, 56–62. [Google Scholar] [CrossRef]
- Akter, A.; Dash, P.; Aktar, A.; Jahan, S.R.; Afrin, S.; Basher, S.R.; Hakim, A.; Lisa, A.K.; Chowdhury, F.; Khan, A.I.; et al. Induction of systemic, mucosal and memory antibody responses targeting Vibrio cholerae O1 O-specific polysaccharide (OSP) in adults following oral vaccination with an oral killed whole cell cholera vaccine in Bangladesh. PLoS Negl. Trop. Dis. 2019, 13, e0007634. [Google Scholar] [CrossRef]
- Baik, Y.O.; Choi, S.K.; Olveda, R.M.; Espos, R.A.; Ligsay, A.D.; Montellano, M.B.; Yeam, J.S.; Yang, J.S.; Park, J.Y.; Kim, D.R.; et al. A randomized, non-inferiority trial comparing two bivalent killed, whole cell, oral cholera vaccines (Euvichol vs Shanchol) in the Philippines. Vaccine 2015, 33, 6360–6365. [Google Scholar] [CrossRef]
- Kozlowski, P.A.; Cu-Uvin, S.; Neutra, M.R.; Flanigan, T.P. Comparison of the oral, rectal, and vaginal immunization routes for induction of antibodies in rectal and genital tract secretions of women. Infect. Immun. 1997, 65, 1387–1394. [Google Scholar] [CrossRef]
- Quiding, M.; Nordstrom, I.; Kilander, A.; Andersson, G.; Hanson, L.A.; Holmgren, J.; Czerkinsky, C. Intestinal immune responses in humans. Oral cholera vaccination induces strong intestinal antibody responses and interferon-gamma production and evokes local immunological memory. J. Clin. Investig. 1991, 88, 143–148. [Google Scholar] [CrossRef]
- Lycke, N.; Lindholm, L.; Holmgren, J. Cholera antibody production in vitro by peripheral blood lymphocytes following oral immunization of humans and mice. Clin. Exp. Immunol. 1985, 62, 39–47. [Google Scholar] [PubMed]
- Kantele, A.; Kantele, J.M.; Savilahti, E.; Westerholm, M.; Arvilommi, H.; Lazarovits, A.; Butcher, E.C.; Mäkelä, P.H. Homing potentials of circulating lymphocytes in humans depend on the site of activation: Oral, but not parenteral, typhoid vaccination induces circulating antibody-secreting cells that all bear homing receptors directing them to the gut. J. Immunol. 1997, 158, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Mora, J.R.; von Andrian, U.H. Differentiation and homing of IgA-secreting cells. Mucosal Immunol. 2008, 1, 96–109. [Google Scholar] [CrossRef]
- Inoue, T.; Kurosaki, T. Memory B cells. Nat. Rev. Immunol. 2024, 24, 5–17. [Google Scholar] [CrossRef]
- Svahn, A.; Linde, A.; Thorstensson, R.; Karlen, K.; Andersson, L.; Gaines, H. Development and evaluation of a flow-cytometric assay of specific cell-mediated immune response in activated whole blood for the detection of cell-mediated immunity against varicella-zoster virus. J. Immunol. Methods 2003, 277, 17–25. [Google Scholar] [CrossRef]
- Helmuth, R.; Achtman, M. Cell-cell interactions in conjugating Escherichia coli: Purification of F pili with biological activity. Proc. Natl. Acad. Sci. USA 1978, 75, 1237–1241. [Google Scholar] [CrossRef]
- Saletti, G.; Çuburu, N.; Yang, J.S.; Dey, A.; Czerkinsky, C. Enzyme-linked immunospot assays for direct ex vivo measurement of vaccine-induced human humoral immune responses in blood. Nat. Protoc. 2013, 8, 1073–1087. [Google Scholar] [CrossRef]
- WHO. Cholera; WHO: Geneva, Switzerland, 2013. [Google Scholar]
- WHO. Cholera Annual Report; WHO: Geneva, Switzerland, 2014. [Google Scholar]
- Clemens, J.D.; Sack, D.A.; Harris, J.R.; Van Loon, F.; Chakraborty, J.; Ahmed, F.; Rao, M.R.; Khan, M.R.; Yunus, M.; Huda, N.; et al. Field trial of oral cholera vaccines in Bangladesh: Results from three-year follow-up. Lancet 1990, 335, 270–273. [Google Scholar] [CrossRef]
- Ivers, L.C.; Teng, J.E.; Lascher, J.; Raymond, M.; Weigel, J.; Victor, N.; Jerome, J.G.; Hilaire, I.J.; Almazor, C.P.; Ternier, R.; et al. Use of oral cholera vaccine in Haiti: A rural demonstration project. Am. J. Trop. Med. Hyg. 2013, 89, 617–624. [Google Scholar] [CrossRef]
- Luquero, F.J.; Grout, L.; Ciglenecki, I.; Sakoba, K.; Traore, B.; Heile, M.; Dialo, A.A.; Itama, C.; Serafini, M.; Legros, D.; et al. First outbreak response using an oral cholera vaccine in Africa: Vaccine coverage, acceptability and surveillance of adverse events, Guinea, 2012. PLoS Negl. Trop. Dis. 2013, 7, e2465. [Google Scholar] [CrossRef]
- Peprah, D.; Palmer, J.J.; Rubin, G.J.; Abubakar, A.; Costa, A.; Martin, S.; Perea, W.; Larson, H.J. Perceptions of oral cholera vaccine and reasons for full, partial and non-acceptance during a humanitarian crisis in South Sudan. Vaccine 2016, 34, 3823–3827. [Google Scholar] [CrossRef] [PubMed]
- Sur, D.; Kanungo, S.; Sah, B.; Manna, B.; Ali, M.; Paisley, A.M.; Niyogi, S.K.; Park, J.K.; Sarkar, B.; Puri, M.K.; et al. Efficacy of a low-cost, inactivated whole-cell oral cholera vaccine: Results from 3 years of follow-up of a randomized, controlled trial. PLoS Negl. Trop. Dis. 2011, 5, e1289. [Google Scholar] [CrossRef] [PubMed]
- Kanungo, S.; Desai, S.N.; Nandy, R.K.; Bhattacharya, M.K.; Kim, D.R.; Sinha, A.; Mahapatra, T.; Yang, J.S.; Lopez, A.L.; Manna, B.; et al. Flexibility of oral cholera vaccine dosing-a randomized controlled trial measuring immune responses following alternative vaccination schedules in a cholera hyper-endemic zone. PLoS Negl. Trop. Dis. 2015, 9, e0003574. [Google Scholar] [CrossRef]
- Capeding, M.R.Z.; Gonzales, M.; Dhingra, M.S.; D’Cor, N.A.; Midde, V.J.; Patnaik, B.N.; Thollot, Y.; Desauziers, E. Safety and immunogenicity of the killed bivalent (O1 and O139) whole-cell cholera vaccine in the Philippines. Hum. Vaccin Immunother. 2017, 13, 2232–2239. [Google Scholar] [CrossRef] [PubMed]
- Weil, A.A.; Arifuzzaman, M.; Bhuiyan, T.R.; LaRocque, R.C.; Harris, A.M.; Kendall, E.A.; Hossain, A.; Tarique, A.A.; Sheikh, A.; Chowdhury, F.; et al. Memory T-cell responses to Vibrio cholerae O1 infection. Infect. Immun. 2009, 77, 5090–5096. [Google Scholar] [CrossRef]
- Bhuiyan, T.R.; Lundin, S.B.; Khan, A.I.; Lundgren, A.; Harris, J.B.; Calderwood, S.B.; Qadri, F. Cholera caused by Vibrio cholerae O1 induces T-cell responses in the circulation. Infect. Immun. 2009, 77, 1888–1893. [Google Scholar] [CrossRef]
| Characteristics of Participants: (N = 60) | ||||
|---|---|---|---|---|
| Single Dose (N = 20) | Two Doses with 14-Day Interval (N = 20) | Two Doses with 30-Day Interval (N = 20) | ||
| Age, [median (range)] | 28.21 [22–45] years | 27.33 [18–44] years | 28.67 [18–42] years | |
| Sex | No. (%) female | 9 (45) | 10 (50) | 11 (55) |
| No. (%) male | 11 (55) | 10 (50) | 9 (45) | |
| Blood type: No. (%) | O | 4 (20) | 8 (40) | 9 (45) |
| A | 5 (25) | 4 (20) | 5 (25) | |
| B | 8 (40) | 7 (35) | 5 (25) | |
| AB | 3 (15) | 1 (5) | 1 (5) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karmakar, P.C.; Rashu, R.; Hoq, M.R.; Salma, U.; Islam, K.; Jahan, N.; Nishat, N.S.; Akter, A.; Jahan, S.R.; Dash, P.; et al. Evaluation of Cholera Antigen-Specific Gut-Homing β7-Positive Antibody-Secreting Cells in the Systemic Circulation of Oral Cholera Vaccinees Receiving Doses at Different Intervals. Vaccines 2025, 13, 919. https://doi.org/10.3390/vaccines13090919
Karmakar PC, Rashu R, Hoq MR, Salma U, Islam K, Jahan N, Nishat NS, Akter A, Jahan SR, Dash P, et al. Evaluation of Cholera Antigen-Specific Gut-Homing β7-Positive Antibody-Secreting Cells in the Systemic Circulation of Oral Cholera Vaccinees Receiving Doses at Different Intervals. Vaccines. 2025; 13(9):919. https://doi.org/10.3390/vaccines13090919
Chicago/Turabian StyleKarmakar, Polash Chandra, Rasheduzzaman Rashu, Mohammad Rubel Hoq, Umme Salma, Kamrul Islam, Nusrat Jahan, Naoshin Sharmin Nishat, Aklima Akter, Sultana Rownok Jahan, Pinki Dash, and et al. 2025. "Evaluation of Cholera Antigen-Specific Gut-Homing β7-Positive Antibody-Secreting Cells in the Systemic Circulation of Oral Cholera Vaccinees Receiving Doses at Different Intervals" Vaccines 13, no. 9: 919. https://doi.org/10.3390/vaccines13090919
APA StyleKarmakar, P. C., Rashu, R., Hoq, M. R., Salma, U., Islam, K., Jahan, N., Nishat, N. S., Akter, A., Jahan, S. R., Dash, P., Saha, A., Ryan, E. T., Qadri, F., & Bhuiyan, T. R. (2025). Evaluation of Cholera Antigen-Specific Gut-Homing β7-Positive Antibody-Secreting Cells in the Systemic Circulation of Oral Cholera Vaccinees Receiving Doses at Different Intervals. Vaccines, 13(9), 919. https://doi.org/10.3390/vaccines13090919







