Long COVID Syndrome Prevalence in 2025 in an Integral Healthcare Consortium in the Metropolitan Area of Barcelona: Persistent and Transient Symptoms
Abstract
1. Introduction
2. Materials and Methods
2.1. Socioeconomic Environment
2.2. Quantification of Long COVID Syndrome Prevalence and Thrombosis
2.3. Survey: Persistent Symptoms
2.4. Statistical Analysis
3. Results
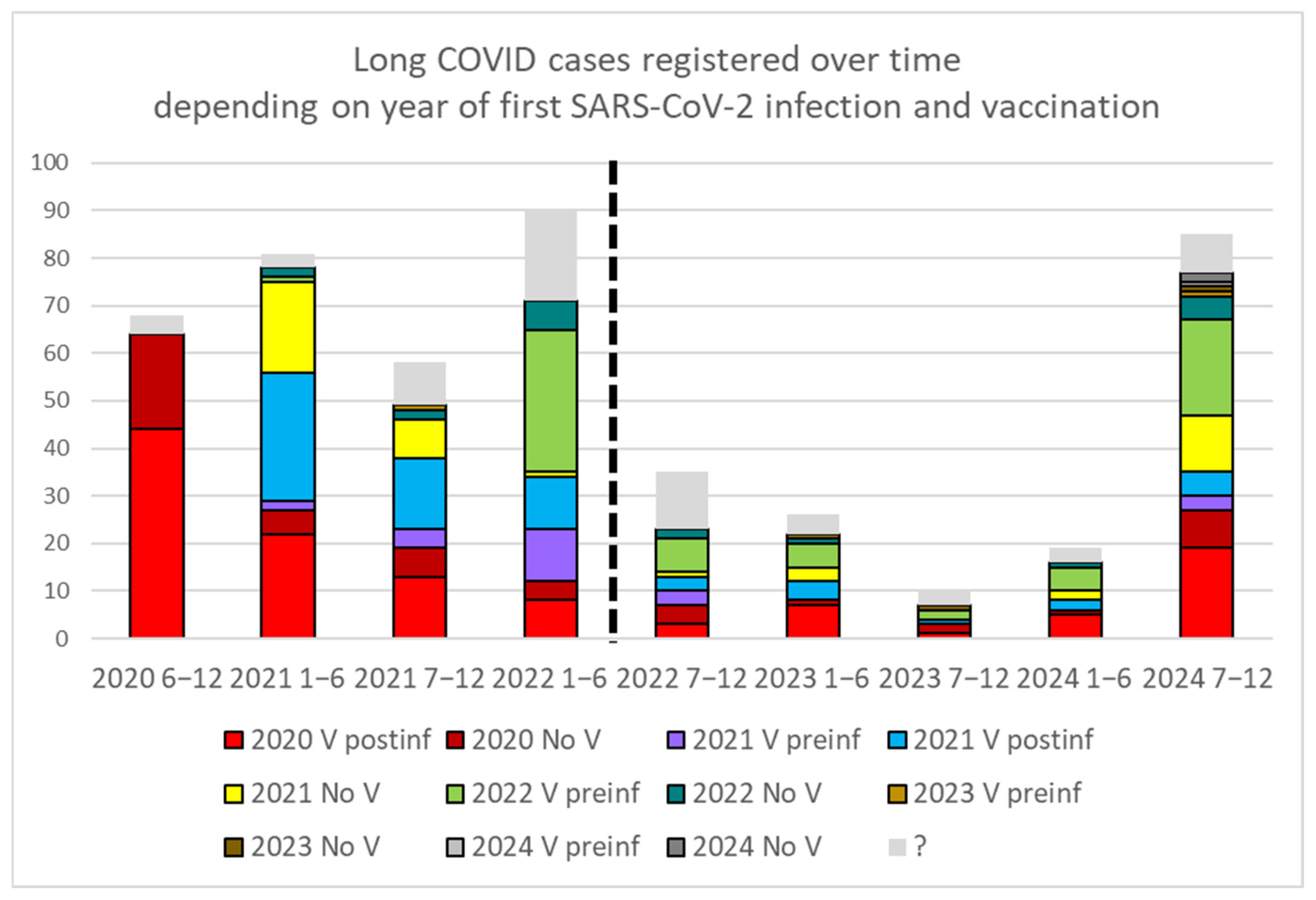
3.1. Prevalence of Long COVID and Number of Infections
3.2. Survey: Reported Persistent and Transient Symptoms
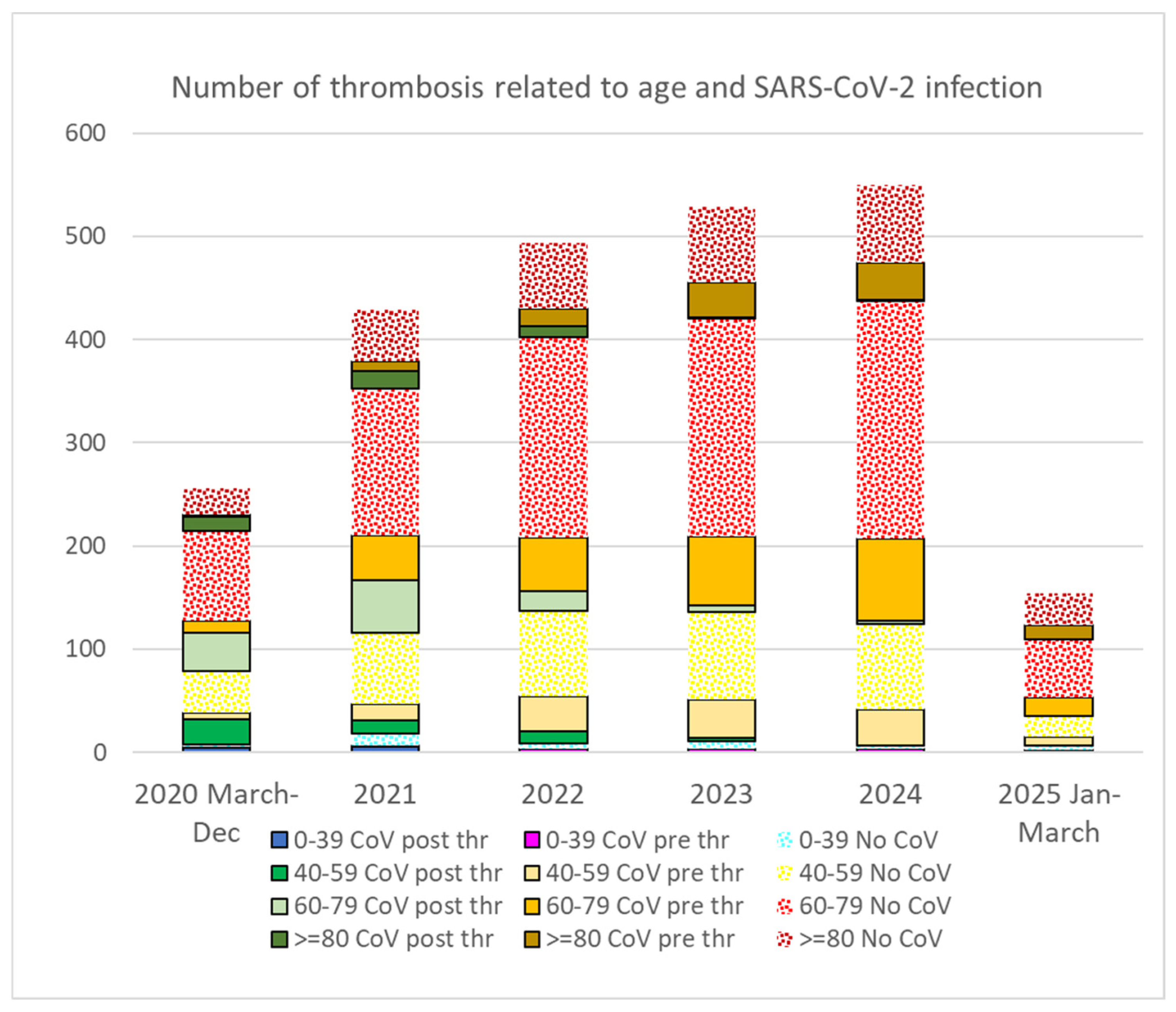
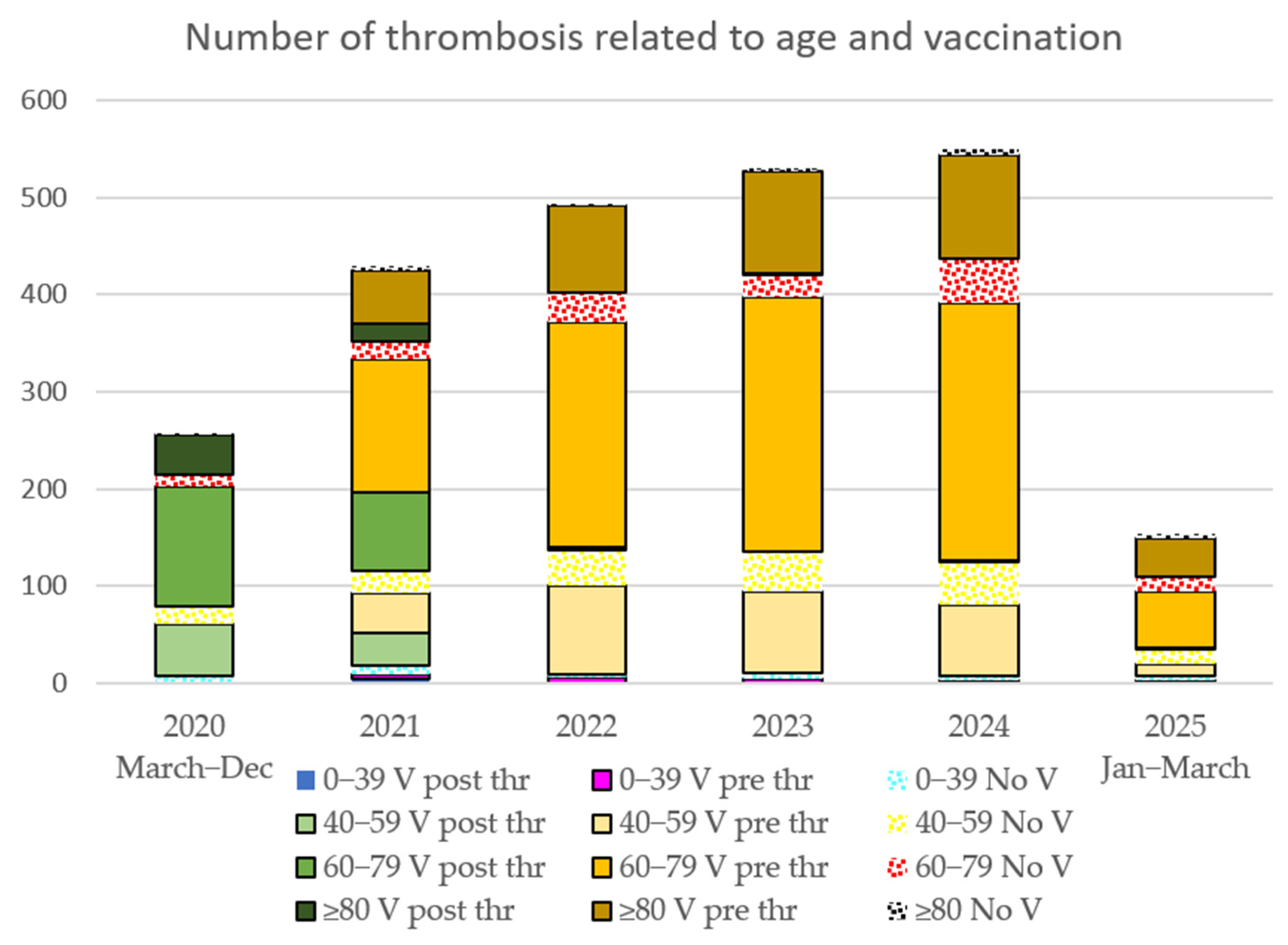
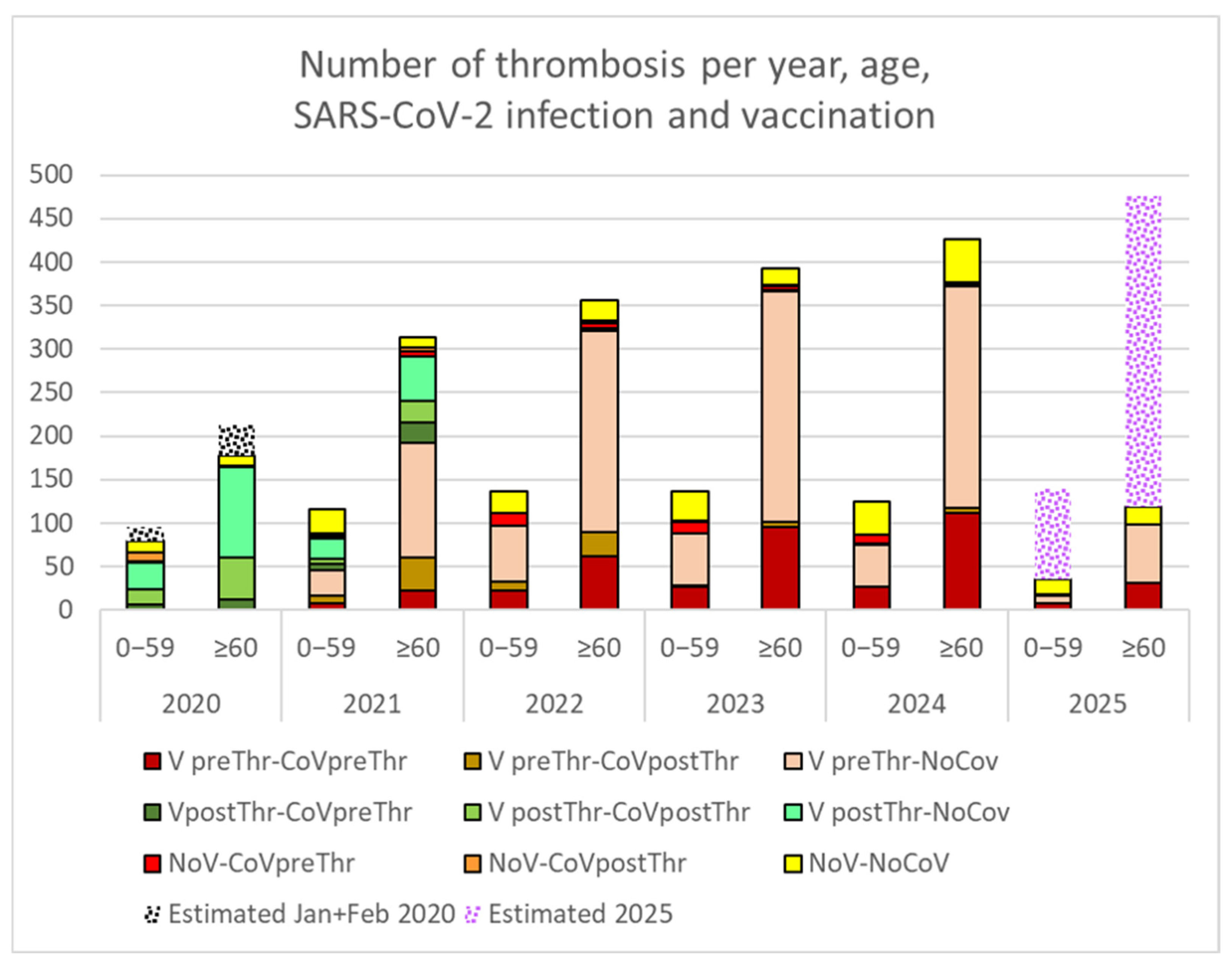
3.3. Thrombotic Events
4. Discussion
4.1. Increased Prevalence with Multiple Infections
4.2. Increase in Thrombotic Events
4.3. Persistent and Transient Symptoms—Functional Implications
4.4. Long COVID, Public Health and Future
4.5. Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LC | Long COVID |
| No V | Non-vaccinated |
| Preinf | Previous to the COVID-19 infection |
| Postinf | Posterior to the COVID-19 infection |
| Pre Thr | Previous to the thrombosis |
| Post Thr | Posterior to the thrombosis |
| CoV | COVID-19 infection |
| CST | Consorci Sanitari de Terrassa |
Appendix A
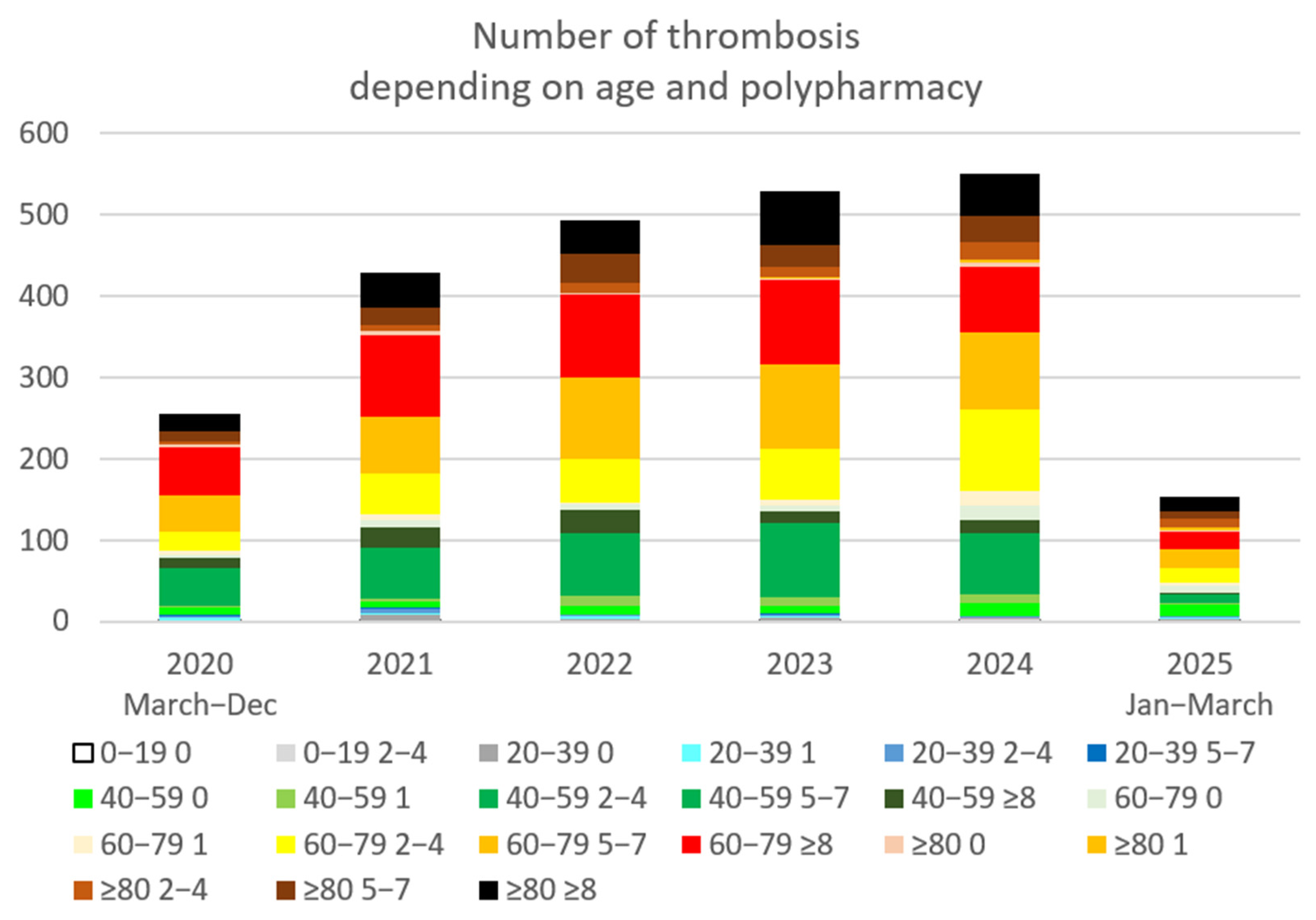
| Age-Polypharmacy | 2020 | 2021 | 2022 | 2023 | 2024 | 2025 | No Thrombus | OR 2024-21/p | |
|---|---|---|---|---|---|---|---|---|---|
| Year of Thrombosis | |||||||||
| 0–39 | |||||||||
| 0 | 2 | 5 | 2 | 4 | 3 | 3 | 76,579 | 0.6 | |
| 1 | 2 | 1 | 1 | 3 | 1 | 7205 | 0.0 | ||
| 2–4 | 1 | 2 | 3 | 1 | 3 | 2 | 3723 | 1.5 | |
| 5–7 | 2 | 1 | 2 | 1 | 1 | 281 | 1.0 | ||
| ≥8 | 53 | ||||||||
| 40–59 | |||||||||
| 0 | 7 | 11 | 10 | 7 | 13 | 13 | 35,889 | 1.2 | |
| 1 | 3 | 13 | 9 | 10 | 3 | 9748 | 3.3 * (p = 0.02) | ||
| 2–4 | 16 | 29 | 40 | 42 | 43 | 10 | 11,572 | 1.5 * (p = 0.026) | |
| 5–7 | 17 | 21 | 21 | 44 | 28 | 1 | 2549 | 1.3 | |
| ≥8 | 7 | 15 | 21 | 9 | 15 | 1 | 796 | 1.0 | |
| 60–79 | |||||||||
| 0 | 6 | 9 | 9 | 7 | 21 | 9 | 6642 | 2.3 * (p = 0.04) | |
| 1 | 5 | 5 | 3 | 8 | 19 | 3 | 4270 | 3.8 * (p = 0.004) | |
| 2–4 | 26 | 54 | 55 | 64 | 101 | 18 | 12,044 | 1.9 * (p = 0.002) | |
| 5–7 | 46 | 68 | 93 | 94 | 89 | 24 | 6653 | 1.3 * (p = 0.047) | |
| ≥8 | 49 | 92 | 90 | 95 | 74 | 21 | 3715 | 0.8 | |
| ≥80 | |||||||||
| 0 | 3 | 6 | 2 | 3 | 4 | 3 | 509 | 0.7 | |
| 1 | 1 | 1 | 2 | 5 | 2 | 338 | 5.0 | ||
| 2–4 | 8 | 12 | 19 | 15 | 22 | 11 | 2147 | 1.8 * (p = 0.04) | |
| 5–7 | 19 | 31 | 51 | 39 | 40 | 9 | 2785 | 1.3 | |
| ≥8 | 39 | 63 | 60 | 81 | 59 | 19 | 2742 | 0.9 | |
| Total general | 256 | 429 | 493 | 529 | 550 | 154 | 190,240 | 1.3 * (p = 0.0001) | |
References
- WHO. Available online: https://www.who.int/europe/news-room/fact-sheets/item/post-covid-19-condition (accessed on 21 July 2025).
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef] [PubMed]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146, Erratum in Nat. Rev. Microbiol. 2023, 21, 408. [Google Scholar] [CrossRef] [PubMed]
- O’Mahoney, L.L.; Routen, A.; Gillies, C.; Jenkins, S.A.; Almaqhawi, A.; Ayoubkhani, D.; Banerjee, A.; Brightling, C.; Calvert, M.; Cassambai, S.; et al. The risk of Long Covid symptoms: A systematic review and meta-analysis of controlled studies. Nat. Commun. 2025, 16, 4249. [Google Scholar] [CrossRef] [PubMed]
- Ceban, F.; Ling, S.; Lui, L.M.; Lee, Y.; Gill, H.; Teopiz, K.M.; Rodrigues, N.B.; Subramaniapillai, M.; Di Vincenzo, J.D.; Cao, B.; et al. Fatigue and cognitive impairment in post-COVID-19 syndrome: A systematic review and meta-analysis. Brain Behav. Immun. 2022, 101, 93–135. [Google Scholar] [CrossRef]
- Ariza, M.; Cano, N.; Segura, B.; Adan, A.; Bargalló, N.; Caldú, X.; Campabadal, A.; Jurado, M.A.; Mataró, M.; Pueyo, R.; et al. Neuropsychological impairment in post-COVID condition individuals with and without cognitive complaints. Front. Aging Neurosci. 2022, 14, 1029842. [Google Scholar] [CrossRef]
- Doskas, T.; Vavougios, G.D.; Kormas, C.; Kokkotis, C.; Tsiptsios, D.; Spiliopoulos, K.C.; Tsiakiri, A.; Christidi, F.; Aravidou, T.; Dekavallas, L.; et al. Neurocognitive Impairment After COVID-19: Mechanisms, Phenotypes, and Links to Alzheimer’s Disease. Brain Sci. 2025, 15, 564. [Google Scholar] [CrossRef]
- Zayet, S.; Zahra, H.; Royer, P.Y.; Tipirdamaz, C.; Mercier, J.; Gendrin, V.; Lepiller, Q.; Marty-Quinternet, S.; Osman, M.; Belfeki, N.; et al. Post-COVID-19 Syndrome: Nine Months after SARS-CoV-2 Infection in a Cohort of 354 Patients: Data from the First Wave of COVID-19 in Nord Franche-Comté Hospital, France. Microorganisms 2021, 9, 1719. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Massey, D.; Saydah, S.; Adamson, B.; Lincoln, A.; Aukerman, D.F.; Berke, E.M.; Sikka, R.; Krumholz, H.M. Prevalence of covid-19 and long covid in collegiate student athletes from spring 2020 to fall 2021: A retrospective survey. BMC Infect. Dis. 2023, 23, 876. [Google Scholar] [CrossRef]
- Rajpal, S.; Tong, M.S.; Borchers, J.; Zareba, K.M.; Obarski, T.P.; Simonetti, O.P.; Daniels, C.J. Cardiovascular Magnetic Resonance Findings in Competitive Athletes Recovering from COVID-19 Infection. JAMA Cardiol. 2021, 6, 116–118, Erratum in JAMA Cardiol. 2021, 6, 123. [Google Scholar] [CrossRef]
- Koutsiaris, A.G.; Karakousis, K. Long COVID Mechanisms, Microvascular Effects, and Evaluation Based on Incidence. Life 2025, 15, 887. [Google Scholar] [CrossRef]
- Eltayeb, A.; Adilović, M.; Golzardi, M.; Hromić-Jahjefendić, A.; Rubio-Casillas, A.; Uversky, V.N.; Redwan, E.M. Intrinsic factors behind long COVID: Exploring the role of nucleocapsid protein in thrombosis. PeerJ 2025, 13, e19429. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Ramasamy, M.N.; Minassian, A.M.; Ewer, K.J.; Flaxman, A.L.; Folegatti, P.M.; Owens, D.R.; Voysey, M.; Aley, P.K.; Angus, B.; Babbage, G.; et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): A single-blind, randomised, controlled, phase 2/3 trial. Lancet 2021, 396, 1979–1993. [Google Scholar] [CrossRef]
- Stephenson, K.E.; Le Gars, M.; Sadoff, J.; de Groot, A.M.; Heerwegh, D.; Truyers, C.; Atyeo, C.; Loos, C.; Chandrashekar, A.; McMahan, K. Immunogenicity of the Ad26.COV2.S Vaccine for COVID-19. JAMA 2021, 325, 1535–1544. [Google Scholar]
- Faksova, K.; Walsh, D.; Jiang, Y.; Griffin, J.; Phillips, A.; Gentile, A.; Kwong, J.C.; Macartney, K.; Naus, M.; Grange, Z.; et al. COVID-19 vaccines and adverse events of special interest: A multinational Global Vaccine Data Network (GVDN) cohort study of 99 million vaccinated individuals. Vaccine 2024, 42, 2200–2211. [Google Scholar] [CrossRef]
- Yardibi, F.; Demirci, S. Global trends and hot spots in cerebral venous sinus thrombosis research over the past 50 years: A bibliometric analysis. Neurol. Res. 2025, 47, 23–34. [Google Scholar] [CrossRef]
- Nitz, J.N.; Ruprecht, K.K.; Henjum, L.J.; Matta, A.Y.; Shiferaw, B.T.; Weber, Z.L.; Jones, J.M.; May, R.; Baio, C.J.; Fiala, K.J.; et al. Cardiovascular Sequelae of the COVID-19 Vaccines. Cureus 2025, 17, e82041. [Google Scholar] [CrossRef] [PubMed]
- Satyam, S.M.; El-Tanani, M.; Bairy, L.K.; Rehman, A.; Srivastava, A.; Kenneth, J.M.; Prem, S.M. Unraveling Cardiovascular Risks and Benefits of COVID-19 Vaccines: A Systematic Review. Cardiovasc. Toxicol. 2025, 25, 306–323. [Google Scholar] [CrossRef]
- Chen, K.; Wang, Z.; Li, J.; Xu, Y.; Gu, S.; Li, H.; Li, J.; Zhang, Y.; Mao, N. Chronic inflammation in Long COVID relationship to autoimmune diseases. Autoimmun. Rev. 2025, 17, 103882. [Google Scholar] [CrossRef]
- Mohammadi, S.; Sisay, M.M.; Saraswati, P.W.; Osman, A.K.; Zuithoff, N.P.A.; Weibel, D.; Sturkenboom, M.; Ahmadizar, F. COVID-19 vaccine safety studies among special populations: A systematic review and meta-analysis of 120 observational studies and randomized clinical trials. Vaccine 2025, 61, 127342. [Google Scholar] [CrossRef] [PubMed]
- Kozłowski, P.; Leszczyńska, A.; Ciepiela, O. Long COVID Definition, Symptoms, Risk Factors, Epidemiology and Autoimmunity: A Narrative Review. Am. J. Med. Open 2024, 11, 100068. [Google Scholar] [CrossRef] [PubMed]
- Huerne, K.; Filion, K.B.; Grad, R.; Ernst, P.; Gershon, A.S.; Eisenberg, M.J. Epidemiological and clinical perspectives of long COVID syndrome. Am. J. Med. Open 2023, 9, 100033. [Google Scholar] [CrossRef] [PubMed]
- Taher, M.K.; Salzman, T.; Banal, A.; Morissette, K.; Domingo, F.R.; Cheung, A.M.; Cooper, C.L.; Boland, L.; Zuckermann, A.M.; Mullah, M.A.; et al. Global prevalence of post-COVID-19 condition: A systematic review and meta-analysis of prospective evidence. Health Promot. Chronic Dis. Prev. Can. 2025, 45, 112–138, Erratum in Health Promot. Chronic Dis. Prev. Can. 2025, 45, 307–308. [Google Scholar] [CrossRef]
- Sk Abd Razak, R.; Ismail, A.; Abdul Aziz, A.F.; Suddin, L.S.; Azzeri, A.; Sha’ari, N.I. Post-COVID syndrome prevalence: A systematic review and meta-analysis. BMC Public Health 2024, 24, 1785. [Google Scholar] [CrossRef]
- Obeidat, M.; Abu Zahra, A.; Alsattari, F. Prevalence and characteristics of long COVID-19 in Jordan: A cross sectional survey. PLoS ONE 2024, 19, e0295969. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Izquierdo-Condoy, J.S.; Fernandez-Naranjo, R.; Vasconez-González, E.; Cordovez, S.; Tello-De-la-Torre, A.; Paz, C.; Delgado-Moreira, K.; Carrington, S.; Viscor, G.; Ortiz-Prado, E. Long COVID at Different Altitudes: A Countrywide Epidemiological Analysis. Int. J. Environ. Res. Public Health 2022, 19, 14673. [Google Scholar] [CrossRef]
- Puigdellívol-Sánchez, A.; Juanes-González, M.; Calderón-Valdiviezo, A.; Valls-Foix, R.; González-Salvador, M.; Lozano-Paz, C.; Vidal-Alaball, J. COVID-19 in Relation to Chronic Antihistamine Prescription. Microorganisms 2024, 12, 2589. [Google Scholar] [CrossRef]
- Puigdellívol-Sánchez, A.; Juanes-González, M.; Calderón-Valdiviezo, A.I.; Losa-Puig, H.; González-Salvador, M.; León-Pérez, M.; Pueyo-Antón, L.; Franco-Romero, M.; Lozano-Paz, C.; Cortés-Borra, A.; et al. COVID-19 Pandemic Waves and 2024–2025 Winter Season in Relation to Angiotensin-Converting Enzyme Inhibitors, Angiotensin Receptor Blockers and Amantadine. Healthcare 2025, 13, 1270. [Google Scholar] [CrossRef]
- Puigdellívol-Sánchez, A.; Juanes-González, M.; Calderón-Valdiviezo, A.; Valls-Foix, R.; González-Salvador, M.; Lozano-Paz, C.; Vidal-Alaball, J. COVID-19 in Relation to Polypharmacy and Immunization (2020–2024). Viruses 2024, 16, 1533. [Google Scholar] [CrossRef]
- Sisó-Almirall, A.; Brito-Zerón, P.; Conangla Ferrín, L.; Kostov, B.; Moragas Moreno, A.; Mestres, J.; Sellarès, J.; Galindo, G.; Morera, R.; Basora, J.; et al. Long COVID-19: Proposed Primary Care Clinical Guidelines for Diagnosis and Disease Management. Int. J. Environ. Res. Public Health 2021, 18, 4350. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- NICE. COVID-19 Rapid Guideline: Managing the Long-Term Effects of COVID-19. Available online: https://www.nice.org.uk/guidance/ng188 (accessed on 9 July 2025).
- Dean, A.G.; Sullivan, K.M.; Soe, M.M. OpenEpi: Open Source Epidemiologic Statistics for Public Health, Versión. Available online: https://www.openepi.com/Menu/OE_Menu.htm (accessed on 30 March 2025).
- Chen, Y.C.; Chiu, C.H.; Chen, C.J. Neurological and psychiatric aspects of long COVID among vaccinated healthcare workers: An assessment of prevalence and reporting biases. J. Microbiol. Immunol. Infect. 2025, 23, S1684-1182(25)00125-2. [Google Scholar] [CrossRef]
- Sterne, J.A.; White, I.R.; Carlin, J.B.; Spratt, M.; Royston, P.; Kenward, M.G.; Wood, A.M.; Carpenter, J.R. Multiple imputation for missing data in epidemiological and clinical research: Potential and pitfalls. BMJ 2009, 338, b2393. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cameli, M.; Pastore, M.C.; Mandoli, G.E.; D’Ascenzi, F.; Focardi, M.; Biagioni, G.; Cameli, P.; Patti, G.; Franchi, F.; Mondillo, S.; et al. COVID-19 and Acute Coronary Syndromes: Current Data and Future Implications. Front. Cardiovasc. Med. 2021, 7, 593496. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.S.; Tauro, L.; Joshi, H.B.; Makhal, A.; Sobczak, T.; Goret, J.; Dewitte, A.; Kaveri, S.; Chakrapani, H.; Matsuda, M.M.; et al. Influence of homocysteine on regulating immunothrombosis: Mechanisms and therapeutic potential in management of infections. Inflamm. Res. 2025, 74, 86. [Google Scholar] [CrossRef]
- Carrera Morodoa, M.; Pérez Orcerob, A.; Ruiz Moreno, J.; Altemir Vidal, A.; Larrañaga Cabrerab, A.; Fernández San Martín, M.I. Prevalencia de la COVID persistente: Seguimiento al año de una cohorte poblacional ambulatoria. Rev. Clin. Med. Fam. 2023, 16, 94–97. [Google Scholar] [CrossRef]
- Pisaturo, M.; Russo, A.; Grimaldi, P.; Monari, C.; Imbriani, S.; Gjeloshi, K.; Ricozzi, C.; Astorri, R.; Curatolo, C.; Palladino, R.; et al. Prevalence, Evolution and Prognostic Factors of PASC in a Cohort of Patients Discharged from a COVID Unit. Biomedicines 2025, 13, 1414. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gordon, D.E.; Jang, G.M.; Bouhaddou, M.; Xu, J.; Obernier, K.; White, K.M.; O’Meara, M.J.; Rezelj, V.V.; Guo, J.Z.; Swaney, D.L. A SARS-CoV-2 Protein Interaction Map Reveals Targets for Drug-Repurposing. Nature 2020, 583, 459–468. [Google Scholar] [CrossRef]
- Morán Blanco, J.I.; Alvarenga Bonilla, J.A.; Fremont-Smith, P.; Villar Gómez de Las Heras, K. Antihistamines as an early treatment for COVID-19. Heliyon 2023, 9, e15772. [Google Scholar] [CrossRef]
- Harandi, A.A.; Pakdaman, H.; Medghalchi, A.; Kimia, N.; Kazemian, A.; Siavoshi, F.; Barough, S.S.; Esfandani, A.; Hosseini, M.H.; Sobhanian, S.A. A randomized open-label clinical trial on the effect of Amantadine on post COVID-19 fatigue. Sci. Rep. 2024, 14, 1343. [Google Scholar] [CrossRef]
- Rejdak, K.; Fiedor, P.; Bonek, R.; Łukasiak, J.; Chełstowski, W.; Kiciak, S.; Dąbrowski, P.; Gala-Błądzińska, A.; Dec, M.; Papuć, E.; et al. Amantadine in unvaccinated patients with early, mild to moderate COVID-19: A randomized, placebo-controlled, double-blind trial. Eur. J. Neurol. 2024, 31, e16045. [Google Scholar] [CrossRef] [PubMed]
- Bramante, C.T.; Beckman, K.B.; Mehta, T.; Karger, A.B.; Odde, D.J.; Tignanelli, C.J.; Buse, J.B.; Johnson, D.M.; Watson, R.H.B.; Daniel, J.J.; et al. Favorable Antiviral Effect of Metformin on SARS-CoV-2 Viral Load in a Randomized, Placebo-Controlled Clinical Trial of COVID-19. Clin. Infect. Dis. 2024, 79, 354–363. [Google Scholar] [CrossRef]
- Bramante, C.T.; Buse, J.B.; Liebovitz, D.M.; Nicklas, J.M.; Puskarich, M.A.; Cohen, K.; Belani, H.K.; Anderson, B.J.; Huling, J.D.; Tignanelli, C.J.; et al. Outpatient treatment of COVID-19 and incidence of post-COVID-19 condition over 10 months (COVID-OUT): A multicentre, randomised, quadruple-blind, parallel-group, phase 3 trial. Lancet Infect. Dis. 2023, 23, 1119–1129, Erratum in Lancet Infect. Dis. 2023, 23, e400. [Google Scholar] [CrossRef] [PubMed]
- Andrews, J.S.; Boonyaratanakornkit, J.B.; Krusinska, E.; Allen, S.; Posada, J.A. Assessment of the Impact of RNase in Patients With Severe Fatigue Related to Post-Acute Sequelae of SARS-CoV-2 Infection: A Randomized Phase 2 Trial of RSLV-132. Clin. Infect. Dis. 2024, 79, 635–642. [Google Scholar] [CrossRef]
- Yotsuyanagi, H.; Ohmagari, N.; Doi, Y.; Yamato, M.; Fukushi, A.; Imamura, T.; Sakaguchi, H.; Sonoyama, T.; Sanaki, T.; Ichihashi, G.; et al. Prevention of post COVID-19 condition by early treatment with ensitrelvir in the phase 3 SCORPIO-SR trial. Antivir. Res. 2024, 229, 105958. [Google Scholar] [CrossRef]
- Geng, L.N.; Bonilla, H.; Hedlin, H.; Jacobson, K.B.; Tian, L.; Jagannathan, P.; Yang, P.C.; Subramanian, A.K.; Liang, J.W.; Shen, S.; et al. Nirmatrelvir-Ritonavir and Symptoms in Adults with Postacute Sequelae of SARS-CoV-2 Infection: The STOP-PASC Randomized Clinical Trial. JAMA Intern. Med. 2024, 184, 1024–1034, Erratum in JAMA Intern. Med. 2024, 184, 1137. [Google Scholar] [CrossRef]
- Tomasa-Irriguible, T.M.; Monfà, R.; Miranda-Jiménez, C.; Morros, R.; Robert, N.; Bordejé-Laguna, L.; Vidal, S.; Torán-Monserrat, P.; Barriocanal, A.M. Preventive Intake of a Multiple Micronutrient Supplement during Mild, Acute SARS-CoV-2 Infection to Reduce the Post-Acute COVID-19 Condition: A Double-Blind, Placebo-Controlled, Randomized Clinical Trial. Nutrients 2024, 16, 1631. [Google Scholar] [CrossRef]
- Yasacı, Z.; Mustafaoglu, R.; Ozgur, O.; Kuveloglu, B.; Esen, Y.; Ozmen, O.; Yalcinkaya, E.Y. Virtual recovery: Efficacy of telerehabilitation on dyspnea, pain, and functional capacity in post-COVID-19 syndrome. Ir. J. Med. Sci. 2025, 194, 631–640. [Google Scholar] [CrossRef]
- Carpallo-Porcar, B.; Calvo, S.; Pérez-Palomares, S.; Blázquez-Pérez, L.; Brandín-de la Cruz, N.; Jiménez-Sánchez, C. Perceptions and Experiences of a Multimodal Rehabilitation Program for People with Post-Acute COVID-19: A Qualitative Study. Health Expect. 2025, 28, e70283. [Google Scholar] [CrossRef] [PubMed]
- Daynes, E.; Evans, R.A.; Greening, N.J.; Bishop, N.C.; Yates, T.; Lozano-Rojas, D.; Ntotsis, K.; Richardson, M.; Baldwin, M.M.; Hamrouni, M.; et al. Post-Hospitalisation COVID-19 Rehabilitation (PHOSP-R): A randomised controlled trial of exercise-based rehabilitation. Eur. Respir. J. 2025, 65, 2402152. [Google Scholar] [CrossRef]
- Seers, K.; Nichols, V.P.; Bruce, J.; Ennis, S.; Heine, P.; Patel, S.; Sandhu, H.K.; Underwood, M.; McGregor, G.; our REGAIN collaborators. Qualitative evaluation of the Rehabilitation Exercise and psycholoGical support After COVID-19 infection (REGAIN) randomised controlled trial (RCT): ‘you are not alone’. BMJ Open 2025, 15, e085950. [Google Scholar] [CrossRef] [PubMed]
- Weix, N.M.; Shake, H.M.; Duran Saavedra, A.F.; Clingan, H.E.; Hernandez, V.C.; Johnson, G.M.; Hansen, A.D.; Collins, D.M.; Pryor, L.E.; Kitchens, R.; et al. Cognitive Interventions and Rehabilitation to Address Long-COVID Symptoms: A Systematic Review. Occup. Ther. J. Res. 2025, 15394492251328310. [Google Scholar] [CrossRef] [PubMed]
- Generalitat de Catalunya. Sistema d’Informació per a la Vigilància d’Infeccions a Catalunya. Available online: https://sivic.salut.gencat.cat/ (accessed on 3 July 2025).
- Alexopoulos, H.; Trougakos, I.P.; Dimopoulos, M.A.; Terpos, E. Clinical usefulness of testing for severe acute respiratory syndrome coronavirus 2 antibodies. Eur. J. Intern. Med. 2023, 107, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Dinnes, J.; Deeks, J.J.; Adriano, A.; Berhane, S.; Davenport, C.; Dittrich, S. Cochrane COVID-19 Diagnostic Test Accuracy Group 2. Rapid point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst. Rev. 2021, 3, CD013705. [Google Scholar]
- World Health Organization. Available online: https://iris.who.int/bitstream/handle/10665/360580/WHO-2019-nCoV-SurveillanceGuidance-2022.2-eng.pdf (accessed on 18 September 2024).
- Ford, N.D.; Baca, S.; Dalton, A.F.; Koumans, E.H.; Raykin, J.; Patel, P.R.; Saydah, S. Use and Characteristics of Clinical Coding for Post-COVID Conditions in a Retrospective US Cohort. J. Public Health Manag. Pract. 2025, 31, E292–E302. [Google Scholar] [CrossRef] [PubMed]
- Hendrix, N.; Parikh, R.V.; Taskier, M.; Walter, G.; Rochlin, I.; Saydah, S.; Koumans, E.H.; Rincón-Guevara, O.; Rehkopf, D.H.; Phillips, R.L. Heterogeneity of diagnosis and documentation of post-COVID conditions in primary care: A machine learning analysis. PLoS ONE 2025, 20, e0324017. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gómez-Carballa, A.; Bello, X.; Pardo-Seco, J.; Pérez del Molino, M.L.; Martiñón-Torres, F.; Salas, A. Phylogeography of SARS-CoV-2 pandemic in Spain: A story of multiple introductions, micro-geographic stratification, founder effects, and super-spreaders. Zool. Res. 2020, 41, 605–620. [Google Scholar] [CrossRef]

| Long COVID | Total | ||||
|---|---|---|---|---|---|
| Pre Survey | Post Survey | Long COVID | No Long COVID | Population | |
| Women/age | 242 | +80 | 322 (3.3‰) | 96,676 | 96,998 |
| 0–29 | 19 | +2 | 21 (0.7‰) | 30,907 | 30,928 |
| 30–59 | 171 | +63 | 234 (5.6‰) | 41,530 | 41,764 |
| ≥60 | 52 | 15 | 67 (2.7‰) | 24,239 | 24,306 |
| Men/age | 134 | +19 | 153 (1.6‰) | 95,500 | 95,653 |
| 0–29 | 16 | +1 | 17 (0.5‰) | 32,850 | 32,867 |
| 30–59 | 90 | +11 | 101 (2.3‰) | 43,054 | 43,155 |
| ≥60 | 28 | +7 | 35 (1.7‰) | 19,596 | 19,631 |
| Total general | 376 | +99 | 475 (2.4‰) | 192,176 | 192,651 |
| n Infection | V | CoV | LC | (%) | OR vs. V preinf | p | OR vs. 1 inf | p |
|---|---|---|---|---|---|---|---|---|
| ? | V | 60,985 | 45 | 0.07% | ||||
| ? | No V | 75,664 | 21 | 0.03% | ||||
| 1 | V preinf | 18,386 | 46 | 0.25% | ||||
| 1 | V postinf | 9889 | 168 | 1.67% | 6.68 | <0.0001 * | ||
| 1 | No V | 21,608 | 94 | 0.43% | 1.8 | 0.001 * | ||
| 2 | V preinf | 2948 | 43 | 1.44% | 5.8 | <0.0001 * | ||
| 2 | V postinf | 335 | 14 | 4.01% | 2.78 | 0.004 * | 2.4 | 0.001 * |
| 2 | No V | 1833 | 18 | 0.97% | 0.67 | 0.07 | 2.2 | 0.001 * |
| ≥3 | V preinf | 355 | 10 | 2.74% | 11 | <0.0001 * | ||
| ≥3 | V postinf | 37 | 8 | 17.78% | 6.49 | <0.0001 * | 10.6 | <0.0001 * |
| ≥3 | No V | 136 | 8 | 5.56% | 2.03 | 0.06 | 12.8 | <0.0001 * |
| Responders with Long COVID Diagnosis: | Yes 702 | Yes, and I Am Still Symptomatic | Yes, but I Already Feel Good | Unsure 527 | No 1998 | |
|---|---|---|---|---|---|---|
| Physical complaints | ||||||
| Anosmia or dysgeusia | 23.5% | 165 | 35 | 17.5% | 76 | 76 |
| Shortness of breath | 31.9% | 224 | 47 | 17.3% | 158 | 136 |
| Headache | 25.1% | 176 | 48 | 21.4% | 155 | 170 |
| Joint pain | 36.5% | 256 | 103 | 28.7% | 259 | 332 |
| Persistent fatigue | 46.9% | 329 | 123 | 27.2% | 323 | 406 |
| Psychological complaints | ||||||
| Memory complaints | 31.9% | 224 | 65 | 22.5% | 164 | 198 |
| Lack of concentration | 33.3% | 234 | 61 | 20.7% | 190 | 224 |
| Depression | 19.2% | 135 | 38 | 22.0% | 119 | 144 |
| Anxiety | 33.2% | 233 | 87 | 27.2% | 219 | 303 |
| Sleep complaints | 32.3% | 227 | 80 | 26.1% | 215 | 299 |
| Functional impairment | ||||||
| Home task | 23.1% | 162 | 31 | 16.1% | 107 | 109 |
| With friends or relatives | 17.9% | 126 | 24 | 16.0% | 90 | 132 |
| Impaired personal hygiene | 5.4% | 38 | 9 | 19.1% | 26 | 33 |
| Work interference | 29.3% | 206 | 53 | 20.5% | 144 | 165 |
| COVID-19-related sick leave | 37.7% | 265 | 132 | 33.2% | 244 | 1002 |
| Number of Infections | Vaccination | Number of Vaccines | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No Vaccines | At Least 1 | 1 | 2 | 3 | >3 | |||||||
| 1 COVID-19 infection | 77 | 1753 | 143 | 691 | 615 | 304 | ||||||
| No | 57 | 1176 | 90 | 439 | 434 | 213 | ||||||
| Unsure | 8 | 277 | 17 | 125 | 95 | 40 | ||||||
| Long COVID still symptomatic | 7 | 9.1% | 185 | 10.6% | 21 | 14.6% | 81 | 11.7% | 51 | 8.2% | 33 | 10.8% |
| Transient long COVID | 5 | 6.5% | 114 | 6.5% | 15 | 10.5% | 46 | 6.7% | 35 | 5.7% | 18 | 5.9% |
| 2 COVID-19 infections | 47 | 962 | 98 | 401 | 356 | 107 | ||||||
| No | 27 | 540 | 47 | 212 | 216 | 65 | ||||||
| Unsure | 6 | 174 | 13 | 76 | 64 | 21 | ||||||
| Long COVID still symptomatic | 9 | 19.14% | 167 | 17.3% | 30 | 30.6% | 81 | 20.1% | 45 | 12.6% | 11 | 10.2% |
| Transient long COVID | 5 | 10.6% | 81 | 8.4% | 8 | 8.2% | 32 | 10.5% | 31 | 8.7% | 10 | 9.3% |
| ≥3 COVID 19 infections | 13 | 173 | 55 | 118 | 99 | 34 | ||||||
| No | 6 | 78 | 21 | 56 | 42 | 15 | ||||||
| Unsure | 0 | 30 | 12 | 20 | 16 | 6 | ||||||
| Long COVID still symptomatic | 4 | 30.7% | 43 | 25.4% | 18 | 32.7% | 29 | 24.5% | 23 | 23.2% | 8 | 23.5% |
| Transient long COVID | 3 | 23.1% | 22 | 12.7% | 4 | 7.3% | 13 | 11.0% | 18 | 18.2% | 5 | 14.7% |
| 2020 | 2021 | 2022 | 2023 | 2024 | 2025 | No Thrombus | OR | p | |
|---|---|---|---|---|---|---|---|---|---|
| V | 2024-21 | ||||||||
| V post Thr | 219 | 137 | 3 | 1 | 3 | 1 | |||
| V pre Thr | 237 | 418 | 454 | 447 | 114 | 1.89 | 0.0000001 * | ||
| 91,235 | |||||||||
| No V | 37 | 55 | 72 | 74 | 100 | 39 | 99,005 | 1.82 | 0.0003 * |
| Thrombus | No Thrombus | OR 2024-21 | p | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 2020 | 2021 | 2022 | 2023 | 2024 | 2025 | ||||
| V | |||||||||
| CoV | |||||||||
| CoV post Thr | 67 | 79 | 37 | 7 | 5 | ||||
| CoV pre Thr | 18 | 62 | 86 | 123 | 139 | 39 | 2.24 | 0.0000001 * | |
| No thrombus | 31,577 | ||||||||
| No Cov | 134 | 233 | 298 | 325 | 306 | 76 | 59,658 | 1.31 | 0.0017 * |
| No V | |||||||||
| CoV | 13 | 16 | 24 | 21 | 14 | 2 | 23,607 | ||
| CoV post Thr | 11 | 7 | 4 | 3 | |||||
| CoV pre Thr | 2 | 9 | 20 | 18 | 14 | 2 | 1.55 | 0.29 | |
| No thrombus | 23,607 | ||||||||
| No Cov | 24 | 39 | 48 | 53 | 86 | 37 | 75,398 | 2.20 | 0.0002 * |
| Total general | 256 | 429 | 493 | 529 | 550 | 154 | 190,240 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arévalo-Genicio, A.; García-Arqué, M.C.; Gragea-Nocete, M.; Llistosella, M.; Moro-Casasola, V.; Pérez-Díaz, C.; Puigdellívol-Sánchez, A.; Roca-Puig, R. Long COVID Syndrome Prevalence in 2025 in an Integral Healthcare Consortium in the Metropolitan Area of Barcelona: Persistent and Transient Symptoms. Vaccines 2025, 13, 905. https://doi.org/10.3390/vaccines13090905
Arévalo-Genicio A, García-Arqué MC, Gragea-Nocete M, Llistosella M, Moro-Casasola V, Pérez-Díaz C, Puigdellívol-Sánchez A, Roca-Puig R. Long COVID Syndrome Prevalence in 2025 in an Integral Healthcare Consortium in the Metropolitan Area of Barcelona: Persistent and Transient Symptoms. Vaccines. 2025; 13(9):905. https://doi.org/10.3390/vaccines13090905
Chicago/Turabian StyleArévalo-Genicio, Antonio, Mª Carmen García-Arqué, Marta Gragea-Nocete, Maria Llistosella, Vanessa Moro-Casasola, Cristina Pérez-Díaz, Anna Puigdellívol-Sánchez, and Ramon Roca-Puig. 2025. "Long COVID Syndrome Prevalence in 2025 in an Integral Healthcare Consortium in the Metropolitan Area of Barcelona: Persistent and Transient Symptoms" Vaccines 13, no. 9: 905. https://doi.org/10.3390/vaccines13090905
APA StyleArévalo-Genicio, A., García-Arqué, M. C., Gragea-Nocete, M., Llistosella, M., Moro-Casasola, V., Pérez-Díaz, C., Puigdellívol-Sánchez, A., & Roca-Puig, R. (2025). Long COVID Syndrome Prevalence in 2025 in an Integral Healthcare Consortium in the Metropolitan Area of Barcelona: Persistent and Transient Symptoms. Vaccines, 13(9), 905. https://doi.org/10.3390/vaccines13090905







