COVID-19 Vaccines: Tolerance of Vaccination in Patients with Allergies
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Clinical Assessments
2.3. Laboratory Assessments
2.4. Questionnaire
2.5. Psychological Assessment
2.6. Statistical Analyses
3. Results
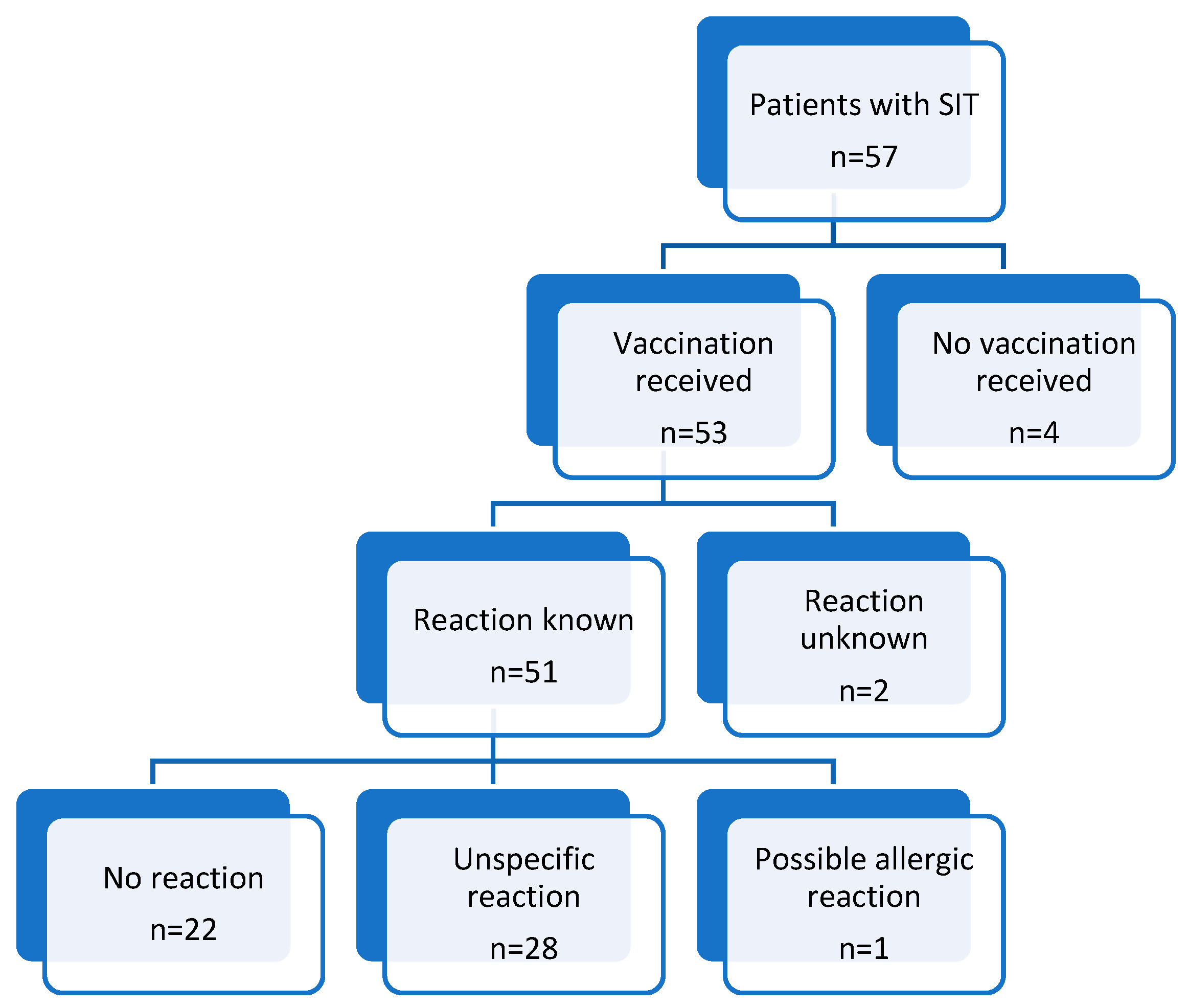
3.1. Most Patients Who Underwent SIT (93%) Received COVID-19 Vaccination; Among Those, 87% Received at Least One Dose of BioNTech/Pfizer Vaccine
3.2. More than Half of the Patients (57%) Reported a Reaction After the Administration of COVID-19 Vaccination
3.3. Only 1 out of 51 Patients Had a Possible Allergic Reaction After the Administration of COVID-19 Vaccination
3.4. Patients with Adverse Reactions After COVID-19 Vaccination Showed No Significant Differences in Clinical Characteristics Compared to Patients with No Reaction
3.5. Previous Adverse Events After Vaccinations and a Sensitization to Aeroallergens Increased the Risk for Reactions to COVID-19 Vaccines
3.6. Antihistamine Intake Was Associated with a Significantly Lower Rate of Unspecific Reactions After COVID-19 Vaccination
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| COVID-19 | Corona Virus Disease 2019 |
| f | Female |
| IgE | Immunoglobulin E |
| IQR | Interquartile Range |
| HADS | Hospital Anxiety and Depression Scale |
| m | Male |
| PEG | Polyethylene Glycol |
| SIT | Specific Immunotherapy |
| SD | Standard Deviation |
| y | Years |
Appendix A

| Vaccinated (n = 53) | Not Vaccinated (n = 4) | Significance p-Value | |
|---|---|---|---|
| Basic characteristics | |||
| Age (y) | |||
| Median (IQR) | 43.0 (27.0) | 41.0 (37.8) | 0.69 |
| Mean ± SD | 46.4 ± 16.7 | 43.8 ± 20.5 | |
| Sex (m:f) | 31:22 | 2:2 | 1.0 |
| Mastocytosis | 3 (5.7%) | 0 (0%) | 1.0 |
| Number of different allergy categories | 0.25 | ||
| Median (IQR) | 1.0 (1.0) | 1.0 (0.0) | |
| Mean ± SD | 1.5 ± 0.8 | 1.0 ± 0.0 | |
| Aeroallergies | 32 (60.4%) | 0 (0%) | 0.03 |
| Food allergies | 6 (11.3%) | 0 (0%) | 1.0 |
| Contact allergies | 1 (1.9%) | 0 (0%) | 1.0 |
| Allergies to oral medication | 8 (15.1%) | 0 (0%) | 1.0 |
| Allergies to injectables | 1 (1.9%) | 0 (0%) | 1.0 |
| Venom allergies | 30 (56.6%) | 4 (100%) | 0.14 |
| Prior anaphylaxis | 30 (56.6%) | 4 (100%) | 0.14 |
| Serology | |||
| Total IgE | 0.18 | ||
| Median (IQR) | 138.0 (199.0) | 60.0 (0.0) | |
| Mean ± SD | 243.8 ± 526.2 | 58.3 ± 36.5 | |
| Tryptase | 0.61 | ||
| Median (IQR) | 5.2 (3.1) | 6.4 (0.0) | |
| Mean ± SD | 6.8 ± 5.9 | 5.9 ± 1.8 | |
| Comorbidities | |||
| Allergic rhinitis | 30 (56.6%) | 0 (0%) | 0.04 |
| Asthma | 9 (17.0%) | 0 (0%) | 1.0 |
| Atopic dermatitis | 6 (11.3%) | 0 (0%) | 1.0 |
| Urticaria | 3 (5.7%) | 0 (0%) | 1.0 |
| Anxiety | 0.59 | ||
| Median (IQR) | 4.0 (5.0) | 4.0 (0.0) | |
| Mean ± SD | 4.8 ± 3.3 | 5.7 ± 2.9 | |
| Depression | 0.51 | ||
| Median (IQR) | 2.0 (4.0) | 5.0 (0.0) | |
| Mean ± SD | 3.0 ± 3.1 | 5.3 ± 2.5 |
| No Reaction | Adverse Reaction n = 29 | |||
|---|---|---|---|---|
| Reaction After 1 Dose, Then No Reaction | No Reaction After 1 Dose, Then Reaction | Reaction After 1 and Other Doses | ||
| Number of Patients | 22 | 7 (24.1%) | 7 (24.1%) | 15 (51.7%) |
| Both Groups | Hymenoptera Allergy | Aeroallergies | Significance | |
|---|---|---|---|---|
| (n = 51) | (n = 27) | (n = 24) | p-Value | |
| Basic characteristics | ||||
| Number of vaccinations | 0.333 | |||
| Median (IQR) | 3.0 (1.0) | 3.0 (1.0) | 3.0 (0.8) | |
| Mean ± SD | 3.2 ± 0.6 | 3.3 ± 0.6 | 3.1 ± 0.7 | |
| Reaction after vaccination | 0.149 | |||
| (unspecific/possibly allergic/no reaction) | 28:1:22 | 13:0:14 | 15:1:8 | |
| Age (y) | <0.001 | |||
| Median (IQR) | 46.0 (27.0) | 54.0 (23.0) | 34.5 (17.8) | |
| Mean ± SD | 46.2 ± 16.8 | 54.4 ± 14.1 | 38.7 ± 15.6 | |
| Sex (m:f) | 30:21 | 16:11 | 14:10 | 0.984 |
| Mastocytosis | 3 (5.9%) | 3 (11.1%) | 0 (0%) | 0.248 |
| Number of different allergy categories | 0.687 | |||
| Median (IQR) | 1.0 (1.0) | 1.0 (1.0) | 1.0 (1.0) | |
| Mean ± SD | 1.5 ± 0.8 | 1.5 ± 0.8 | 1.4 ± 0.7 | |
| Aeroallergies | 30 (58.8%) | 6 (22.2%) | 24 (100%) | <0.001 |
| Food allergies | 5 (9.8%) | 2 (7.4%) | 3 (12.5%) | 0.643 |
| Contact allergies | 1 (2.0%) | 1 (3.7%) | 0 (0%) | 1.000 |
| Allergies to oral medication | 8 (15.7%) | 4 (14.8%) | 4 (16.7%) | 0.718 |
| Allergies to injectables | 1 (2.0%) | 1 (3.7%) | 0 (0%) | 1.000 |
| Venom allergies | 30 (58.8%) | 27 (100%) | 3 (12.5%) | <0.001 |
| Prior anaphylaxis | 29 (56.9%) | 25 (92.6%) | 4 (16.7%) | <0.001 |
| Comorbidities | ||||
| Allergic rhinitis | 28 (54.9%) | 4 (14.8%) | 24 (100%) | <0.001 |
| Asthma | 9 (17.6%) | 1 (3.7%) | 8 (33.3%) | 0.007 |
| Atopic dermatitis | 6 (11.8%) | 0 (0%) | 6 (25.0%) | 0.005 |
| Urticaria | 3 (5.9%) | 2 (7.4%) | 1 (4.2%) | 1.000 |
| Anxiety | 0.575 | |||
| Median (IQR) | 4.0 (4.75) | 3.0 (5.0) | 4.0 (3.0) | |
| Mean ± SD | 4.7 ± 3.3 | 4.4 ± 3.4 | 5.0 ± 3.2 | |
| (n = 48) | (n = 25) | (n = 23) | ||
| Depression | 0.373 | |||
| Median (IQR) | 2.0 (4.0) | 1.0 (4.5) | 2.0 (4.0) | |
| Mean ± SD | 3.0 ± 3.1 | 2.6 ± 2.8 | 3.4 ± 3.4 | |
| (n = 48) | (n = 25) | (n = 23) | ||
| COVID-19 infection (before/after/both/no) | 2:21:4:22 | 1:11:1:12 | 1:10:3:10 | 0.350 |
| (n = 49) | (n = 25) | |||
| Serology | ||||
| Total IgE | 0.006 | |||
| Median (IQR) | 132.0 (199.0) | 70.0 (118.0) | 203.0 (324.5) | |
| Mean ± SD | 239.1 ± 508.1 | 123.1 ± 137.3 | 369.5 ± 711.6 | |
| Tryptase | 0.075 | |||
| Median (IQR) | 5.1 (3.1) | 5.7 (5.4) | 4.1 (2.7) | |
| Mean ± SD | 6.7 ± 5.9 | 8.2 ± 7.3 | 4.7 ± 2.0 | |
| (n = 49) | (n = 22) | |||
| Prior Vaccinations | ||||
| Reactions to other vaccines (e.g., influenza) | 10:1:39 | 3:0:23 | 7:1:16 | 0.033 |
| (unspecific/possibly allergic/no reaction) | (n = 50) | (n = 26) | ||
| Concerns before vaccination | 0.300 | |||
| Median (IQR) | 0.0 (1.0) | 0.0 (1.0) | 1.0 (1.8) | |
| Mean ± SD | 0.7 ± 0.9 | 0.9 ± 1.0 | 0.9 ± 1.0 | |
| Prophylactic medication with antihistamines | 7 (13.7%) | 5 (18.5%) | 2 (8.7%) | 0.430 |
| (n = 23) |
References
- Puxkandl, V.; Bangerl, T.; Hanfstingl, K.; Guenova, E.; Hoetzenecker, W.; Altrichter, S. Second-dose COVID-19 vaccines are well tolerated in patients with allergic reactions to the first dose—A single center experience. World Allergy Organ. J. 2022, 15, 100654. [Google Scholar] [CrossRef]
- Copaescu, A.M.; Rosa Duque, J.S.; Phillips, E.J. What have we learned about the allergenicity and adverse reactions associated with the severe acute respiratory syndrome coronavirus 2 vaccines: One year later. Ann. Allergy Asthma Immunol. 2022, 129, 40–51. [Google Scholar] [CrossRef]
- Shimabukuro, T.; Nair, N. Allergic Reactions Including Anaphylaxis After Receipt of the First Dose of Pfizer-BioNTech COVID-19 Vaccine. J. Am. Med. Assoc. 2021, 325, 780. [Google Scholar] [CrossRef]
- CDC COVID-19 Response Team; Food and Drug Administration. Allergic Reactions Including Anaphylaxis After Receipt of the First Dose of Pfizer-BioNTech COVID-19 Vaccine—United States, December 14–23, 2020. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Asperti, C.; Benanti, G.; Ramirez, G.A.; Russo, M.; Vai, B.; Bramé, B.; Viapiana, N.; Nannipieri, S.; Cilona, M.B.; Mazzetti, M.; et al. Interactions between Severe Allergy and Anxiety in Anti-SARS-CoV-2 Vaccinees. Vaccines 2022, 10, 2047. [Google Scholar] [CrossRef] [PubMed]
- Jaggers, J.; Wolfson, A.R. mRNA COVID-19 Vaccine Anaphylaxis: Epidemiology, Risk Factors, and Evaluation. Curr. Allergy Asthma Rep. 2023, 23, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Garvey, L.H.; Nasser, S. Anaphylaxis to the first COVID-19 vaccine: Is polyethylene glycol (PEG) the culprit? Br. J. Anaesth. 2021, 126, e106–e108. [Google Scholar] [CrossRef]
- Kayode, O.S.; Nakonechna, A.; Siew, L.Q.C.; Dziadzio, M.; Kennard, L.; Rutkowski, K.; Mirakian, R.; Wagner, A. Polyethylene glycol hypersensitivity, patient outcomes in a 7-year retrospective study. Ann. Allergy. Asthma Immunol. 2024, 133, 93–100. [Google Scholar] [CrossRef]
- Macy, E.; Pandya, S.; Sheikh, J.; Burnette, A.; Shi, J.M.; Chung, J.; Gin, N.; Crawford, W.; Zhang, J. Population-Based Incidence, Severity, and Risk Factors Associated with Treated Acute-Onset COVID-19 mRNA Vaccination–Associated Hypersensitivity Reactions. J. Allergy Clin. Immunol. Pract. 2022, 10, 827–836. [Google Scholar] [CrossRef]
- Shavit, R.; Maoz-Segal, R.; Iancovici-Kidon, M.; Offengenden, I.; Haj Yahia, S.; Machnes Maayan, D.; Lifshitz-Tunitsky, Y.; Niznik, S.; Frizinsky, S.; Deutch, M.; et al. Prevalence of Allergic Reactions After Pfizer-BioNTech COVID-19 Vaccination Among Adults with High Allergy Risk. JAMA Netw. Open 2021, 4, e2122255. [Google Scholar] [CrossRef]
- Ring, J.; Messmer, K. Incidence and Severity of Anaphylactoid Reactions to Colloid Volume Substitutes. Lancet 1977, 309, 466–469. [Google Scholar] [CrossRef]
- Valent, P.; Akin, C.; Hartmann, K.; Alvarez-Twose, I.; Brockow, K.; Hermine, O.; Niedoszytko, M.; Schwaab, J.; Lyons, J.J.; Carter, M.C.; et al. Updated Diagnostic Criteria and Classification of Mast Cell Disorders: A Consensus Proposal. HemaSphere 2021, 5, e646. [Google Scholar] [CrossRef]
- Zigmond, A.S.; Snaith, R.P. The Hospital Anxiety and Depression Scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef]
- Batac, A.L.R.; Merrill, K.A.; Askin, N.; Golding, M.A.; Abrams, E.M.; Bégin, P.; Ben-Shoshan, M.; Ladouceur, E.; Roos, L.E.; Protudjer, V.; et al. Vaccine confidence among those living with allergy during the COVID pandemic (ACCORD): A scoping review. J. Allergy Clin. Immunol. Glob. 2023, 2, 100079. [Google Scholar] [CrossRef] [PubMed]
- Kogseder, N.; Puxkandl, V.; Hoetzenecker, W.; Altrichter, S. Vaccine hesitancy in patients presenting to a specialized allergy center: Clinical relevant sensitizations, impact on mental health and vaccination rates. Front. Immunol. 2024, 15, 1324987. [Google Scholar] [CrossRef] [PubMed]
- Leru, P.M.; Anton, V. Role of Allergist Advice in Determining Personal Decisions for COVID-19 Vaccination of People with a History of Allergies. Cureus 2022, 14, e23156. [Google Scholar] [CrossRef]
- Hudson, A.; Montelpare, W.J. Predictors of Vaccine Hesitancy: Implications for COVID-19 Public Health Messaging. Int. J. Environ. Res. Public Health 2021, 18, 8054. [Google Scholar] [CrossRef]
- Tavanaei Tamanaei, T.; Oghazian, M.B.; Mojtabaee, M.; Faregh, M.; Oghazian, S.; Tavana, E.; Hoseinzadeh, A.; Haghighi, R. Self-Reported Adverse Events Following COVID-19 Vaccination Among Medical Sciences Students After a Symptomatology Training Program: A Cross-Sectional Study. Health Sci. Rep. 2025, 8, e70492. [Google Scholar] [CrossRef]
- Fitzpatrick, T.; Yamoah, P.; Lacuesta, G.; Sadarangani, M.; Cook, V.; Pourshahnazari, P.; Kalicinsky, C.; Upton, J.E.M.; Cameron, S.B.; Zaborniak, K.; et al. Revaccination outcomes among adolescents and adults with suspected hypersensitivity reactions following COVID-19 vaccination: A Canadian immunization research network study. Vaccine 2024, 42, 126078. [Google Scholar] [CrossRef] [PubMed]
- Gold, M.S.; Amarasinghe, A.; Greenhawt, M.; Kelso, J.M.; Kochhar, S.; Yu-Hor Thong, B.; Top, K.A.; Turner, P.J.; Worm, M.; Law, B. Anaphylaxis: Revision of the Brighton collaboration case definition. Vaccine 2023, 41, 2605–2614. [Google Scholar] [CrossRef]
- Risma, K.A. COVID-19 mRNA vaccine allergy. Curr. Opin. Pediatr. 2021, 33, 610–617. [Google Scholar] [CrossRef]
- Filon, F.L.; Lazzarato, I.; Patriarca, E.; Iavernig, T.; Peratoner, A.; Perri, G.; Ponis, G.; Rocco, G.; Cegolon, L. Allergic Reactions to COVID-19 Vaccination in High-Risk Allergic Patients: The Experience of Trieste University Hospital (North-Eastern Italy). Vaccines 2022, 10, 1616. [Google Scholar] [CrossRef]
- Blumenthal, K.G.; Robinson, L.B.; Camargo, C.A.; Shenoy, E.S.; Banerji, A.; Landman, A.B.; Wickner, P. Acute Allergic Reactions to mRNA COVID-19 Vaccines. JAMA 2021, 325, 1562. [Google Scholar] [CrossRef] [PubMed]
- Żurek, W.; Baron, M.; Moos, Ł.; Kapeluszna, K.; Starczewska-Dymek, L.; Brzoza, Z. Vaccination against the SARS-CoV-2 virus in patients undergoing Hymenoptera venom immunotherapy. Adv. Dermatol. Allergol. 2024, 41, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Teufelberger, A.R.; Dan, A.-R.; Irmler, L.; Wolf, P.; Kränke, B. COVID-19 vaccines: Anaphylaxis and anxiety: A case study from an allergy unit. Wien. Klin. Wochenschr. 2024, 136, 590–597. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Patients with SIT (n = 57) |
|---|---|
| Age | |
| Median (IQR) | 43.0 (28.0) |
| Mean ± SD | 46.2 ± 16.8 |
| Sex (m:f) | 33:24 |
| Mastocytosis, n (%) | 3 (5.3%) |
| Number of pos. Allergy Categories * | |
| Median (IQR) | 1.0 (1.0) |
| Mean ± SD | 1.4 ± 0.7 |
| Prior Anaphylaxis, n (%) | 34 (59.6%) |
| No Reaction | Unspecific Reaction | Possible Allergic Reaction | Significance | |
|---|---|---|---|---|
| (n = 22) | (n = 28) | (n = 1) | p-Value | |
| Basic characteristics | ||||
| Number of vaccinations | 0.099 | |||
| Median (IQR) | 3.0 (1.0) | 3.0 (0.0) | 2.0 | |
| Mean ± SD | 3.3 ± 0.6 | 3.1 ± 0.6 | ||
| Age (y) | ||||
| Median (IQR) | 54.0 (22.0) | 40.5 (28.8) | 39.0 | 0.199 |
| Mean ± SD | 51.6 ± 15.6 | 43.8 ± 17.2 | ||
| Sex (m:f) | 13:9 | 17:11 | 0:1 | 0.479 |
| Allergy group | 0.278 | |||
| Hymenoptera venom | 14 (63.3%) | 13 (46.4%) | 0 (0%) | |
| Aeroallergen | 8 (36.65%) | 15 (53.6%) | 1 (100%) | |
| Serology | ||||
| Total IgE | 0.43 | |||
| Median (IQR) | 94.0 (352.3) | 135.0 (175.0) | 332.0 | |
| Mean ± SD | 199.7 ± 187.6 | 266.6 ± 669.5 | ||
| Tryptase | (n = 21) | (n = 27) | ||
| Median (IQR) | 5.1 (3.2) | 5.2 (3.9) | 4.1 | 0.857 |
| Mean ± SD | 6.2 ± 6.1 | 7.1 ± 5.8 | ||
| Comorbidities | ||||
| Allergic rhinitis | 9 (40.9%) | 18 (64.3%) | 1 (100%) | 0.169 |
| Asthma | 4 (18.2%) | 5 (17.9%) | 0 (0%) | 0.896 |
| Atopic dermatitis | 1 (4.5%) | 5 (17.9%) | 0 (0%) | 0.326 |
| Urticaria | 2 (9.1%) | 1 (3.6%) | 0 (0%) | 0.69 |
| Mastocytosis | 1 (4.5%) | 2 (7.1%) | 0 (0%) | 0.899 |
| Anxiety | (n = 19) | 0.331 | ||
| Median (IQR) | 5.0 (5.0) | 4.0 (3.8) | 11.0 | |
| Mean ± SD | 4.6 ± 3.3 | 4.6 ± 3.2 | ||
| Depression | (n = 19) | 0.229 | ||
| Median (IQR) | 2.0 (5.0) | 2.0 (3.0) | 11.0 | |
| Mean ± SD | 3.1 ± 2.9 | 2.6 ± 2.9 | ||
| Concerns before vaccination | <0.001 | |||
| Median (IQR) | 0.0 (1.3) | 0.0 (1.0) | 4.0 | |
| Mean ± SD | ±0.8 | 0.6 ± 0.8 | ||
| COVID-19 infection before vaccination | (n = 21) | (n = 27) | 0.725 | |
| 2 (9.5%) | 4 (14.8%) | 0 (0%) | ||
| Prior reactions to medication | ||||
| Anamnestic allergic reactions after oral medication | 4 (18.2%) | 3 (10.7%) | 1 (100%) | 0.05 |
| Anamnestic allergic reactions after injectable medication | 0 (0%) | 1 (3.6%) | 0 (0%) | 0.658 |
| Anamnestic unspecific reactions to other vaccines (e.g., influenza) | (n = 27) | |||
| 1 (4.5%) | 9 (33.3%) | 0 (0%) | <0.001 | |
| Prior anaphylaxis | ||||
| Any prior anaphylaxis | 15 (68.2%) | 13 (46.4%) | 1 (100%) | 0.213 |
| Anamnestic anaphylaxis to other vaccines (e.g., influenza) | 0 | 0 | 1 (100%) | <0.001 |
| Premedication | ||||
| Prophylactic medication with antihistamines | (n = 21) 5 (23.8%) | 1 (3.6%) | 1 (100%) | 0.006 |
| Patients in Hymenoptera Allergy Group (n = 27) | Patients in Aeroallergy Group (n = 24) | Significance p | |
|---|---|---|---|
| No reaction | 14 (51.9%) | 8 (33.3%) | 0.201 |
| Unspecific reaction | 13 (48.1%) | 15 (62.5%) | 0.705 |
| Possible allergic reaction | 0 (0%) | 1 (4.2%) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kogseder, N.; Puxkandl, V.; Hötzenecker, W.; Altrichter, S. COVID-19 Vaccines: Tolerance of Vaccination in Patients with Allergies. Vaccines 2025, 13, 904. https://doi.org/10.3390/vaccines13090904
Kogseder N, Puxkandl V, Hötzenecker W, Altrichter S. COVID-19 Vaccines: Tolerance of Vaccination in Patients with Allergies. Vaccines. 2025; 13(9):904. https://doi.org/10.3390/vaccines13090904
Chicago/Turabian StyleKogseder, Natalie, Viktoria Puxkandl, Wolfram Hötzenecker, and Sabine Altrichter. 2025. "COVID-19 Vaccines: Tolerance of Vaccination in Patients with Allergies" Vaccines 13, no. 9: 904. https://doi.org/10.3390/vaccines13090904
APA StyleKogseder, N., Puxkandl, V., Hötzenecker, W., & Altrichter, S. (2025). COVID-19 Vaccines: Tolerance of Vaccination in Patients with Allergies. Vaccines, 13(9), 904. https://doi.org/10.3390/vaccines13090904







