Abstract
Objectives: Hepatitis E virus (HEV) infection is associated with severe hepatitis and high mortality rates, yet vaccination coverage remains suboptimal. Investigating public preferences for HEV vaccination is critical for developing targeted prevention strategies. This study employed a discrete choice experiment (DCE) to quantify attribute preferences and willingness-to-pay (WTP) for HEV vaccination among Chinese residents (in Shaanxi Province, for example), aiming to inform evidence-based immunization policy optimization. Methods: A cross-sectional survey recruited 3300 participants using stratified random sampling. The vaccine attributes—protective efficacy, duration of protection, and out-of-pocket cost—were identified using a systematic literature review and expert consultation. A comparative analysis of preference characteristics was conducted using conditional logit (Model 1) and mixed logit (Model 2) regression models. Population heterogeneity in vaccination preferences was further analyzed using the conditional logit framework, with marginal WTP estimated using parameter coefficients. Results: Among 3199 valid responses, duration of protection (Model 2: 10-years; β = 0.456, p < 0.001) and out-of-pocket cost (Model 2: 2000–3000 CNY; β = −0.179, p < 0.001) significantly influenced vaccination decisions. Preference heterogeneity was observed: women of childbearing age prioritized longer protection (10 years; β = 0.677, p < 0.001) and were sensitive to the cost of 1000–2000 CNY (β = 0.169, p = 0.011), while urban residents valued extended protection more than rural counterparts. Conclusions: Protection duration emerged as the primary determinant of HEV vaccination preference. Policy recommendations include implementing tiered pricing strategies and targeted health education campaigns emphasizing long-term protection benefits to enhance vaccine uptake and affordability.
1. Introduction
Viral hepatitis, as one of the critical global public health challenges, directly impacts the achievement of the United Nations Sustainable Development Goals. Hepatitis E virus (HEV) infection, recognized as the leading cause of acute sporadic viral hepatitis worldwide, has persistently suffered from severe underestimation of its disease burden. HEV spreads via fecal–oral transmission, bloodborne routes, and zoonotic pathways, exhibiting endemic prevalence in low-resource settings and low-to-middle-income countries [1]. Annually, HEV accounts for approximately 20 million infections worldwide, including 3.3 million symptomatic cases and 44,000 fatalities [2]. Despite the generally self-limiting course and favorable prognosis observed in most immunocompetent infected individuals [3], HEV infection can lead to catastrophic outcomes in specific high-risk populations. Pregnant women, chronic liver disease patients, and immunocompromised individuals face elevated risks of fulminant hepatitis, hepatic failure, and mortality. Notably, third-trimester HEV infections incur case fatality rates of 20–25% [4], which is much higher than in the general population. It is also significantly associated with adverse pregnancy outcomes such as preterm birth, stillbirth, and vertical transmission. In China, the disease burden of hepatitis E is also not negligible. Since 2012, the incidence rate of hepatitis E in China (2.02 per 100,000) has surpassed that of hepatitis A (1.81 per 100,000) [5]. The substantial burden of this disease is further exacerbated by high hospitalization costs and the lack of specific therapeutic agents [6]. Shaanxi Province serves as a crucial region in Northwest China due to its high representativeness in terms of China’s geography, demographics, and socioeconomic characteristics. Geographically spanning both northern and southern China, Shaanxi encompasses the urbanization of the Guanzhong Plain, the features of the Loess Plateau in northern Shaanxi, and the mountainous terrain of the Qinba Mountains in southern Shaanxi. Its population structure, economic development level, distribution of healthcare resources, and ethnic composition (predominantly Han Chinese with some minority groups) collectively make it a microcosm of China. The global seroprevalence of anti-HEV IgG is 12.47% [7], indicating widespread prior infection. A 2017 seroepidemiological survey in Shaanxi Province of China revealed a significantly higher prevalence of 15.40% among the general population [8], classifying it as a high-endemic region for hepatitis E. However, underdiagnosis due to suboptimal HEV detection sensitivity likely underestimates disease burden [9,10], underscoring the urgency for enhanced vaccination in high-risk populations and foodborne transmission control.
No HEV-specific antiviral therapies have been globally approved, leaving supportive care as the primary clinical intervention [9,11]. Vaccination thus emerges as the cornerstone strategy for HEV containment. Notably, China has achieved a groundbreaking milestone in this field with the domestically developed recombinant hepatitis E vaccine (expressed in Escherichia coli system) pioneered by Xiamen University and collaborators, which received initial regulatory approval in 2012 for individuals aged 16 years and above. Pivotal phase III clinical trial data and extended follow-up studies demonstrated 86.8% protective efficacy against symptomatic hepatitis E over 4.5 years post-vaccination, accompanied by robust immunogenicity evidenced by a seroconversion rate exceeding 98% following the complete vaccination schedule [12]. This vaccine represents the world’s first and currently sole licensed prophylactic HEV vaccine, marking a significant milestone in global public health. WHO’s 2015 position paper endorses its protective efficacy in healthy Chinese populations aged 16–65 and advocates context-specific immunization strategies [13]. Globally, systematic vaccination campaigns achieved 58% full vaccination coverage in South Sudan’s high-endemicity zones [14] and Bentiu region has reached 95% coverage in displaced persons camps (27,000 cases) by April 2022 [15]. In contrast, China reports only 8.81% vaccine uptake in Shanghai [16], with regional data elsewhere remaining unreported. This mismatch between immunological protection and disease burden is most pronounced among women of childbearing age, a critical target group given their elevated mortality risk during late pregnancy. Despite this urgency, HEV vaccination uptake remains suboptimal due to multifaceted barriers including vaccine safety concerns, fragmented health communication, and reproductive health considerations.
Understanding individual preferences is paramount for optimizing resource allocation and enhancing intervention uptake in public health policymaking and health economic evaluations. This is particularly pertinent to vaccination decisions, where success critically depends on individuals’ voluntary engagement and active participation.
Discrete choice experiments (DCEs) have emerged as a robust tool for vaccine preference research, simulating real-world decision-making contexts. Spanish studies reveal parental prioritization of vaccine safety versus clinicians’ efficacy focus [17]. New Zealand research shows that the risk of adverse reactions to vaccines is the attribute that the general public cares about most, but adolescents are more concerned about the burden of disease [18]. Guizhou’s DCE application highlights parental sensitivity to pneumococcal vaccine pricing and accessibility [19]. Over 45 DCE studies in the past five years have mapped preferences for influenza and HPV vaccines across diverse populations [20]. These studies not only validate the robustness and cross-cultural applicability of DCE, but also accumulate rich practical experience. However, HEV vaccine preference research remains conspicuously absent. Given China’s unique position as the sole country with a licensed HEV vaccine globally, coupled with the pronounced disparity between high disease burden and low vaccination uptake, this research gap becomes particularly critical to address. Consequently, this study focuses on Shaanxi Province—a representative high-endemic region for hepatitis E in China—employing a rigorous discrete choice experiment (DCE) methodology integrated with advanced econometric models (e.g., mixed logit models) to propose a systematic analysis of the preference characteristics of Shaanxi residents for HEV vaccination, quantify the impact of vaccine attributes on decision making, estimate the willingness-to-pay (WTP), and dissect subgroup heterogeneity. The findings are anticipated to provide pivotal scientific evidence for developing precision-targeted and effective HEV vaccination promotion strategies and immunization policies in Shaanxi Province and nationwide. By identifying key attributes and population characteristics that drive or hinder vaccination uptake, the results will directly inform policymakers to optimize vaccine attributes (e.g., enhancing communication strategies to emphasize safety evidence), refine pricing and financing mechanisms, improve accessibility and convenience of vaccination services, and design tailored health education and social mobilization programs for specific subpopulations (particularly high-risk groups with low willingness such as women of childbearing age).
Ultimately, this research will contribute substantially to increasing HEV vaccine coverage, reducing morbidity and mortality among high-risk populations, and alleviating the disease burden of this long-neglected major public health challenge.
2. Materials and Methods
2.1. Participant Recruitment
The study utilized data from Shaanxi Province’s 2017 hepatitis B seroepidemiological survey to select 10 districts/counties across 8 cities (Yan’an City, Yulin City, Xi’an City, Baojing City, Xianyang City, Weinan City, Shangluo City, and Ankang City), spanning three geographical regions (Guanzhong, Northern Shaanxi, and Southern Shaanxi), as detailed in Figure 1. Sampling sites encompassed urban communities and rural townships with varying urbanization levels, socioeconomic structures, healthcare resource allocations, and population densities to comprehensively represent HEV vaccination demand.
Figure 1.
Geographical distribution map of sampling districts and counties in Shaanxi Province.
(1) Recruitment Protocol
This study employed a stratified random sampling approach to select representative residents at the county level. Initially, within each of the 10 pre-selected counties/districts, three administrative units (i.e., administrative villages or urban communities) were randomly chosen as primary sampling sites using simple random sampling. Subsequently, within each selected village/community, eligible permanent resident registries (household registration or residency records) were obtained from local village/neighborhood committees or community health centers to serve as sampling frames. Predetermined numbers of target households were then systematically selected from these registries using computer-generated random number tables.
(2) Inclusion Criteria
Research subjects must meet all of the following conditions:
- (a)
- Permanent residency: Continuous residence ≥6 months in the sampled administrative village/community as of the survey date, irrespective of household registration status;
- (b)
- Age range: 15 to 66 years inclusive, verified through government-issued identification or household registration documents;
- (c)
- Informed consent: After trained investigators comprehensively explained the study purpose, procedures, potential benefits/risks (primarily time commitment and privacy safeguards), voluntary participation, right to withdraw unconditionally, and confidentiality measures in a clear, non-suggestive manner, participants provided written informed consent. For non-literate individuals, impartial witnesses attested to the complete disclosure process before participants affixed their thumbprint, with both investigator and witness countersigning.
(3) Exclusion Criteria
Those who meet any of the following conditions will be excluded:
- (a)
- Comprehension and communication barriers: Individuals with severe hearing, linguistic, or cognitive impairments (e.g., intellectual disability, advanced dementia) were excluded if investigators assessed them as incapable of comprehending survey content or engaging in basic communication during the consent process;
- (b)
- Psychiatric disorders: Clinically diagnosed patients with severe mental illnesses (e.g., schizophrenia, bipolar disorder in acutely symptomatic phase) exhibiting unstable conditions that could impair rational decision making or study understanding.
2.2. Study Design
2.2.1. Attribute and Level Identification
A systematic literature review [21,22,23] identified vaccine efficacy, cost, duration of protection, and adverse event risk as primary determinants of public vaccination preferences. Five candidate attributes were initially proposed based on contextual analysis of 10 Shaanxi counties: protective efficacy, duration of protection, severe adverse event rate, vaccination site, and out-of-pocket cost. To ensure the attributes and levels employed in the DCE authentically, effectively, relevantly, and actionably reflect the actual preferences and decision trade-offs of the target population in Shaanxi Province, the research team implemented a rigorous two-phase screening and validation protocol:
Phase 1: Expert Panel Review
A six-member advisory panel was convened, comprising authorities with extensive expertise in infectious disease control, vaccinology, health economics, epidemiology, public health policy, and primary healthcare management. Panelists were recruited from provincial Centers for Disease Control and Prevention (CDC), university schools of public health, and primary healthcare institutions within sampled counties to ensure diversified perspectives. Focus group discussions were conducted to critically evaluate the scientific validity, practical relevance, and potential implementation challenges of proposed attributes.
Regarding the “serious adverse event incidence rate” attribute, the panel presented the following key arguments during in-depth deliberation:
- (a)
- Post-marketing surveillance data from long-term, large-scale HEV vaccination programs (e.g., the China-licensed vaccine [13]) demonstrate an incidence rate orders of magnitude lower than common vaccines.
- (b)
- China’s established and rigorous Adverse Events Following Immunization (AEFI) surveillance system ensures robust safety monitoring, maintaining relatively high public trust.
The consensus indicated that retaining this attribute would likely fail to discriminate preferences while unnecessarily inflating questionnaire complexity and respondent cognitive burden. Consequently, the panel strongly recommended its exclusion—a recommendation subsequently implemented.
Phase 2: Pilot Survey and Cognitive Testing
The retained attributes (efficacy, duration, location, and out-of-pocket cost) and their levels underwent empirical testing within the target population to assess comprehensibility, realism, acceptability, and discriminatory power. One representative county (encompassing urban and rural settings) was purposefully selected from the 10 sampled counties in Shaanxi Province. Approximately 100 eligible residents were recruited using convenience sampling supplemented by quota sampling to achieve balanced age, gender, and urban–rural distribution.
Participants first completed the draft questionnaire containing DCE choice sets. Subsequently, trained researchers conducted one-on-one cognitive interviews. Feedback indicated the following:
- (a)
- Most participants clearly understood “efficacy,” “duration,” and “cost” attributes.
- (b)
- Regarding “vaccination location,” respondents widely reported minimal perceived differences in convenience (e.g., distance, time cost) across healthcare tiers (village clinics, township health centers, county hospitals) due to Shaanxi’s well-established primary healthcare network. Typical responses included the following: “Similar convenience regardless of site” or “Routine vaccination occurs at nearest local clinics.”
This finding suggested limited discriminatory utility for the location attribute. Consequently, the decision was made to exclude “vaccination location” to streamline the questionnaire, focus on core decision drivers, and enhance data validity and reliability.
Through a rigorous tripartite screening protocol encompassing a literature review, expert panel review, and target population pilot validation, three key attributes that best capture the fundamental determinants of hepatitis E vaccination decision making among Shaanxi residents were ultimately identified for the formal discrete choice experiment (Supplementary Table S1).
2.2.2. DCE Framework
A full factorial design generated 18 theoretical vaccine profiles (2 efficacy levels × 3 duration levels × 3 cost levels).
Given that a full factorial design comprising 18 vaccine profiles would generate 153 combinations if employing an initial choice set framework with two randomly paired profiles sampled without replacement—significantly increasing respondent burden—this study adopted a D-optimal design strategy to balance methodological rigor and respondent experience. Specifically, the %ChoicEff macro in SAS 9.4 software was utilized to screen 20 statistically optimal choice sets from the full factorial design space, achieving a D-efficiency score of 14.30. Each choice set included two vaccine profiles with differing attribute levels and an “opt-out” option, ensuring pronounced inter-profile attribute variability while controlling cognitive burden. Example choice sets are provided in Supplementary Table S2.
To enhance data collection efficiency, the 20 choice sets were partitioned into four randomized blocks (5 sets per block) using a randomized block design.
2.2.3. Questionnaire Development and Piloting
A structured questionnaire was developed through literature synthesis, semi-structured interviews, and expert consultations. Closed-ended questions were prioritized for analytical efficiency. The questionnaire comprised:
- Instructions: Study background, objectives, and completion guidelines.
- Demographics: Age, gender, education, income, etc.
- Health status: Chronic liver disease history, prior vaccination experience.
- Vaccination intent: Willingness to receive HEV vaccination.
- DCE section: Presented choice sets requiring participants to select preferred alternatives.
A pilot study involving 100 participants in Huayin County of Shaanxi Province assessed attribute interpretability and questionnaire feasibility. Attributes and levels were refined based on pretest outcomes, while ambiguous or inappropriate items were revised to enhance logical coherence and respondent clarity.
2.3. Sample Size Estimation
The precision and stability of parameter estimation in DCE, a stated-preference methodology grounded in random utility theory, are highly dependent on sample size. This study integrated two mainstream approaches for sample size determination.
First, following the empirical rule proposed by Johnson and Orme [24]—widely adopted in DCE research—the minimum sample size was calculated using the formula:
where t denotes the number of choice sets (5), a the alternatives per choice set (2), and c the maximum attribute level count (3). Substituting these parameters yielded a minimum requirement of n > 150, but this only meets the most basic requirements for model parameter estimation. However, it is generally insufficient for complex analyses or detecting small effect sizes between attribute levels with high precision.
Given that empirical rules provide only a baseline threshold, this study further referenced empirical DCE studies and scholarly recommendations. As noted by Sargazi et al. [25] and Hofman et al. [26], multiple simulation analyses and practical DCE implementations suggest that sample sizes of 300–400 participants (or generally >300) provide adequate statistical power for robust and reliable parameter estimation. This approach prioritizes analytical feasibility and result stability, complementing the lower bound derived from empirical formulas.
Based on the above dual criteria, this study adopted a stratified sampling strategy:
- (a)
- Basic sample: 300 residents were sampled from each of the 10 sample counties, for a total of 3000 people.
- (b)
- Professional sample: An additional 300 healthcare workers were selected.
The total sample size is 3300 individuals, which significantly exceeds the minimum sample requirement (150). This design meets the needs for obtaining robust parameter estimates, exploring potential preference heterogeneity, conducting subgroup comparison analyses, and maintaining high statistical test power.
2.4. Data Collection
This study collected data through an electronic questionnaire system. Survey points were set up in vaccination clinics at county-level hospitals, township health centers, and community health service centers. Participants scanned a QR code or clicked on a dedicated link to access the online platform and complete the questionnaire. Trained investigators provided standardized instructions on study objectives and completion protocols. To ensure questionnaire quality, questionnaires were considered invalid under the following criteria: (1) logical inconsistencies were identified if respondents refused a clearly dominant alternative within any DCE choice set (e.g., selecting “80–90% protective efficacy + 10-year duration + 2000–3000 CNY” while rejecting “90–100% protective efficacy + 30-year duration + 0–1000 CNY”); (2) critical data omissions were deemed invalid if respondents selected “Opt-out” for all choice sets. Of 3300 distributed questionnaires, 3199 valid responses were retained after excluding invalid submissions (logical inconsistencies or critical data omissions), yielding a 96.94% valid response rate.
2.5. Data Analysis Methods
2.5.1. Descriptive Statistics
Statistical analyses were conducted using Stata 17.0. Continuous variables were summarized as mean (standard deviation, SD), while categorical variables were reported as frequency (n) and percentage (%).
2.5.2. Mixed Logit Model
The mixed logit model was employed as the core analytical method, incorporating random parameters to capture individual preference heterogeneity. This model was selected based on its significantly superior goodness-of-fit compared to the conditional logit model, as evidenced by lower Akaike Information Criterion (AIC)/Bayesian Information Criterion (BIC) values.
Random parameters were specified as follows:
- (a)
- Random parameters: protective efficacy, duration of protection, and out-of-pocket cost;
- (b)
- Distributional assumption: All random parameters were assumed to follow a normal distribution;
- (c)
- Correlation structure: Random parameters were specified as mutually independent, resulting in a diagonal covariance matrix (implemented by omitting the “corr” option in Stata17.0’s “mixlogit” command);
- (d)
- Variance estimation: The model estimated the standard deviation and statistical significance of each random parameter.
The model was fitted using simulated maximum likelihood estimation (SMLE). Estimation efficiency was enhanced through 500 simulation repetitions, achieved by setting “nrep(500)’’ in Stata 17.0. Covariates included in the model are detailed in Table 1. Subsequently, interaction terms between covariates and subgroup variables were introduced to identify between-group differences. Statistical significance (p < 0.05) of interaction coefficients and their 95% confidence intervals (CI) served as the criteria for interpretation.

Table 1.
Included variables.
2.5.3. WTP Analysis
Marginal WTP was calculated via the mixed logit model using the formula:
where x denotes vaccine attributes, βx the regression coefficient for attribute x, and βcost the coefficient for out-of-pocket cost. Furthermore, this formula requires the cost variable to exhibit linear (continuous) effects within the utility function. Given that the cost data in this study were collected in categorical interval format, they were converted into a continuous variable during subsequent analyses to approximate compliance with this assumption. Specifically, midpoint imputation was applied for conversion (e.g., the interval “A–B” was assigned the value (A + B)/2).
Mixed logit models were used in all analyses exploring subgroup preference heterogeneity or residential heterogeneity in vaccine preferences for the hepatitis E vaccine.
2.6. Ethics
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the First Affiliated Hospital of Xi’an Jiaotong University (protocol code XJTU1AF2025LSYY-021). All participant information was maintained under strict confidentiality, with questionnaires identified solely by research codes. The linkage table connecting personal identifiers to research data was encrypted and stored separately. Participants retained the unconditional right to withdraw from the study at any stage without incurring adverse consequences. Findings will be reported exclusively in aggregated form, ensuring no individual-level information is disclosed.
3. Results
3.1. Participant Characteristics
The study cohort comprised 3199 participants with a mean age of 38.11 ± 13.83 years. Females accounted for 51.58% of the sample, with 79.27% residing in rural areas. Educational attainment was predominantly at the junior high school level (31.20%). Annual income distribution revealed 34.82% earning between 10,000 and 30,000 CNY. Occupational profiles indicated industrial/commercial workers and production practitioners as the largest group (65.74%), followed by students (14.07%), with retirees representing the smallest proportion (1.25%). Hepatitis-related medical history was rare, with 2.00% reporting familial hepatitis history and 0.53% having personal hepatitis diagnoses. Detailed demographic characteristics are presented in Table 2.

Table 2.
Demographic characteristics of subjects.
3.2. Attribute-Specific Influences on Vaccination Decisions
The goodness-of-fit of the conditional logit model (Model 1) and mixed logit model (Model 2) is presented in Table 3. Duration of protection emerged as the dominant attribute, particularly favoring 10-year coverage (Model 1: β = 0.407, p < 0.001; Model 2: β = 0.456, p < 0.001). Out-of-pocket cost exhibited consistent negative associations across models. Protective efficacy showed statistically non-significant effects in both models (Table 4 and Table 5).

Table 3.
Model goodness-of-fit.

Table 4.
Influence of vaccine attributes on vaccine choice preferences based on the conditional logit model (Model 1).

Table 5.
Influence of vaccine attributes on vaccine choice preferences based on the mixed logit model (Model 2).
3.3. WTP Analysis for Hepatitis E Vaccine
WTP estimates revealed limited economic valuation for efficacy improvements (80–90% to 90–100%: WTP = 0.102 CNY, p = 0.802). Conversely, extended duration of protection (5 to 10 years) generated substantial WTP increments (7.077 CNY, p < 0.001), indicating strong preference for prolonged coverage (Table 6). This result is calculated based on the converted continuous cost variable.

Table 6.
Calculation of willingness-to-pay based on mixed logit model.
3.4. Subgroup Preference Heterogeneity
Both population groups exhibited a highly significant preference for longer protection durations (10 years/30 years vs. 5 years; p < 0.001), indicating that extended protection duration is a core attribute for improving vaccination willingness (Figure 2 and Figure 3). In contrast, enhanced efficacy (90–100% vs. 80–90%) showed no significant impact in either group (healthcare workers: p = 0.101; women of childbearing age: p = 0.928).
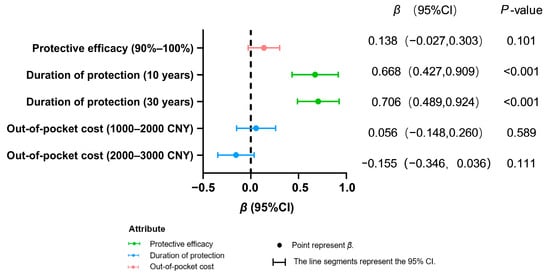
Figure 2.
Preference analysis of health workers’ decision making on hepatitis E vaccination.
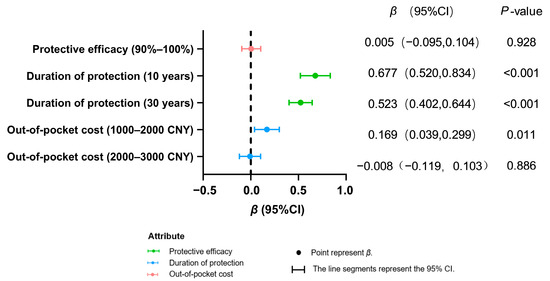
Figure 3.
Preference analysis of women of childbearing age for decision making on hepatitis E vaccination.
Among healthcare workers, increased cost (2000–3000 CNY vs. 0–1000 CNY) demonstrated no significant negative effect (p = 0.111). For women of childbearing age, greater acceptance was observed for moderate costs (1000–2000 CNY; β = 0.169, p = 0.011), while no significant resistance emerged toward higher costs (2000–3000 CNY: β = −0.155, p = 0.111). This result is based on calculations using the mixed logit model.
3.5. Residential Heterogeneity in Vaccine Preferences
Residents from both rural and urban areas showed no preference for paying 1000–2000 CNY for vaccination (p = 0.345 vs. p = 0.644, respectively). Compared to short-term protection (5 years), both groups exhibited a significant preference for longer protection durations (10 years/30 years; p < 0.001 for both). Regarding enhanced efficacy (90–100% vs. 80–90%), rural residents showed no response (β = −0.010, p = 0.739), while urban residents demonstrated a non-significant positive trend (β = 0.106, p = 0.168) (Table 7). This result is based on calculations using the mixed logit model.

Table 7.
Analysis of heterogeneity in hepatitis E vaccine choice preferences among residents of different places of residence.
4. Discussion
The duration of protection is a core factor influencing the preference for hepatitis E vaccines. The study reveals that protection periods of 10 and 30 years are particularly favored by residents, with a pronounced preference for the 10-year duration. This tendency might be related to the cost reduction associated with long-lasting vaccines, a pattern also confirmed in other vaccine studies: parents were willing to pay more for a protection period exceeding 10 years for pneumococcal vaccines [19], protection duration was the primary concern in the Australian meningitis vaccine survey [27], and both Chinese and American residents showed a preference for long-lasting vaccines in the choice of COVID-19 vaccines [28]. From a public health perspective, extending the vaccine protection period has dual benefits. On one hand, long-lasting vaccines can increase vaccination willingness by reducing the frequency of vaccinations; on the other hand, enduring protective effects can enhance public trust in vaccine efficacy, thereby creating a virtuous cycle of “high trust-high vaccination rates.”
Optimizing the out-of-pocket mechanism for hepatitis E vaccination to enhance long-term protection awareness and increase willingness-to-pay. This study reveals distinct characteristics of group sensitivity to out-of-pocket costs associated with hepatitis E vaccination. Economic burdens significantly impact vaccination decisions, with low-income groups being particularly sensitive. This finding is consistent with numerous prior studies [29,30,31]. Data indicate that 2000 CNY may represent a critical psychological threshold. When the cost is below this level, the essential demand for the vaccine or the positive perception of other attributes (such as long-term protection) effectively reduces price resistance; however, once this threshold is surpassed, sensitivity to price markedly increases. This suggests that controlling costs within a reasonable range can effectively eliminate economic barriers. In other studies, the positive influence of vaccine efficacy on willingness-to-pay has been corroborated. A study conducted in the United States [32] demonstrated that when vaccine efficacy rises from 50% to 95%, the willingness-to-pay increases by 21.7%, with similar trends observed in COVID-19 vaccine data [33]. This study does not observe similar results. The potential reason may be that the increase in vaccine efficacy (from 80–90% to 90–100%) fails to significantly enhance the respondents’ willingness-to-pay. The relationship between duration of protection and willingness-to-pay is nonlinear: the price premium for extending protection from 5 years to 10 years is higher than that for extending it from 10 years to 30 years. This may relate to health planning cycles (such as risk windows at specific age intervals) and expectations of technological advancements. The risk aversion associated with medium- to long-term immunity directly translates into payment incentives; however, excessively long protection periods may lead to diminishing marginal utility due to a disconnect from actual needs.
Understanding the vaccination needs for hepatitis E among women of childbearing age is vital for enhancing health education and effectively promoting tailored vaccination programs. This demographic plays a crucial role in hepatitis E prevention, and the existing literature suggests that vaccination behaviors are significantly influenced by individual characteristics such as income and education levels [34,35,36]. However, there is a lack of explicit mention in the literature regarding their preferences for vaccine efficacy and protection duration. This study identifies important insights regarding the vaccination demand among women of childbearing age: this group exhibits a stronger sensitivity to vaccine protection duration (10 years or 30 years) and out-of-pocket cost than to the efficacy range (80–90% vs. 90–100%). However, when costs increase, especially when prices exceed 2000 CNY, vaccination willingness decreases significantly (β = −0.008, p = 0.886). Such preferences are closely linked to their unique reproductive health needs; opting for long-lasting vaccines can reduce the frequency of required vaccinations and provide a sustained immune barrier for their families. Additionally, in the context of rising medical expenses and childcare costs, economic accessibility emerges as a critical factor in their decision-making process. Based on these findings, a “demand-driven” intervention strategy is recommended: health education should emphasize the long-term protective benefits of vaccines, and precise recommendations are implemented through counseling by medical personnel to communicate birth plans and economic status.
It is important to provide precise and personalized vaccination programs informed by heterogeneity in population preferences. While previous studies [37,38,39,40] primarily examined disparities in vaccination rates between urban and rural populations, this research delves deeper into the differing preferences for vaccine characteristics among these groups. The data show that there is consensus among urban and rural groups on two aspects: the value of long-term protection is generally recognized and both are sensitive to out-of-pocket costs. Based on these findings, differentiated strategies are recommended. Specifically, mid-term protective vaccines (5–10 years) should be promoted in rural areas, supported by community health workers leading public education on “illustrating vaccine efficacy”; meanwhile, urban areas should prioritize long-lasting vaccines and implement a tiered medical insurance reimbursement structure to alleviate financial burdens, thus enhancing vaccination efficiency and reducing health equity gaps.
A stratified sampling strategy encompassing 10 urban and rural districts/counties with heterogeneous socioeconomic profiles in Shaanxi Province ensured geographical representativeness. In the experimental design phase, a full factorial design and D-optimal design were utilized to select the 20 choice sets with optimal statistical efficiency. Block design was applied to balance the complexity of the options, ensuring analytical power while reducing cognitive load on respondents. In terms of quality control, the research team conducted a pre-survey in Huayin County to refine the attribute settings of the questionnaire, providing standardized training to enumerators to ensure response consistency. Data collection adopted a mixed-mode approach, integrating online and offline methods, where the mandatory options of the electronic questionnaire was combined with on-site guidance to effectively avoid invalid responses and missing key information. Dual-entry verification and quality audits enhanced data integrity and accuracy.
However, this study presents several limitations. Methodologically, the DCE requires respondents to make trade-off decisions across multiple attributes, which may pose barriers to understanding for individuals with lower educational backgrounds. Although the questionnaire design was improved through a pre-survey, completely eliminating the risk of cognitive bias remains challenging, particularly regarding potential biases in decision making. Geographically, while the sample encompasses various regions of Shaanxi Province, caution should be exercised when extrapolating findings to other settings due to the specific characteristics of healthcare resource allocation in these areas.
A limitation of this study stems from the approach to cost data collection. Cost information was obtained as predefined intervals rather than precise continuous values. Although these intervals were converted into a continuous variable (using midpoint imputation) for WTP calculations during analysis, this transformation relies on assumptions regarding the cost distribution within intervals (presumed to be uniform). Consequently, the reported WTP values should be interpreted as approximations of the true continuous marginal WTP. Future research designs should prioritize collecting more precise cost data (e.g., actual payment amounts or finer cost levels) or employ specialized modeling techniques for interval data (e.g., interval regression) to enhance the precision of WTP estimates. Nevertheless, the WTP estimates presented herein retain significant value for comparing the relative importance of attributes and informing management decisions.
5. Conclusions
This study identifies the duration of protection as the key influencing factor in HEV vaccination decisions. The public shows a strong preference for vaccines that provide long-term protection for 10 years, while improvements in protective efficacy do not significantly affect their choices. Vaccination willingness decreases significantly when out-of-pocket costs exceed 2000 CNY, indicating pronounced economic sensitivity. Women of childbearing age are particularly focused on long-term protection, while both urban and rural residents acknowledge the importance of protection duration. Policy recommendations include the following: (1) prioritizing vaccines with ≥10-year protection; (2) tiered pricing strategies to mitigate financial barriers; (3) demographic-specific immunization programs.
Future research should consider expanding the sample size and geographic coverage, exploring additional attributes and their levels, employing advanced modeling techniques (e.g., latent class analysis) to refine preference heterogeneity mapping, and integrating long-term follow-up surveys to provide a comprehensive assessment of vaccination preferences and their influencing factors.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/vaccines13090906/s1, Table S1: Attributes and levels of included vaccines; Table S2: Example of questionnaire choice set.
Author Contributions
Conceptualization, C.Z., Z.Z., W.H., S.Z. (Shaobai Zhang), Q.W., and L.Z.; Methodology, Y.C. and Z.Z.; Software, Y.C.; Formal Analysis, Y.C. and Z.Z.; Investigation, Y.C., C.Z., S.Z. (Sidi Zhao), and Q.W.; Resources, Q.W.; Data Curation, Y.C. and C.Z.; Writing—Original Draft Preparation, Y.C., D.Z., and S.Z. (Sidi Zhao); Writing-Review and Editing, C.Z., Z.Z., W.H., S.Z. (Shaobai Zhang), Q.W., and L.Z.; Supervision, C.Z., W.H., D.Z., S.Z. (Shaobai Zhang), and Q.W.; Project Administration, C.Z., W.H., D.Z., and Q.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Xiamen Innovax Biotech Co., Ltd., grant number HXDSH20231374.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the First Affiliated Hospital of Xi’an Jiaotong University (protocol code XJTU1AF2025LSYY-021; Approval Date: 18 April 2025).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding authors.
Acknowledgments
We would like to express our sincere gratitude to Xiamen Innovax Biotech Co., Ltd. for their generous financial support; to the Shaanxi Provincial CDC for their coordinated leadership; and to the CDCs of Ankang, Baoji, Shangluo, Weinan, Xi’an, Xianyang, Yulin, and Yan’an for their collaborative efforts. Special thanks to all the grassroots staff and volunteers for their hard work, which ensured the smooth implementation of the program.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Zhou, L.Z.; Zheng, M.H.; Li, S.W.; Xia, N.S. Epidemiological characteristics of hepatitis E and progress in vaccine development. J. Xiamen Univ. (Nat. Sci.) 2024, 63, 378–386. [Google Scholar]
- World Health Organization. Hepatitis E. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-E (accessed on 11 March 2025).
- Songtanin, B.; Molehin, A.J.; Brittan, K.; Manatsathit, W.; Nugent, K. Hepatitis E Virus Infections: Epidemiology, Genetic Diversity, and Clinical Considerations. Viruses 2023, 15, 1389. [Google Scholar] [CrossRef]
- Pérez-Gracia, M.T.; Suay-García, B.; Mateos-Lindemann, M.L. Hepatitis E and pregnancy: Current state. Rev. Med. Virol. 2017, 27, e1929. [Google Scholar] [CrossRef]
- Dong, D.B.; Zou, S.Q.; Tang, S. Spatio-temporal epidemiological characterisation of viral hepatitis incidence in China, 2009–2019. Mod. Prev. Med. 2024, 51, 595–601. [Google Scholar]
- Kong, D.G.; Tang, W.F.; Chen, J.; Hu, Q. Attitude toward Hepatitis E vaccine and influence factors among urban residents in Wuhan. J. Public Health Prev. Med. 2018, 29, 41–44. [Google Scholar]
- Li, P.; Liu, J.; Li, Y.; Su, J.; Ma, Z.; Bramer, W.M.; Cao, W.; de Man, R.A.; Peppelenbosch, M.P.; Pan, Q. The global epidemiology of hepatitis E virus infection: A systematic review and meta-analysis. Liver Int. Off. J. Int. Assoc. Study Liver 2020, 40, 1516–1528. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Liu, X.Y.; Zhou, T.T.; Wang, R.Z.; Si, Y.; Hu, W.J. Seroprevalence of Hepatitis E Antibody among a 1-60-year-old Population of Shaanxi Province in 2017. Chin. J. Vaccines Immun. 2022, 28, 15–18. [Google Scholar]
- Li, L.J. Expert consensus on the process of in-hospital screening and management of viral hepatitis E in China. J. Clin. Hepatol. 2023, 39, 785–794. [Google Scholar]
- Tang, Z.M.; Ge, S.X. Research progress in laboratory diagnosis of hepatitis E virus infection. J. Xiamen Univ. (Nat. Sci.) 2024, 63, 345–356. [Google Scholar]
- Almeida, P.H.; Matielo, C.E.L.; Curvelo, L.A.; Rocco, R.A.; Felga, G.; Della Guardia, B.; Boteon, Y.L. Update on the management and treatment of viral hepatitis. World J. Gastroenterol. 2021, 27, 3249–3261. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, X.F.; Huang, S.J.; Wu, T.; Hu, Y.M.; Wang, Z.Z.; Wang, H.; Jiang, H.M.; Wang, Y.J.; Yan, Q.; et al. Long-term efficacy of a hepatitis E vaccine. N. Engl. J. Med. 2015, 372, 914–922. [Google Scholar] [CrossRef]
- WHO. Hepatitis E vaccine: WHO position paper, May 2015—Recommendations. Vaccine 2016, 34, 304–305. [Google Scholar] [CrossRef] [PubMed]
- Nesbitt, R.C.; Asilaza, V.K.; Gignoux, E.; Koyuncu, A.; Gitahi, P.; Nkemenang, P.; Duncker, J.; Antier, Z.; Haile, M.; Gakima, P.; et al. Vaccination coverage and adverse events following a reactive vaccination campaign against hepatitis E in Bentiu displaced persons camp, South Sudan. PLoS Neglected Trop. Dis. 2024, 18, e0011661. [Google Scholar] [CrossRef]
- Ciglenecki, I.; Rumunu, J.; Wamala, J.F.; Nkemenang, P.; Duncker, J.; Nesbitt, R.; Gignoux, E.; Newport, T.; Heile, M.; Jamet, C.; et al. The first reactive vaccination campaign against hepatitis E. Lancet Infect. Dis. 2022, 22, 1110–1111. [Google Scholar] [CrossRef]
- Yan, R.; Sun, X.D.; Li, Z.; Zhang, L.P.; Chen, H.H.; Wu, X.T.; Du, Y.; Jin, B.F.; Mei, K.W. Uptake of nine vaccines and influencing factors of vaccine hesitancy among adults in Minhang district, Shanghai. Chin. J. Viral Dis. 2023, 13, 278–285. [Google Scholar]
- Martinón-Torres, F.; de Miguel, Á.G.; Ruiz-Contreras, J.; Vallejo-Aparicio, L.A.; García, A.; Gonzalez-Inchausti, M.C.; de Gomensoro, E.; Kocaata, Z.; Gabás-Rivera, C.; Comellas, M.; et al. Societal Preferences for Meningococcal B Vaccination in Children: A Discrete Choice Experiment in Spain. Infect. Dis. Ther. 2023, 12, 157–175. [Google Scholar] [CrossRef]
- Chan, A.H.Y.; Tao, M.; Marsh, S.; Petousis-Harris, H. Vaccine decision making in New Zealand: A discrete choice experiment. BMC Public Health 2024, 24, 447. [Google Scholar] [CrossRef]
- Zhang, M.X.; Xu, S.F.; Xiong, H.Y. Selection preference study of 13-valent pneumococcal conjugate vaccine based on discrete choice experiment in Guizhou province. Health Dev. Policy Res. 2023, 26, 35–41. [Google Scholar]
- Michaels-Igbokwe, C.; MacDonald, S.; Currie, G.R. Individual Preferences for Child and Adolescent Vaccine Attributes: A Systematic Review of the Stated Preference Literature. Patient 2017, 10, 687–700. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.W.; Guo, N.; Wang, J.; Wang, C.; Ma, H. The Application of Discrete Choice Experiment in the Study of Preferences and Demands for Vaccination. Health Dev. Policy Res. 2016, 35, 5–7. [Google Scholar]
- Yang, Y.Z.; Hou, X.; Jia, Y.C.; Kang, W.; Wen, Y.; Hao, L.X. Scoping review of factors influencing vaccination preferences based on discrete choice experiments. Chin. J. Vaccines Immun. 2023, 29, 698–707. [Google Scholar]
- Zhu, S.; Chang, J.; Fang, Y. Application of discrete choice experiments in immunization service researches. Chin. J. Vaccines Immun. 2017, 23, 235–240. [Google Scholar]
- de Bekker-Grob, E.W.; Donkers, B.; Jonker, M.F.; Stolk, E.A. Sample Size Requirements for Discrete-Choice Experiments in Healthcare: A Practical Guide. Patient 2015, 8, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Sargazi, N.; Takian, A.; Yaseri, M.; Daroudi, R.; Ghanbari Motlagh, A.; Nahvijou, A.; Zendehdel, K. Mothers’ preferences and willingness-to-pay for human papillomavirus vaccines in Iran: A discrete choice experiment study. Prev. Med. Rep. 2021, 23, 101438. [Google Scholar] [CrossRef] [PubMed]
- Hofman, R.; de Bekker-Grob, E.W.; Richardus, J.H.; de Koning, H.J.; van Ballegooijen, M.; Korfage, I.J. Have preferences of girls changed almost 3 years after the much debated start of the HPV vaccination program in The Netherlands? A discrete choice experiment. PLoS ONE 2014, 9, e104772. [Google Scholar] [CrossRef]
- Marshall, H.S.; Chen, G.; Clarke, M.; Ratcliffe, J. Adolescent, parent and societal preferences and willingness to pay for meningococcal B vaccine: A Discrete Choice Experiment. Vaccine 2016, 34, 671–677. [Google Scholar] [CrossRef]
- Li, X.; Yang, L.; Tian, G.; Feng, B.; Jia, X.; He, Z.; Liu, T.; Zhao, X.; Huang, M.; Yu, W.; et al. Understanding influencing attributes of COVID-19 vaccine preference and willingness-to-pay among Chinese and American middle-aged and elderly adults: A discrete choice experiment and propensity score matching study. Front. Public Health 2023, 11, 1067218. [Google Scholar] [CrossRef]
- Yin, H.; Zhang, X.; Wang, J.H.; Wu, Q.H.; Shan, L.H.; Gao, L.J.; Chen, G.Y.; Chen, R.H.; Pan, L.; Xu, J.; et al. Self-paid direct medical expense and economic risk among hospitalized chronic disease patients in China: 2013. Chin. J. Public Health 2021, 37, 618–622. [Google Scholar]
- Sarker, A.R.; Ali, S.M.Z.; Ahmed, M.; Chowdhury, S.M.Z.I.; Ali, N. Out-of-pocket payment for healthcare among urban citizens in Dhaka, Bangladesh. PLoS ONE 2022, 17, e0262900. [Google Scholar] [CrossRef]
- Huang, A.D.; Xu, X.; Tang, L.; Huang, L.F.; Zhang, X.; Zhou, Y.; Zhang, Q.; Zhou, Z.M.; Wang, Y.; Wang, X.Q.; et al. Parental preferences for childhood vaccination regimens with diphtheria, tetanus, and acellular pertussis; hepatitis B; inactivated poliovirus; and Haemophilus influenzae type b containing vaccines: A discrete choice experiment in selected cities. Chin. J. Vaccines Immun. 2024, 30, 127–133. [Google Scholar]
- Carpio, C.E.; Coman, I.A.; Sarasty, O.; García, M. COVID-19 Vaccine Demand and Financial Incentives. Appl. Health Econ. Health Policy 2021, 19, 871–883. [Google Scholar] [CrossRef] [PubMed]
- Neumann-Böhme, S.; Sabat, I.; Brinkmann, C.; Attema, A.E.; Stargardt, T.; Schreyögg, J.; Brouwer, W. Jumping the Queue:Willingness to Pay for Faster Access to COVID-19 Vaccines in Seven European Countries. Pharmacoeconomics 2023, 41, 1389–1402. [Google Scholar] [CrossRef]
- Wang, H.B.; Yang, J.; Hu, C.F.; Zhou, L.F.; Lu, B.J.; Xu, M. Influencing factors of human papilloma virus vaccination in the female population in Liuzhou. J. China Med. Univ. 2025, 54, 185–189. [Google Scholar] [CrossRef]
- Qian, H.K.; Ji, W.Y.; Suo, L.D. Investigation Report and Analysis Knowledge, attitudes and vaccination status regarding HPV vaccines among female healthcare workers at vaccination clinics in Beijing city: A survey report. Chin. J. Public Health 2024, 40, 1487–1492. [Google Scholar]
- Guo, F.; Guo, Y.L. Influencing factors of cognitive level of human papillomavirus vaccination in women of childbearing age. Med. J. Chin. People’s Health 2025, 37, 7–10. [Google Scholar]
- Wassie, G.T.; Ambelie, Y.A.; Adebabay, T.; Yeshiwas, A.G.; Fenta, E.T.; Abebe, E.C.; Wassie, G.T.; Adella, G.A.; Anley, D.T. COVID-19 vaccine uptake and its associated factors among adult population in Dangila district, Awi Zone, Northwest Ethiopia: A mixed method study. PLoS ONE 2024, 19, e0302531. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.T.; Lu, M.X.; Dou, Q.H.; Wang, C.S.; Xiao, Z.P.; Wang, Y.; Zhang, M.Y.; Ji, Y.F.; Bai, Y.R.; Zhang, X.X.; et al. Analysis of hepatitis B vaccination rates and influencing factors for timely first dose among low-birth-weight newborns in Henan Province. Mod. Dis. Control Prev. 2025, 36, 9–13. [Google Scholar]
- Xu, Q.Q.; Huang, Z.S.; Meng, X.; Li, S.L. Epidemiological characteristics of varicella and the coverage of varicella vaccine among population aged 1–17 from 2005 to 2021 in Zibo City. Prog. Microbiol. Immunol. 2025, 53, 67–72. [Google Scholar]
- Li, M.C.; Tao, J.; Zhang, R.; Wu, Y.M.; Hee, Z.K.; Wu, C. Factors influencing influenza vaccination coverage among kindergarten and primary school children in Zhejiang Province, 2023. Shanghai J. Prev. Med. 2024, 37, 23–28. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).