Safety and Immunogenicity of Vaccines in Children with Kaposiform Hemangioendothelioma Receiving Sirolimus: A Prospective Study
Abstract
1. Introduction
2. Methods
2.1. General Information
2.2. Subject Grouping
2.3. Vaccination and Catch-Up
2.4. Observation Indicators
2.5. Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics
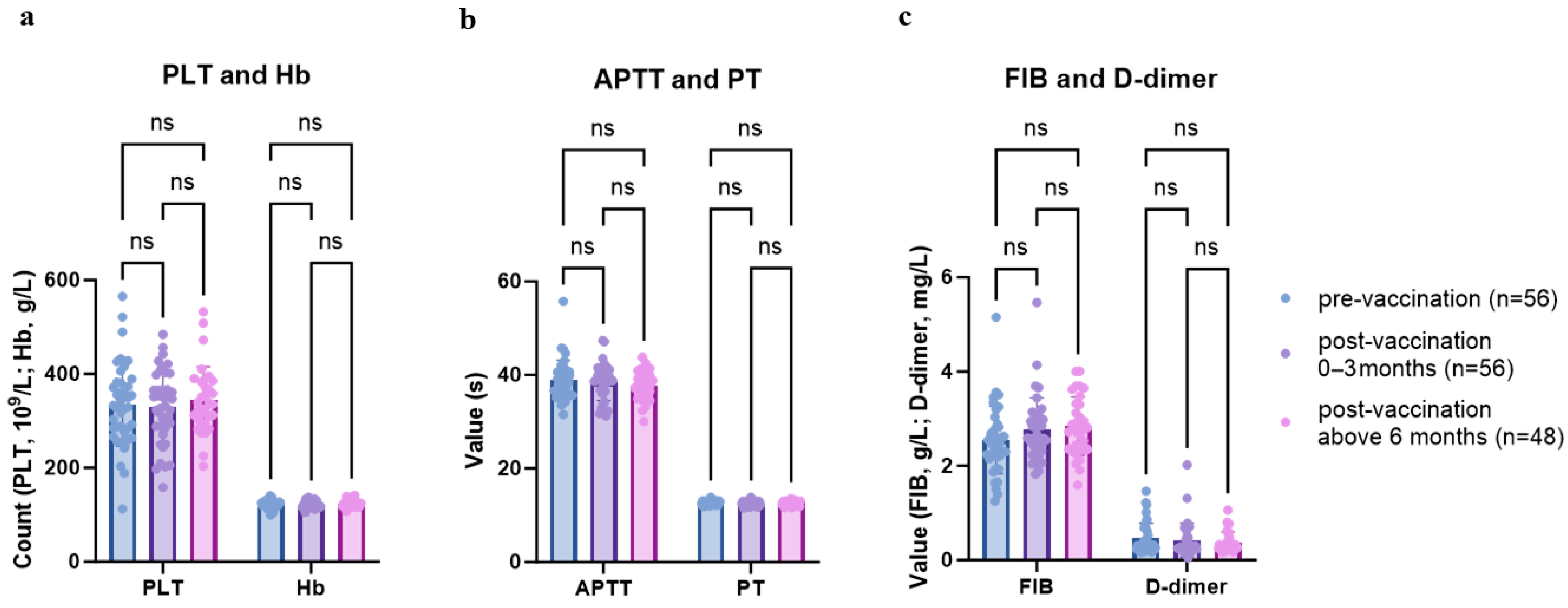
3.2. Safety Evaluation of Vaccination
3.3. Effectiveness Evaluation of Vaccination
4. Discussion
4.1. Safety Evaluation of Vaccination
4.2. Effectiveness Evaluation of Vaccination
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Full terms |
| APTT | Activated Partial Thromboplastin Time |
| bOPV | bivalent Oral Polio Vaccine |
| DTaP | Diphtheria and Tetanus Toxoid with Acellular Pertussis Vaccine |
| DTP | Diphtheria Tetanus Pertussis Vaccine |
| FIB | Fibrinogen |
| HBs-Ab | Hepatitis B Surface Antibody |
| IgG | Immunoglobulin G |
| JE | Japanese Encephalitis |
| KHE | Kaposiform Hemangioendothelioma |
| KMP | Kasabach–Merritt Phenomenon |
| MMR | Measles, Mumps, and Rubella Vaccine |
| MRI | Nuclear Magnetic Resonance Imaging |
| mTOR | Mammalian target of Rapamycin |
| PT | Prothrombin Time |
| VPI | Vaccine-Preventable Infections |
References
- Ji, Y.; Chen, S.; Yang, K.; Xia, C.; Li, L. Kaposiform hemangioendothelioma: Current knowledge and future perspectives. Orphanet J. Rare Dis. 2020, 15, 39. [Google Scholar] [CrossRef]
- Li, K. Current status and recent advances in drug therapies of Kaposiform hemangioendothelioma. J. Clin. Pediatr. Surg. 2019, 18, 626–628. [Google Scholar] [CrossRef]
- Glanz, J.M.; McClure, D.L.; Magid, D.J.; Daley, M.F.; France, E.K.; Salmon, D.A.; Hambidge, S.J. Parental refusal of pertussis vaccination is associated with an increased risk of pertussis infection in children. Pediatrics 2009, 123, 1446–1451. [Google Scholar] [CrossRef] [PubMed]
- Glanz, J.M.; McClure, D.L.; O’Leary, S.T.; Narwaney, K.J.; Magid, D.J.; Daley, M.F.; Hambidge, S.J. Parental decline of pneumococcal vaccination and risk of pneumococcal related disease in children. Vaccine 2011, 29, 994–999. [Google Scholar] [CrossRef] [PubMed]
- Feldman, A.G.; Curtis, D.J.; Moore, S.L.; Kempe, A. Under-immunization of pediatric transplant recipients: A call to action for the pediatric community. Pediatr. Res. 2020, 87, 277–281. [Google Scholar] [CrossRef]
- Hill, H.A.; Elam-Evans, L.D.; Yankey, D.; Singleton, J.A.; Kang, Y. Vaccination Coverage Among Children Aged 19-35 Months—United States, 2016. MMWR Morb. Mortal. Wkly. Rep. 2017, 66, 1171–1177. [Google Scholar] [CrossRef] [PubMed]
- Feldman, A.G.; Beaty, B.L.; Curtis, D.; Juarez-Colunga, E.; Kempe, A. Incidence of Hospitalization for Vaccine-Preventable Infections in Children Following Solid Organ Transplant and Associated Morbidity, Mortality, and Costs. JAMA Pediatr. 2019, 173, 260–268. [Google Scholar] [CrossRef]
- Vascular Tumor and Vascular Malformation Group; Plastic Surgery Branch; Chinese Medical Association. Guidelines for the Diagnosis and Treatment of Hemangiomas and Vascular Malformations (2019 Edition). Zu Zhi Gong Cheng Yu Chong Jian Wai Ke Za Zhi (In Chinese). 2019, 15, 277–317. [Google Scholar] [CrossRef]
- Zhou, J.; Qiu, T.; Zhang, Z.; Lan, Y.; Huo, R.; Xiang, B.; Chen, S.; Qiu, L.; Xia, C.; Xu, X.; et al. Consensus statement for the diagnosis, treatment, and prognosis of kaposiform hemangioendothelioma. Int. J. Cancer 2025, 156, 1986–1994. [Google Scholar] [CrossRef]
- Gao, M.X.; Wang, N.; Tian, J.; Ma, Z.H.; Wu, Y.; Wang, X.Y.; Zhang, S.Y.; Yang, M.Y.; Wang, W.B.; Huang, Z.Y. Factors associated with timely vaccination of pertussis-containing vaccines in children born from 2019 to 2023, Shanghai. Zhonghua Liu Xing Bing Xue Za Zhi 2024, 45, 1216–1223. [Google Scholar] [CrossRef]
- Mao, B.; Zhang, Q.; Ma, L.; Zhao, D.S.; Zhao, P.; Yan, P. Overview of Research into mTOR Inhibitors. Molecules 2022, 27, 5295. [Google Scholar] [CrossRef] [PubMed]
- Shan, Y.; Tian, R.; Gao, H.; Zhang, L.; Li, J.; Xie, C.; Liang, Y.; Chen, Y.; Wang, J.; Xu, M.; et al. Sirolimus for the treatment of kaposiform hemangioendothelioma: In a trough level-dependent way. J. Dermatol. 2021, 48, 1201–1209. [Google Scholar] [CrossRef] [PubMed]
- Alanko, S.; Pelliniemi, T.T.; Salmi, T.T. Recovery of blood B-lymphocytes and serum immunoglobulins after chemotherapy for childhood acute lymphoblastic leukemia. Cancer 1992, 69, 1481–1486. [Google Scholar] [CrossRef] [PubMed]
- Rubin, L.G.; Levin, M.J.; Ljungman, P.; Davies, E.G.; Avery, R.; Tomblyn, M.; Bousvaros, A.; Dhanireddy, S.; Sung, L.; Keyserling, H.; et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin. Infect. Dis. 2014, 58, 309–318. [Google Scholar] [CrossRef]
- Bochennek, K.; Allwinn, R.; Langer, R.; Becker, M.; Keppler, O.T.; Klingebiel, T.; Lehrnbecher, T. Differential loss of humoral immunity against measles, mumps, rubella and varicella-zoster virus in children treated for cancer. Vaccine 2014, 32, 3357–3361. [Google Scholar] [CrossRef]
- Shams Shahemabadi, A.; Salehi, F.; Hashemi, A.; Vakili, M.; Zare, F.; Esphandyari, N.; Kashanian, S. Assessment of antibody titers and immunity to Hepatitis B in children receiving chemotherapy. Iran. J. Ped Hematol. Oncol. 2012, 2, 133–139. [Google Scholar]
- Perkins, G.B.; Tunbridge, M.J.; Chai, C.S.; Hope, C.M.; Yeow, A.E.L.; Salehi, T.; Singer, J.; Shi, B.; Masavuli, M.G.; Mekonnen, Z.A.; et al. Mechanistic Target of Rapamycin Inhibitors and Vaccine Response in Kidney Transplant Recipients. J. Am. Soc. Nephrol. 2025. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.Y.; Subjeck, J.R.; Shrikant, P.A.; Kim, H.L. Temsirolimus, an mTOR inhibitor, enhances anti-tumour effects of heat shock protein cancer vaccines. Br. J. Cancer 2011, 104, 643–652. [Google Scholar] [CrossRef]
- Niu, H.; Bai, C.; Zhu, B.; Zhang, Y. Rapamycin improves the long-term T-cell memory and protective efficacy of tuberculosis subunit vaccine. Microb. Pathog. 2024, 190, 106631. [Google Scholar] [CrossRef]
- World Health Organization. Tetanus vaccines: WHO position paper, February 2017—Recommendations. Vaccine 2018, 36, 3573–3575. [Google Scholar] [CrossRef]
- Dembiński, Ł.; Krzesiek, E.; Klincewicz, B.; Grzybowska-Chlebowczyk, U.; Demkow, U.; Banaszkiewicz, A.; Radzikowski, A. Immunogenicity of Diphtheria Booster Vaccination in Adolescents with Inflammatory Bowel Disease. Pediatr. Infect. Dis. J. 2020, 39, 244–246. [Google Scholar] [CrossRef] [PubMed]




| Features | Numerical Value |
|---|---|
| Sex (number, n) | |
| male | 32 |
| female | 24 |
| Age (mean ± SD 1, year) | 4.7 ± 1.6 |
| 0–1 year (number, n) | 13 |
| 1–3 year | 20 |
| >3 year | 23 |
| Age of onset (mean ± SD, year) | 0.59 ± 1.22 |
| >1 month (number, n) | 16 |
| 1 month–1 year | 20 |
| >1 year | 20 |
| Age of initiation of sirolimus treatment (mean ± SD, year) | 1.18 ± 1.44 |
| Duration of sirolimus administration (mean ± SD, month) | 18.2 ± 13.8 |
| Previous occurrence of KMP (number, n) | |
| Yes | 28 |
| No | 28 |
| Platelets, hemoglobin and coagulation before catcj-up vaccination | |
| PLT 2 count (mean ± SD, 109/L) | 334.7 ± 88.2 |
| Hb 3 concentration (mean ± SD, g/L) | 121.1 ± 8.5 |
| APTT 4 (mean ± SD, s) | 38.9 ± 4.2 |
| PT 5 (mean ± SD, s) | 12.6 ± 0.5 |
| fibrinogen (mean ± SD, g/L) | 2.54 ± 0.71 |
| D-dimer (mean ± SD, mg/L) | 0.45 ± 0.31 |
| Occurrences (n, %) | |
|---|---|
| Mild Adverse Reaction | |
| Pain at the injection site | 15 (1.9%) |
| Redness at the injection site | 75 (9.6%) |
| Swelling at the injection site | 39 (5%) |
| fever | 8 (1%) |
| Severe Adverse Reaction | |
| Severe allergy | 0 (0%) |
| Shock | 0 (0%) |
| Lesion enlargement | 0 (0%) |
| Pain at the site of the lesion | 0 (0%) |
| Type of Vaccine | DTaP Routine Vaccination Group (n, %) | DTaP Catch-Up Vaccination Group (n, %) | ||
|---|---|---|---|---|
| Post-Vaccination 0–3 Months (n = 10) | Post-Vaccination Above 6 Months (n = 8) | Post-Vaccination 0–3 Months (n = 24) | Post-Vaccination Above 6 Months (n = 18) | |
| Pertussis | 10 (100%) | 8 (100%) | 24 (100%) | 18 (100%) |
| Diphtheria | 9 (90%) | 7 (87.5%) | 23 (95.8%) | 16 (88.9%) |
| Tetanus | 9 (90%) | 1 (12.5%) | 24 (100%) | 2 (11.1%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, J.; Yuan, Z.; Ding, Y.; Wang, Z.; Yao, W.; Li, J.; Zeng, M.; Li, K. Safety and Immunogenicity of Vaccines in Children with Kaposiform Hemangioendothelioma Receiving Sirolimus: A Prospective Study. Vaccines 2025, 13, 903. https://doi.org/10.3390/vaccines13090903
Yuan J, Yuan Z, Ding Y, Wang Z, Yao W, Li J, Zeng M, Li K. Safety and Immunogenicity of Vaccines in Children with Kaposiform Hemangioendothelioma Receiving Sirolimus: A Prospective Study. Vaccines. 2025; 13(9):903. https://doi.org/10.3390/vaccines13090903
Chicago/Turabian StyleYuan, Junhong, Zhenxiang Yuan, Yingjing Ding, Zuopeng Wang, Wei Yao, Jingjing Li, Mei Zeng, and Kai Li. 2025. "Safety and Immunogenicity of Vaccines in Children with Kaposiform Hemangioendothelioma Receiving Sirolimus: A Prospective Study" Vaccines 13, no. 9: 903. https://doi.org/10.3390/vaccines13090903
APA StyleYuan, J., Yuan, Z., Ding, Y., Wang, Z., Yao, W., Li, J., Zeng, M., & Li, K. (2025). Safety and Immunogenicity of Vaccines in Children with Kaposiform Hemangioendothelioma Receiving Sirolimus: A Prospective Study. Vaccines, 13(9), 903. https://doi.org/10.3390/vaccines13090903






