Immune Response to Childhood Vaccination in Vertically Infected People Living with HIV: A Long-Term Evaluation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Data Collection and Variable Definition
- CD4+ T-cell counts were retrieved for both the year 2023 and at the time of the last of the primary series.
- HIV viral load was recorded for both the year 2023 and at the time of the last dose of the primary series.
- HIV disease stage prior to antiretroviral therapy (ART) initiation and in 2023 was determined according to CDC criteria.
2.3. Serological Assays
2.4. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
3.2. Vaccination Coverage
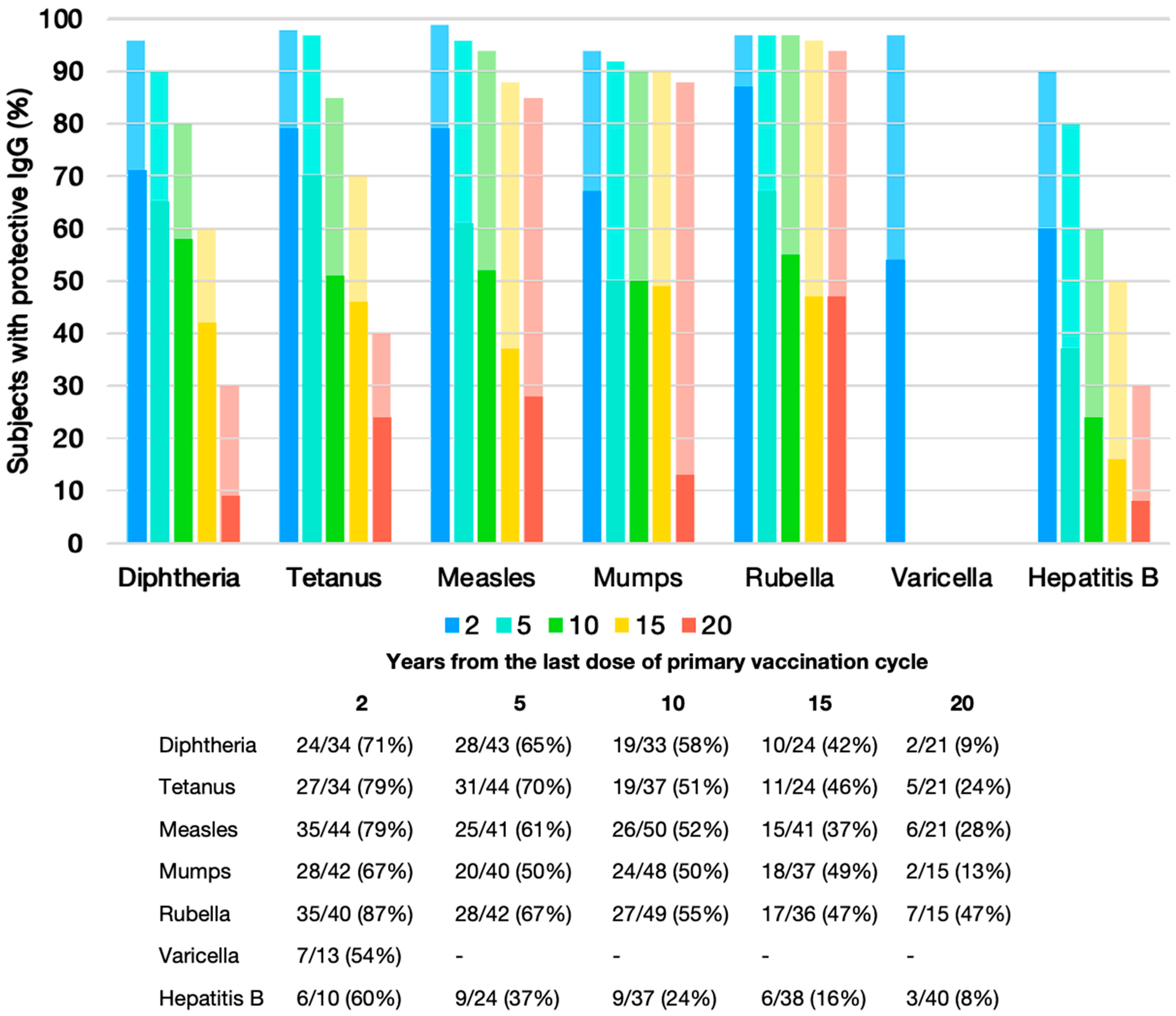
3.3. Seroprotection at Specific Time Points
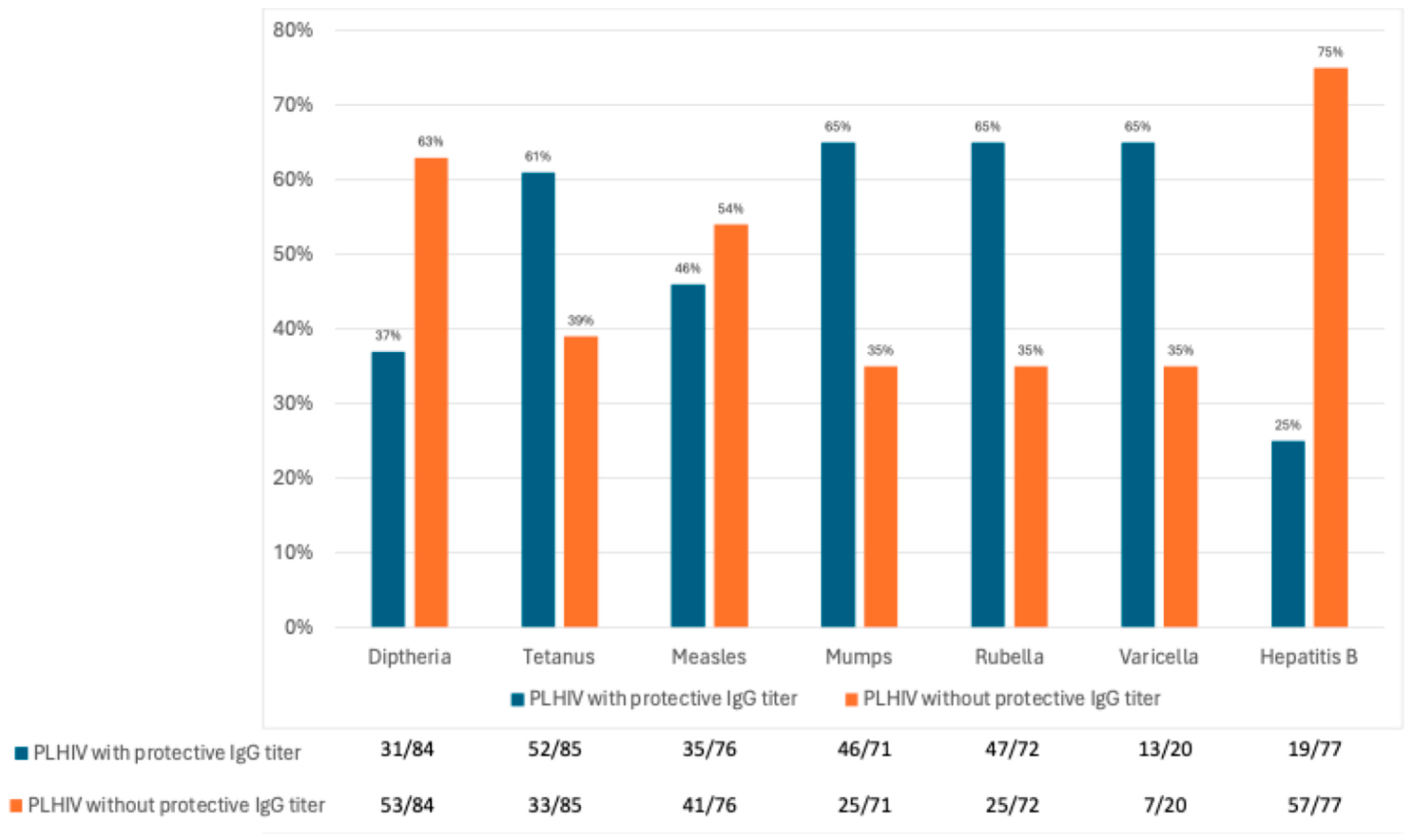
3.4. Seroprotection Rates in 2023
3.5. Multivariate Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ART | Antiretroviral Therapy |
| cART | Combination Antiretroviral Therapy |
| VL | Viral Load |
| CI | Confidence Interval |
| CDC | Center for Disease Control and Prevention |
| ELISA | Enzyme Linked Immunosorbent Assay |
| IQR | Interquartile Range |
| MMRV | Measles, Mumps, Rubella, and Varicella |
| OR | Odds Ratio |
| PLHIV | People Living with HIV |
| RT-PCR | Real Time—Polymerase Chain Reaction |
References
- WHO Epidemiological Fact Sheet, HIV Statistic, Globally and by WHO Region. 2023. Available online: https://cdn.who.int/media/docs/default-source/hq-hiv-hepatitis-and-stis-library/j0294-who-hiv-epi-factsheet-v7.pdf (accessed on 28 June 2024).
- Trickey, A.; Sabin, C.A.; Burkholder, G.; Crane, H.; d’Arminio Monforte, A.; Egger, M.; Gill, M.J.; Grabar, S.; Guest, J.L.; Jarrin, I.; et al. Life Expectancy after 2015 of Adults with HIV on Long-Term Antiretroviral Therapy in Europe and North America: A Collaborative Analysis of Cohort Studies. Lancet HIV 2023, 10, e295–e307. [Google Scholar] [CrossRef] [PubMed]
- Slaymaker, E.; Todd, J.; Marston, M.; Calvert, C.; Michael, D.; Nakiyingi-Miiro, J.; Crampin, A.; Lutalo, T.; Herbst, K.; Zaba, B. How Have ART Treatment Programmes Changed the Patterns of Excess Mortality in People Living with HIV? Estimates from Four Countries in East and Southern Africa. Glob. Health Action 2014, 7, 22789. [Google Scholar] [CrossRef] [PubMed]
- Menson, E.N.; Mellado, M.J.; Bamford, A.; Castelli, G.; Duiculescu, D.; Marczyńska, M.; Navarro, M.L.; Scherpbier, H.J.; Heath, P.T. Guidance on Vaccination of HIV-Infected Children in Europe. HIV Med. 2012, 13, 333–336. [Google Scholar] [CrossRef]
- Siberry, G.K.; Abzug, M.J.; Nachman, S.; Brady, M.T.; Dominguez, K.L.; Handelsman, E.; Mofenson, L.M.; Nesheim, S.; Panel on Opportunistic Infections in HIV-Exposed; National Institutes of Health; et al. Guidelines for the Prevention and Treatment of Opportunistic Infections in HIV-Exposed and HIV-Infected Children: Recommendations from the National Institutes of Health, Centers for Disease Control and Prevention, the HIV Medicine Association of the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the American Academy of Pediatrics. Pediatr. Infect. Dis. J. 2013, 32 (Suppl. 2), 1–166. [Google Scholar] [CrossRef]
- Cheng, Z.; Lin, P.; Cheng, N. HBV/HIV Coinfection: Impact on the Development and Clinical Treatment of Liver Diseases. Front. Med. 2021, 8, 713981. [Google Scholar] [CrossRef]
- Irina, E.; Srdan, M.; Monique, M. HIV/AIDS treatment and care: Clinical protocols for the WHO European Region. World Health Organization. Regional Office for Europe. 2007. Available online: https://iris.who.int/handle/10665/341846 (accessed on 15 August 2025).
- Rubin, L.G.; Levin, M.J.; Ljungman, P.; Davies, E.G.; Avery, R.; Tomblyn, M.; Bousvaros, A.; Dhanireddy, S.; Sung, L.; Keyserling, H.; et al. 2013 IDSA Clinical Practice Guideline for Vaccination of the Immunocompromised Host. Clin. Infect. Dis. 2014, 58, 309–318. [Google Scholar] [CrossRef]
- Toti, M.; Carosi, G.; Consiglieri, A.P.; Alberici, F.; Azzini, M.; Cargnel, A.; Monforte, A.; De Lalla, F.; De, F.; Esposito, R.R.; et al. Linee Guida Italiane Sull’utilizzo Della Terapia Antiretrovirale e la Gestione Diagnostico-Clinica Delle Persone con Infezione da HIV-1; Ministero della Salute: Roma, Italy, 2010. [Google Scholar]
- Pozniak, A.; Hospital, W.; Alison Rodger, L. British HIV Association Guidelines on the Use of Vaccines in HIV-Positive Adults 2015 Bhiva Guidelines on the Use of Vaccines in HIV—Positive Adults 2015 Writing Group; British HIV Association (BHIVA): Hertfordshire, UK, 2015. [Google Scholar]
- Sutcliffe, C.G.; Moss, W.J. Do Children Infected with HIV Receiving HAART Need to Be Revaccinated? Lancet Infect. Dis. 2010, 10, 630–642. [Google Scholar] [CrossRef]
- Kernéis, S.; Launay, O.; Turbelin, C.; Batteux, F.; Hanslik, T.; Boëlle, P.Y. Long-Term Immune Responses to Vaccination in HIV-Infected Patients: A Systematic Review and Meta-Analysis. Clin. Infect. Dis. 2014, 58, 1130–1139. [Google Scholar] [CrossRef]
- Bekker, V.; Scherpbier, H.; Pajkrt, D.; Jurriaans, S.; Zaaijer, H.; Kuijpers, T.W. Persistent Humoral Immune Defect in Highly Active Antiretroviral Therapy-Treated Children with HIV-1 Infection: Loss of Specific Antibodies against Attenuated Vaccine Strains and Natural Viral Infection. Pediatrics 2006, 118, 315–322. [Google Scholar] [CrossRef]
- Farquhar, C.; Wamalwa, D.; Selig, S.; John-Stewart, G.; Mabuka, J.; Majiwa, M.; Sutton, W.; Haigwood, N.; Wariua, G.; Lohman-Payne, B. Immune Responses to Measles and Tetanus Vaccines among Kenyan Human Immunodeficiency Virus Type 1 (HIV-1)-Infected Children Pre- and Post-Highly Active Antiretroviral Therapy and Revaccination. Pediatr. Infect. Dis. J. 2009, 28, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Melvin, A.J.; Mohan, K.M. Response to Immunization with Measles, Tetanus, and Haemophilus Influenzae Type b Vaccines in Children Who Have Human Immunodeficiency Virus Type 1 Infection and are Treated with Highly Active Antiretroviral Therapy. Pediatrics 2003, 111, 641–644. [Google Scholar] [CrossRef]
- Bruzzese, E.; Pagano, F.; Diana, A.; Punzi, L.; Guarino, A. Protection of Vaccine Preventable Diseases in a Population of Hiv-Infected Children: A 3 Years Prospective Study. Vaccines 2021, 9, 1331. [Google Scholar] [CrossRef] [PubMed]
- Cagigi, A.; Cotugno, N.; Giaquinto, C.; Nicolosi, L.; Bernardi, S.; Rossi, P.; Douagi, I.; Palma, P. Immune Reconstitution and Vaccination Outcome in HIV-1 Infected Children: Present Knowledge and Future Directions. Hum. Vaccines Immunother. 2012, 8, 1784–1794. [Google Scholar] [CrossRef]
- Pensieroso, S.; Cagigi, A.; Palma, P.; Nilsson, A.; Capponi, C.; Freda, E.; Bernardi, S.; Thorstensson, R.; Chiodi, F.; Rossi, P. Timing of HAART Defines the Integrity of Memory B Cells and the Longevity of Humoral Responses in HIV-1 Vertically-Infected Children. Proc. Natl. Acad. Sci. USA 2009, 106, 7939–7944. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef]
- Wodi, A.P.; Murthy, N.; Mcnally, V.; Matthew; Daley, F.; Cineas, S. Advisory Committee on Immunization Practices Recommended Immunization Schedule for Children and Adolescents Aged 18 Years or Younger-United States, 2024. MMWR Morb. Mortal Wkly. Rep. 2024, 73, 6–10. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kovac, M.; Rathi, N.; Kuriyakose, S.; Hardt, K.; Schwarz, T.F. Immunogenicity and Reactogenicity of a Decennial Booster Dose of a Combined Reduced-Antigen-Content Diphtheria-Tetanus-Acellular Pertussis and Inactivated Poliovirus Booster Vaccine (DTpa-IPV) in Healthy Adults. Vaccine 2015, 33, 2594–2601. [Google Scholar] [CrossRef]
- Tomovici, A.; Barreto, L.; Zickler, P.; Meekison, W.; Noya, F.; Voloshen, T.; Lavigne, P. Humoral Immunity 10 Years after Booster Immunization with an Adolescent and Adult Formulation Combined Tetanus, Diphtheria, and 5-Component Acellular Pertussis Vaccine. Vaccine 2012, 30, 2647–2653. [Google Scholar] [CrossRef]
- Mcquillan, G.M.; Kruszon-Moran, D.; Deforest, A.; Chu, S.Y.; Wharton, M. Serologic Immunity to Diphtheria and Tetanus in the United States Background: Serologic Data on Diseases That Are Preventable By. Ann. Intern. Med. 2002, 136, 660–666. [Google Scholar] [CrossRef]
- Brandon, D.; Kimmel, M.; Kuriyakose, S.O.; Kostanyan, L.; Mesaros, N. Antibody Persistence and Safety and Immunogenicity of a Second Booster Dose Nine Years after a First Booster Vaccination with a Reduced Antigen Diphtheria-Tetanus-Acellular Pertussis Vaccine (Tdap) in Adults. Vaccine 2018, 36, 6325–6333. [Google Scholar] [CrossRef] [PubMed]
- Coppola, N.; Corvino, A.R.; De Pascalis, S.; Signoriello, G.; Di Fiore, E.; Nienhaus, A.; Sagnelli, E.; Lamberti, M. The Long-Term Immunogenicity of Recombinant Hepatitis B Virus (HBV) Vaccine: Contribution of Universal HBV Vaccination in Italy. BMC Infect. Dis. 2015, 15, 149. [Google Scholar] [CrossRef] [PubMed]
- Gara, N.; Abdalla, A.; Rivera, E.; Zhao, X.; Werner, J.M.; Liang, T.J.; Hoofnagle, J.H.; Rehermann, B.; Ghany, M.G. Durability of Antibody Response against Hepatitis B Virus in Healthcare Workers Vaccinated as Adults. Clin. Infect. Dis. 2015, 60, 505–513. [Google Scholar] [CrossRef]
- Carryn, S.; Feyssaguet, M.; Povey, M.; Di Paolo, E. Long-Term Immunogenicity of Measles, Mumps and Rubella-Containing Vaccines in Healthy Young Children: A 10-Year Follow-Up. Vaccine 2019, 37, 5323–5331. [Google Scholar] [CrossRef]
- Bianchi, F.P.; Mascipinto, S.; Stefanizzi, P.; De Nitto, S.; Germinario, C.; Tafuri, S. Long-Term Immunogenicity after Measles Vaccine vs. Wild Infection: An Italian Retrospective Cohort Study. Hum. Vaccines Immunother. 2021, 17, 2078–2084. [Google Scholar] [CrossRef]
- Lewnard, J.A.; Grad, Y.H. Vaccine Waning and Mumps Re-Emergence in the United States. Sci. Transl. Med. 2018, 10, eaao5945. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention; Hall, E.; Wodi, A.P.; Hamborsky, J.; Morelli, V.; Schillie, S. Epidemiology and Prevention of Vaccine-Preventable Diseases, 14th ed.; Public Health Foundation: Washington, DC, USA, 2021.
- Vashishtha, V.M.; Kumar, P. The Durability of Vaccine-Induced Protection: An Overview. Expert Rev. Vaccines 2024, 23, 389–408. [Google Scholar] [CrossRef] [PubMed]
- Ministro della Salute Le Novità Del Decreto-Legge Sui Vaccini: Decreto-Legge 7 Giugno 2017, Disposizioni Urgenti in Materia Di Prevenzione Vaccinale. Available online: https://www.normattiva.it/uri-res/N2Ls?urn:nir:stato:decreto.legge:2017-06-07;73!vig (accessed on 6 August 2025).
- Istituto Superiore di Sanità Varicella Vaccination Coverage in Italy. Available online: https://www.epicentro.iss.it/varicella/aggiornamenti (accessed on 6 August 2025).
- Bruce, M.G.; Bruden, D.; Hurlburt, D.; Zanis, C.; Thompson, G.; Rea, L.; Toomey, M.; Townshend-Bulson, L.; Rudolph, K.; Bulkow, L.; et al. Antibody Levels and Protection after Hepatitis B Vaccine: Results of a 30-Year Follow-up Study and Response to a Booster Dose. J. Infect. Dis. 2016, 214, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Spina, F.G.; Gouvea, A.; Succi, R.C.d.M.; Calanca, F.; Weckx, L.Y.; Terreri, M.T.; Takano, M.A.S.; de Moraes-Pinto, M.I. Immune Response to a Tdap Booster in Vertically HIV-Infected Adolescents. Vaccine 2018, 36, 5609–5616. [Google Scholar] [CrossRef] [PubMed]
- Sticchi, L.; Bruzzone, B.; Caligiuri, P.; Rappazzo, E.; Casto, M.L.; De Hoffer, L.; Gustinetti, G.; Viscoli, C.; Di Biagio, A. Seroprevalence and Vaccination Coverage of Vaccine-Preventable Diseases in Perinatally HIV-1-Infected Patients. Hum. Vaccines Immunother. 2015, 11, 263–269. [Google Scholar] [CrossRef]
- Abzug, M.J.; Warshaw, M.; Rosenblatt, H.M.; Levin, M.J.; Nachman, S.A.; Pelton, S.I.; Borkowsky, W.; Fenton, T. Immunogenicity and Immunologic Memory after Hepatitis B Virus Booster Vaccination in HIV-Infected Children Receiving Highly Active Antiretroviral Therapy. J. Infect. Dis. 2009, 200, 935–946. [Google Scholar] [CrossRef]
- Titanji, K.; De Milito, A.; Cagigi, A.; Thorstensson, R.; Grützmeier, S.; Atlas, A.; Hejdeman, B.; Kroon, F.P.; Lopalco, L.; Nilsson, A.; et al. Loss of Memory B Cells Impairs Maintenance of Long-Term Serologic Memory during HIV-1 Infection. Blood 2006, 108, 1580–1587. [Google Scholar] [CrossRef]
- Lok, J.J.; Bosch, R.J.; Benson, C.A.; Collier, A.C.; Robbins, G.K.; Shafer, R.W.; Hughes, M.D. Long-Term Increase in CD4+ T-Cell Counts during Combination Antiretroviral Therapy for HIV-1 Infection. AIDS 2010, 24, 1867–1876. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, I.; Tarancon-Diez, L.; Vázquez-Alejo, E.; Jiménez De Ory, S.; Sainz, T.; Apilanez, M.; Epalza, C.; Guillén, S.; Ramos, J.T.; Díez, C.; et al. Innate and Adaptive Abnormalities in Youth with Vertically Acquired HIV through a Multicentre Cohort in Spain Integrated in the Translational Research Network in Pediatric Infectious Diseases (RITIP) 1. J. Int. AIDS Soc. 2021, 24, e25804. [Google Scholar] [CrossRef] [PubMed]
| Demographic Characteristics | HIV-Positive Subjects (n = 85) |
|---|---|
| Sex | 47 F (55%); 38 M (45%) |
| Age (years), median (IQR) | 24 (16, 31) |
| Country of birth, n (%) | |
| Italy | 41 (48%) |
| Africa | 28 (33%) |
| Others | 16 (19%) |
| HIV infection stage before starting ART, n (%) | |
| CDC 1 | 32 (38%) |
| CDC 2 | 21 (24%) |
| CDC 3 | 32 (38%) |
| HIV infection stage in 2023, n (%) | |
| CDC 1 | 30 (36%) |
| CDC 2 | 21 (24%) |
| CDC 3 | 34 (40%) |
| Undetectable VL, n (%) | 78 (92%) |
| VL (cp/mL) among subjects with unsuppressed VL in 2023, median (IQR) | 6410 (59, 133,000) |
| CD4+ cell/mm3 in 2023, median (IQR) | 793 (614, 1026) |
| CD4+ percentage in 2023, median (IQR) | 36.5 (30.5, 41.8) |
| Subjects in ART in 2023, n (%) | 85 (100%) |
| ART duration in 2023 (years), median (IQR) | 21 (14, 24) |
| Age at ART initiation (months), median (IQR) | 53 (12, 118) |
| Initiation of ART within the first 12 months of life, n (%) | |
| Yes | 23 (27%) |
| No | 58 (68%) |
| Not available | 4 (5%) |
| Subjects with a Completed Vaccine Schedule, n (%) | Subjects with an Incomplete Vaccine Schedule, n (%) | Subjects Without Any Dose of Vaccine, n (%) | |
|---|---|---|---|
| Anti-Diphtheria n = 85 | 62 (73.0%) | 22 (26.0%) | 1 (1.0%) |
| Anti-Tetanus, n = 85 | 62 (73.0%) | 23 (27.0%) | 0 (0.0%) |
| Anti-Measles, n = 85 | 61 (72.0%) | 16 (19.0%) | 8 (9.0%) |
| Anti-Mumps, n = 85 | 54 (64.0%) | 17 (20.0%) | 14 (16.0%) |
| Anti-Rubella, n = 85 | 54 (64.0%) | 18 (20.0%) | 13 (16.0%) |
| Anti-Varicella, n = 85 | 16 (19.0%) | 4 (5.0%) | 65 (76.0%) |
| Anti-Hepatitis B, n = 79 | 74 (94.0%) | 3 (3.5%) | 2 (2.5%) |
| Anti- Diphtheria and Anti- Tetanus N = 76 | Anti- Measles N = 67 | Anti-Mumps and Rubella N = 62 | Anti- Varicella N = 19 | Anti- Hepatitis B N = 68 | |
|---|---|---|---|---|---|
| Subjects with primary vaccination series while on ART | |||||
| only the last dose, n (%) | 30 (40%) | 23 (34%) | 23 (37%) | 3 (16%) | 14 (21%) |
| complete series, n (%) | 17 (22%) | 25 (37%) | 25 (40%) | 13 (68%) | 18 (26%) |
| Subjects with no doses of primary vaccination series while on ART, n (%) | 29 (38%) | 19 (28%) | 14 (23%) | 3 (16%) | 36 (53%) |
| Diphtheria | Tetanus | Measles | ||||
| OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value | |
| ART start within 12 months of life | 0.97 (0.72–1.30) | 0.825 | 1.04 (0.79–1.37) | 0.759 | 1.24 (0.89–1.73) | 0.206 |
| CDC3 stadium in 2023 | 0.85 (0.67–1.09) | 0.213 | 0.78 (0.62–0.98) | 0.039 | 1.12 (0.84–1.50) | 0.444 |
| CD4 for the last dose of the primary series | 1.00 (1.00–1.00) | 0.289 | 1.00 (1.00–1.00) | 0.182 | 1.00 (1.00–1.00) | 0.079 |
| Viral load groups for the last dose of the primary series | ||||||
| <50 copies/mL (ref) | ref | ref | ref | |||
| 50 < copies/mL < 200 | 0.76 (0.50–1.17) | 0.223 | 0.73 (0.49–1.09) | 0.128 | 1.18 (0.67–2.07) | 0.574 |
| >200 copies/mL | 0.85 (0.62–1.16) | 0.314 | 1.06 (0.79–1.43) | 0.683 | 1.00 (0.72–1.40) | 0.999 |
| Years from the last dose of the primary series | 0.98 (0.97–1.00) | 0.028 | 0.98 (0.96–0.99) | 0.003 | 0.96 (0.94–0.99) | 0.003 |
| Complete vaccination | 0.92 (0.71–1.13) | 0.547 | 1.10 (0.86–1.42) | 0.454 | 0.76 (0.52–1.10) | 0.153 |
| Male sex | 0.92 (0.71–1.13) | 0.377 | 1.17 (0.94–1.45) | 0.173 | 1.02 (0.77–1.34) | 0.914 |
| Mumps | Rubella | HBV | ||||
| OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | |
| ART start within 12 months of life | 0.97 (0.72–1.31) | 0.843 | 1.12 (0.81–1.55) | 0.492 | 1.19 (0.83–1.71) | 0.343 |
| CDC3 stadium in 2023 | 1.00 (0.76–1.30) | 0.971 | 0.85 (0.60–1.20) | 0.358 | 1.07 (0.80–1.44) | 0.635 |
| CD4 for the last dose of the primary series | 0.99 (0.99–1.00) | 0.017 | 1.08 (0.81–1.46) | 0.595 | 1.00 (1.00–1.00) | 0.964 |
| Viral load groups for the last dose of the primary series | ||||||
| <50 copies/mL (ref) | ref | ref | ref | |||
| 50 < copies/mL < 200 | 0.53 (0.32–0.86) | 0.016 | 1.00 (1.00–1.00) | 0.97 | 0.97 (0.62–1.51) | 0.878 |
| >200 copies/mL | 0.70 (0.52–0.95) | 0.026 | 1.47 (0.85–2.55) | 0.176 | 0.76 (0.52–1.11) | 0.161 |
| Years from the last dose of the primary series | 0.98 (0.96–1.00) | 0.086 | 0.96 (0.93–0.98) | 0.001 | 0.99 (0.97–1.01) | 0.271 |
| Complete vaccination | 0.81 (0.58–1.13) | 0.221 | 1.09 (0.79–1.52) | 0.605 | 0.60 (0.35–1.03) | 0.075 |
| Male sex | 1.14 (0.88–1.48) | 0.335 | 0.99 (0.75–1.31) | 0.954 | 0.82 (0.62–1.08) | 0.159 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zin, A.; Barbieri, E.; Brigadoi, G.; Berlese, A.; Chiusaroli, L.; Mengato, D.; Francavilla, A.; Giaquinto, C.; Donà, D.; Rampon, O. Immune Response to Childhood Vaccination in Vertically Infected People Living with HIV: A Long-Term Evaluation. Vaccines 2025, 13, 871. https://doi.org/10.3390/vaccines13080871
Zin A, Barbieri E, Brigadoi G, Berlese A, Chiusaroli L, Mengato D, Francavilla A, Giaquinto C, Donà D, Rampon O. Immune Response to Childhood Vaccination in Vertically Infected People Living with HIV: A Long-Term Evaluation. Vaccines. 2025; 13(8):871. https://doi.org/10.3390/vaccines13080871
Chicago/Turabian StyleZin, Annachiara, Elisa Barbieri, Giulia Brigadoi, Andrea Berlese, Lorenzo Chiusaroli, Daniele Mengato, Andrea Francavilla, Carlo Giaquinto, Daniele Donà, and Osvalda Rampon. 2025. "Immune Response to Childhood Vaccination in Vertically Infected People Living with HIV: A Long-Term Evaluation" Vaccines 13, no. 8: 871. https://doi.org/10.3390/vaccines13080871
APA StyleZin, A., Barbieri, E., Brigadoi, G., Berlese, A., Chiusaroli, L., Mengato, D., Francavilla, A., Giaquinto, C., Donà, D., & Rampon, O. (2025). Immune Response to Childhood Vaccination in Vertically Infected People Living with HIV: A Long-Term Evaluation. Vaccines, 13(8), 871. https://doi.org/10.3390/vaccines13080871






