1. Introduction
Tuberculosis (TB) is a potentially fatal and life-threatening infectious disease caused by
Mycobacterium tuberculosis (MTB). Although pulmonary TB mostly affects the lungs, extrapulmonary TB can also affect other organs. Transmission occurs via the inhalation of airborne droplets released by individuals with active TB, posing a significant public health threat, particularly in overcrowded and resource-limited settings. There are two primary types of TB: latent TB, in which a person is infected but shows no symptoms, and active TB, which is typified by clinical signs like fever, weight loss, and a persistent cough. Although latent cases do not present immediate clinical signs, they carry the risk of progressing to active disease and may contribute to ongoing transmission [
1].
Globally, TB remains one of the top ten causes of death from infectious disease. According to the World Health Organization, an estimated 10.6 million people contracted TB in 2022, resulting in approximately 1.3 million deaths [
2]. Despite improvements in diagnosis and treatment, TB continues to burden low- and middle-income countries due to factors such as late detection, drug resistance, and asymptomatic transmission [
3].
Thailand is classified among the 30 high TB burden countries by the WHO, with an estimated incidence of over 150 cases per 100,000 population annually [
4]. Thailand presents a unique and complex context for TB control, shaped by several intersecting demographic, social, and health system factors. One of the most critical challenges is the country’s rapidly aging population, which increases the prevalence of latent TB infection (LTBI). LTBI occurs when a person is infected with
MTB but does not have active TB disease. The bacteria remain dormant (inactive) in the body and cause no symptoms. However, LTBI is clinically important because it represents a reservoir from which active TB disease can emerge [
2] and the risk of reactivation due to age-related immunosenescence and comorbidities. Additionally, Thailand experiences significant internal migration, particularly from rural to urban areas, often leading to overcrowded living conditions and limited healthcare continuity, both of which contribute to increased TB transmission risks. Cross-border migration, especially along the western and northeastern borders, further complicates disease surveillance and treatment continuity, as many migrants may have limited access to healthcare services or face legal and language barriers. These population movements heighten the risk of TB spread, and challenge national efforts toward disease elimination.
Moreover, there are notable regional disparities in healthcare infrastructure and resource allocation, which result in uneven access to TB diagnosis, treatment, and follow-up care across provinces. Rural and underserved areas often struggle with healthcare workforce shortages and inadequate laboratory capacity, delaying detection and contributing to poorer treatment outcomes. Despite significant advances in TB control, including Directly Observed Treatment Shortcourse (DOTS) implementation and Bacillus Calmette–Guerin (BCG) vaccination programs, the persistence of asymptomatic carriers and undetected latent TB infections remains a major obstacle to achieving TB elimination [
2,
5]. These silent carriers may unknowingly spread the disease, complicating control measures and increasing the risk of outbreaks, particularly in urban and marginalized communities [
6]. These complexities necessitate a context-specific modeling approach to evaluate the potential impact of various intervention strategies and inform evidence-based public health policies tailored to Thailand’s epidemiological and socio-demographic landscape.
Vaccination, along with other intervention strategies such as the treatment of latent and active TB, plays a critical role in reducing TB incidence and mortality. Emerging TB vaccines, when integrated with improved case detection, preventive therapy, and strategies to enhance treatment adherence, provide a comprehensive approach to controlling both asymptomatic and symptomatic transmission [
7,
8,
9]. Collectively, these interventions not only mitigate the overall disease burden but also interrupt transmission chains within the population, contributing to more effective TB control.
Comprehensive knowledge of vaccine and treatment approaches is necessary to effectively combat TB. Mathematical modeling can provide critical insights into how different levels of investment and intervention coverage can influence TB incidence and prevalence in both short- and long-term timeframes. Previous modeling studies have estimated the importance of integrated, multi-component strategies for reductions in disease burden compared to single-policy approaches [
3,
5,
9,
10], but have not explicitly investigated the impact of vaccination and treatment on the dynamics of asymptomatic and symptomatic TB in Thailand.
This study presents a robust mathematical modeling framework designed to evaluate the impact of vaccination coverage and targeted public health interventions on the transmission dynamics of both asymptomatic and symptomatic TB in Thailand. The model integrates key epidemiological features of TB, including latent infection, asymptomatic carriers, progression to active disease, vaccination, and treatment interventions. By simulating a range of control scenarios over a 15-year horizon, the framework facilitates an extensive evaluation of the effectiveness of single and combined strategies, such as increased vaccination coverage, improved treatment for latent and active TB infections, and their respective programmatic scales.
2. Methods and Materials
In this study, we developed a compartmental deterministic model to characterize the transmission dynamics and control strategies of TB in Thailand. The model explicitly incorporates both asymptomatic and symptomatic transmission pathways, as well as the impact of vaccination on disease progression and control.
The total population is divided into six mutually exclusive compartments: susceptible individuals
, vaccinated individuals
, latent individuals
, asymptomatic infectious individuals
, symptomatic infectious individuals
, and recovered individuals
. At the given time
, the total population
is expressed as:
The model assumes that the total population size remains constant over time, with the inflow of new births balancing natural deaths. Natural death refers to the baseline mortality in the population that occurs independently of the disease under study. In our proposed model, it is typically represented by a constant per capita death rate, denoted by , and affects all compartments equally. This population assumption simplifies the demographic dynamics, allowing a focused investigation of TB transmission and intervention effects within a stable population framework. By incorporating both vaccination strategies and distinct infectious states, the model aims to provide deeper insights into the relative contributions of asymptomatic and symptomatic transmission to the overall TB burden and to evaluate the potential impact of vaccination and treatment on disease control in the Thai population.
In the model, susceptible individuals enter the population at a constant recruitment rate denoted by , which represents births or the influx of new individuals into the system, and all compartments are subject to natural mortality at the rate . Susceptible individuals face the risk of infection through effective contact with either symptomatic infectious individuals or asymptomatic infectious individuals .
The force of infection, which defines the per capita rate at which susceptible individuals acquire infection, is given by:
where
is the transmission rate of TB and
represents the modulation parameter that adjusts the relative infectiousness of asymptomatic individuals compared to symptomatic ones. A lower value of
indicates that asymptomatic individuals are less infectious, while
would imply they are equally infectious as symptomatic cases.
Following exposure, susceptible individuals who become infected transition to the latent compartment , which represents individuals who have been infected but are not yet capable of transmitting the disease. This latent stage is critical in TB epidemiology, as it captures the delay between initial infection and the onset of infectiousness, allowing for the possibility of either progression to active disease or maintenance in a non-infectious state. Latently infected individuals progress to either asymptomatic or symptomatic TB. Specifically, a fraction of latent individuals develops symptomatic infection , while the remaining develops asymptomatic infection , where is the overall activation rate from latency. Again, latent individuals may recover at a rate and move to . Asymptomatic individuals may naturally recover at a rate , or progress to symptomatic TB at a rate . Symptomatic individuals may recover at a treatment-induced rate and naturally recover at a rate , or die from TB-induced complications at a rate . Additionally, symptomatic individuals move to at a rate .
In this model, vaccination is represented as a single-dose preventive strategy aimed at reducing susceptibility to TB infection. Susceptible individuals are vaccinated at a rate and subsequently enter the vaccinated compartment . We assume homogeneous vaccination among the susceptible population, without distinguishing between prevaccinated and unvaccinated individuals. While this simplifies the system, it may overlook behavioral heterogeneity in vaccine uptake. Introducing separate compartments for these subgroups would enhance realism but is unlikely to alter the qualitative dynamics significantly, as the key distinction lies in the infection rate rather than in structural model flows. However, vaccination does not confer lifelong immunity. Vaccinated individuals are assumed to lose vaccine-induced protection over time and transition to the recovered compartment at a rate , reflecting waning immunity or partial protection mechanisms. Both vaccinated and recovered individuals are assumed to have temporary immunity. Due to immune decline, vaccinated individuals may return to the susceptible class at a rate , while recovered individuals lose natural immunity and re-enter the susceptible pool at a rate .
The resulting system of nonlinear ordinary differential equations is used to explore the dynamic behavior of the TB epidemic under different levels of vaccination, treatment, and detection. The structure of the model is represented by the flow diagram shown in
Figure 1, which illustrates the transmission and control pathways of TB. The transmission dynamics of TB are described by the following system of nonlinear ordinary differential equations derived from the formulated deterministic model.
With the following positive initial conditions,
the boundedness of the solution (for all
) of the proposed model (2) with the initial conditions (3) can be easily performed.
2.1. Boundedness
In this section, we analyze the model (2) to determine the biological feasible solution set. The following theorem assures that the solutions of the system are bounded in the set with the non-negative conditions.
Theorem 1 The feasible solution set of the system (2) subjected to the initial conditions (3) which initiate in are uniformly bounded in Γ, where is the positively invariant region.
Proof. Using the non-negative initial conditions of (3) in the system (2), it is observed that each of the dynamical variables remains non-negative. So, adding each of the equations of the system (2), we obtain the total population size,
, which satisfies in the absence of death due to TB, then we get
Integrating the above equation, we have = constant. □
Accordingly, given the assumption of a constant total population size, it follows that all feasible solutions of the dynamical variables are bounded within a positively invariant region.
2.2. Basic Reproduction Number
The basic reproduction number, commonly denoted as , is a foundational metric in the study of infectious disease dynamics. It quantifies the average number of secondary infections generated by one primary infectious individual in a completely susceptible population. This parameter serves as a threshold indicator that helps determine whether an infectious disease is likely to spread or die out in a given population. When , each infected person is expected to infect more than one individual, leading to potential epidemic expansion and sustained transmission within the population. This scenario signals a public health concern and necessitates immediate intervention to curb disease spread. Conversely, if , the infection fails to replace itself over time, resulting in a declining trajectory of disease cases. Under such conditions, the pathogen is likely to fade out from the population. In the special case where , the infection maintains a steady-state transmission, implying that the number of new cases remains constant over time without exponential growth or decline.
The model consists of three uninfected states
, and
and three infected states
. The model has six distinct states in such a scenario, while the overall population stays constant. At the free steady diseases
. Therefore,
,
, and
. Hence, for
, we have the following system:
Our framework ODE (4) is the subsystem for infection. It only defines the creation of new infections and modifications to the conditions of previously existing infections. Suppose we set
where T stands for transpose. We now want to express the subsystem as follows:
We found the two matrices from Equation (4) by polarizing the transmission events from each other proceedings. When we designate the affected states as having indices
and
, the rate at which an individual state j generates individuals in the system’s infected state
is known as the entry
. Thus, in the case of subsystem (4), we get
Here, K is the next-generation matrix [
10] with an essential minus sign.
A single infected person can create an average of one new infection, which is represented by the dominating eigenvalue of K in this instance. Thus, the basic reproduction number is
where
2.3. Model Evaluation
The following section provides a comprehensive overview of the foundational components of the proposed TB transmission dynamics framework (2).
Steady-State Analysis: Disease-Free Equilibrium and Endemic Equilibrium
In this section, we present the system properties of two biologically and mathematically meaningful equilibrium states of the proposed model. The first is the disease-free equilibrium (DFE), representing a scenario in which the basic reproduction number
is less than one. Under this condition, the infection cannot establish itself or persist in the population, and the disease eventually dies out. The second is the endemic equilibrium, which arises when
, indicating that the infection can sustain transmission and remain prevalent within the population over time. It is important to highlight that the disease-free equilibrium always exists for system (2), regardless of parameter values, as it corresponds to a state where no individuals are infected making it a fundamental reference point for model behavior. The existence and stability of these equilibria provide critical insight into the potential outcomes of TB dynamics under varying epidemiological conditions.
where
,
,
and
We may also determine the diseases-endemic equilibrium from Equation (2).
where
where
,
,
,
Therefore, Equations (11) and (12) show that the endemic equilibrium (i.e., exists) if .
4. Discussion and Conclusions
Tuberculosis (TB) continues to pose a significant public health challenge globally, including in Thailand. Despite progress in recent years, the transmission dynamics and epidemiological patterns of TB within the Thai context remain unclear. This limits the ability to fully evaluate the effectiveness of ongoing interventions or to design context-specific strategies. In response to the persistent burden of TB, the Thai government has implemented a series of national intervention programs aimed at eradicating the disease. Key initiatives have encompassed the provision of free diagnostic and treatment services, Bacillus Calmette–Guérin (BCG) vaccination, management of both active and latent tuberculosis infections, strengthened case detection and reporting mechanisms, and the formulation of clinical and public health guidelines in alignment with international standards.
While these efforts have led to notable improvements in TB control—reflected in declining incidence and mortality rates in many regions—significant challenges persist. Critical gaps remain in case detection, particularly among asymptomatic individuals, and in ensuring continuity of care for those undergoing treatment. Furthermore, delays in accessing timely diagnosis and care, suboptimal vaccination coverage in specific populations, poor treatment adherence, and the growing threat of drug-resistant TB strains continue to undermine the overall effectiveness of control strategies.
In this study, we developed and analyzed a compartmental mathematical model of TB transmission dynamics, explicitly incorporating the effects of vaccination to better understand the epidemiological landscape in Thailand. The model was structured to capture key aspects of TB progression, including latent infection, active disease, diagnosis, treatment, vaccination, and immunization strategies, and estimated the basic reproduction number ().
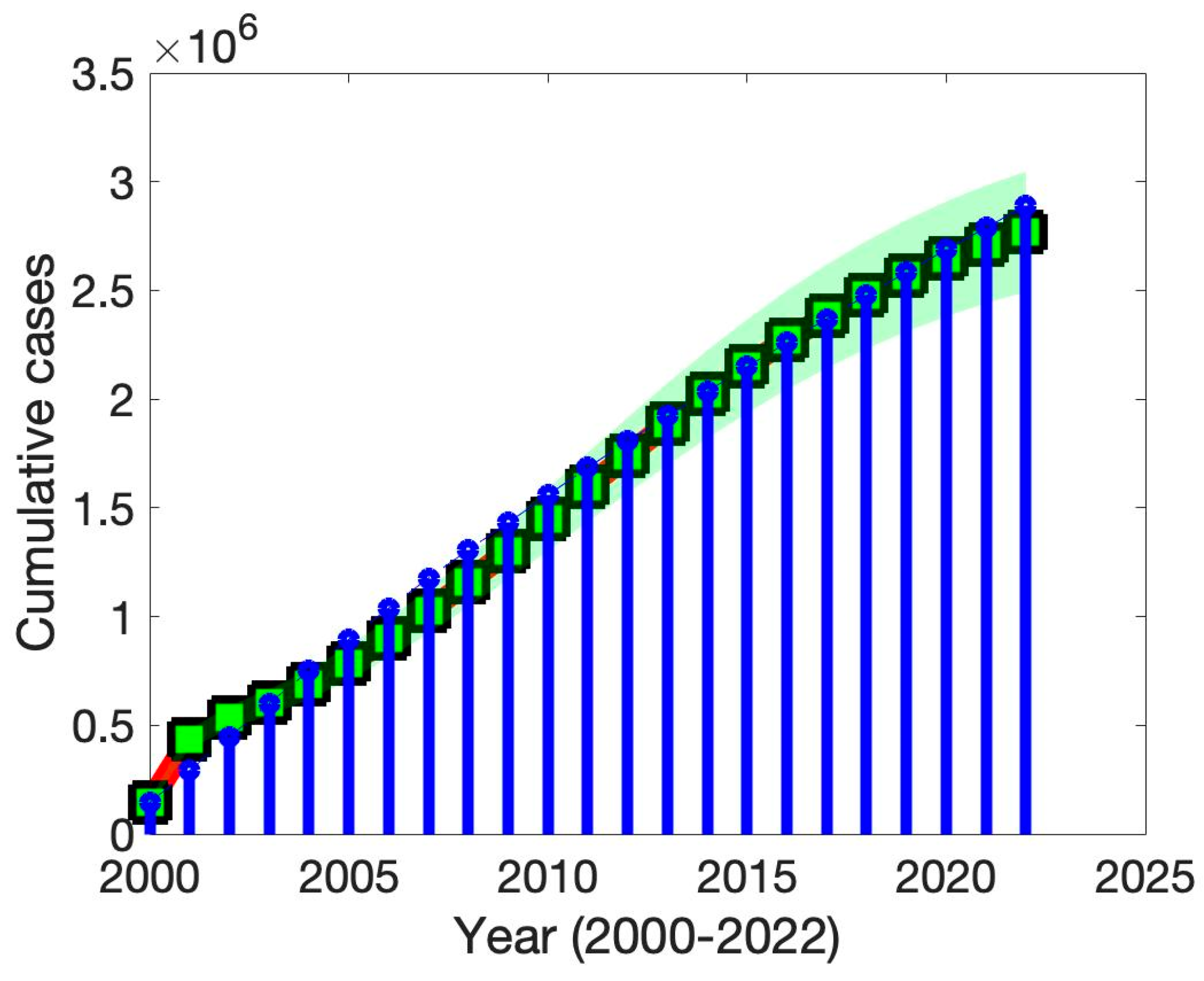
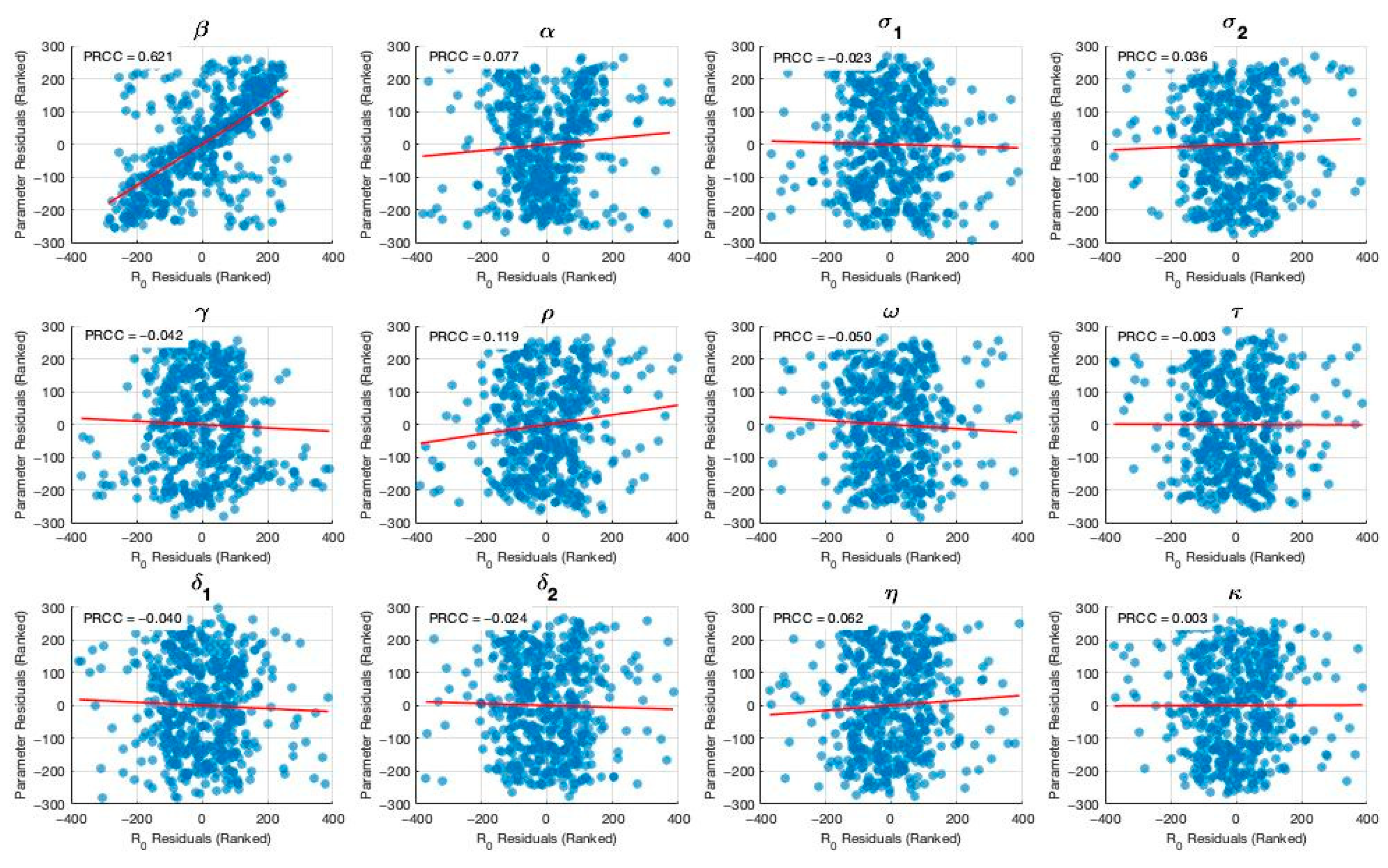
To validate and ensure the empirical relevance of our model, we performed calibration using reported TB incidence data from Thai government surveillance sources. Model parameters were either derived from data, the literature, or model fitting. In addition, we conducted a comprehensive sensitivity analysis of the basic reproduction number with respect to key epidemiological and programmatic parameters. The results demonstrated that TB transmission rate, along with the efficacy and coverage of treatment for both latent and active TB, had the greatest influence on . These findings suggest that targeted efforts to reduce transmission and scale up effective treatment interventions—particularly among latent TB carriers—could significantly lower the reproduction number.
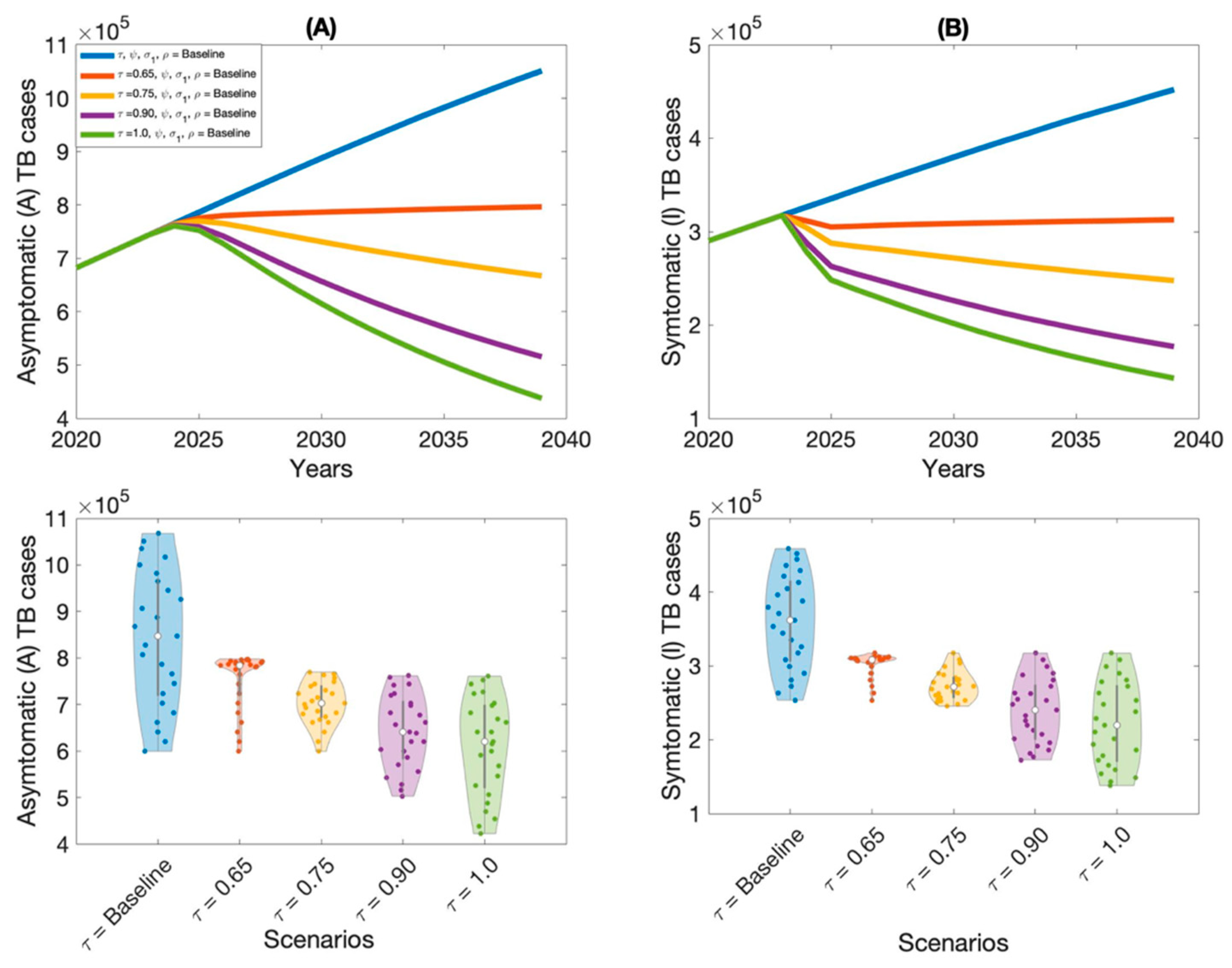
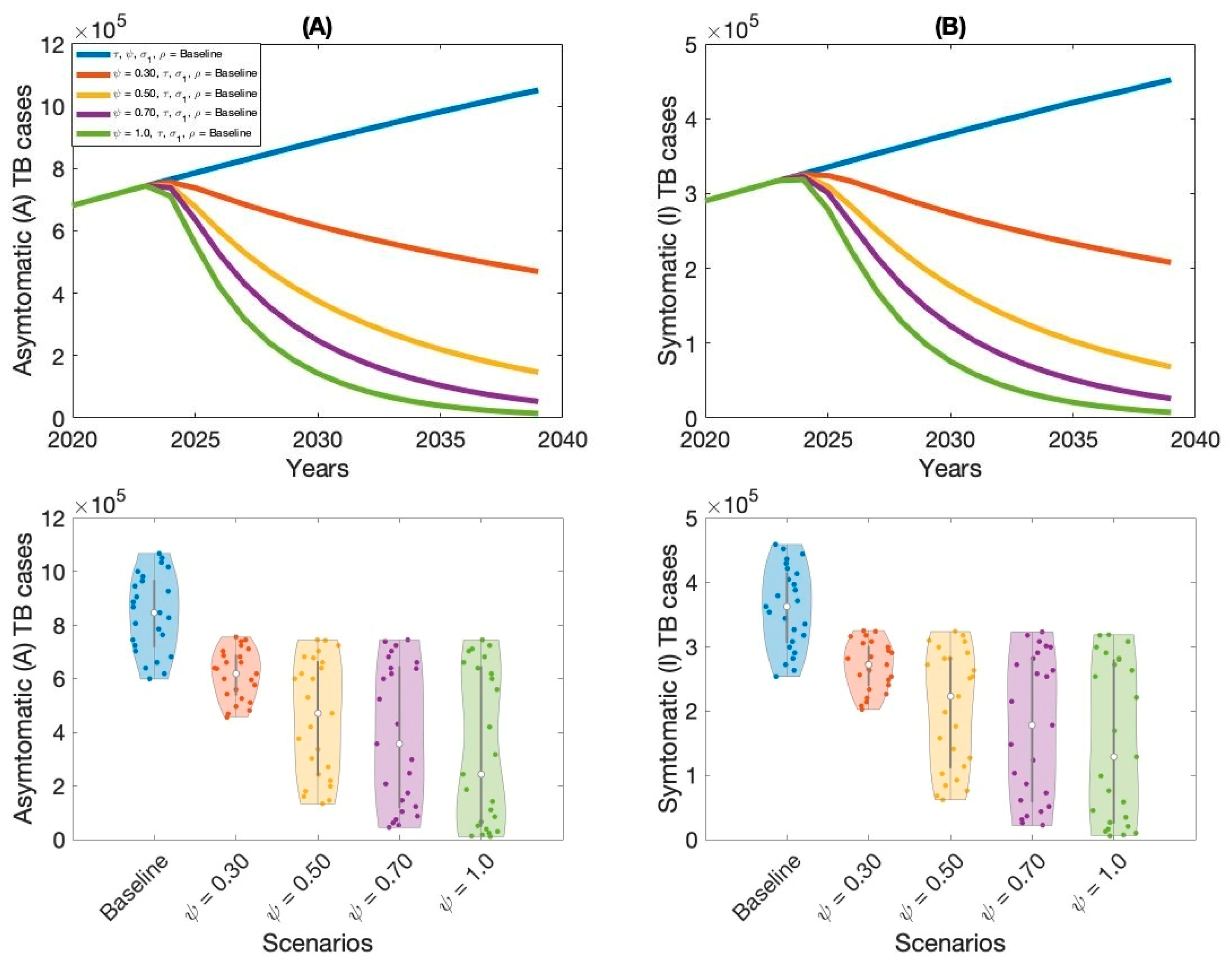
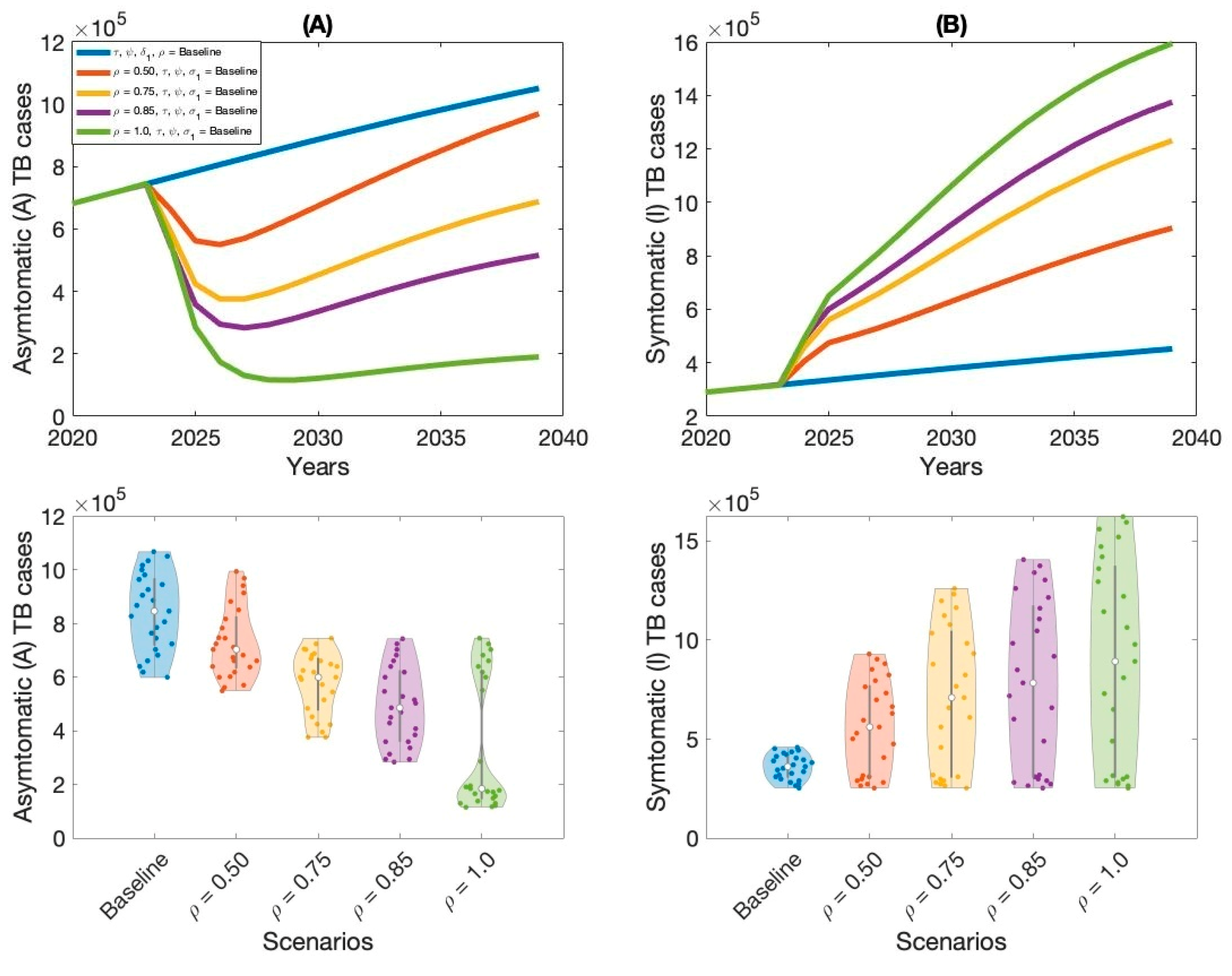
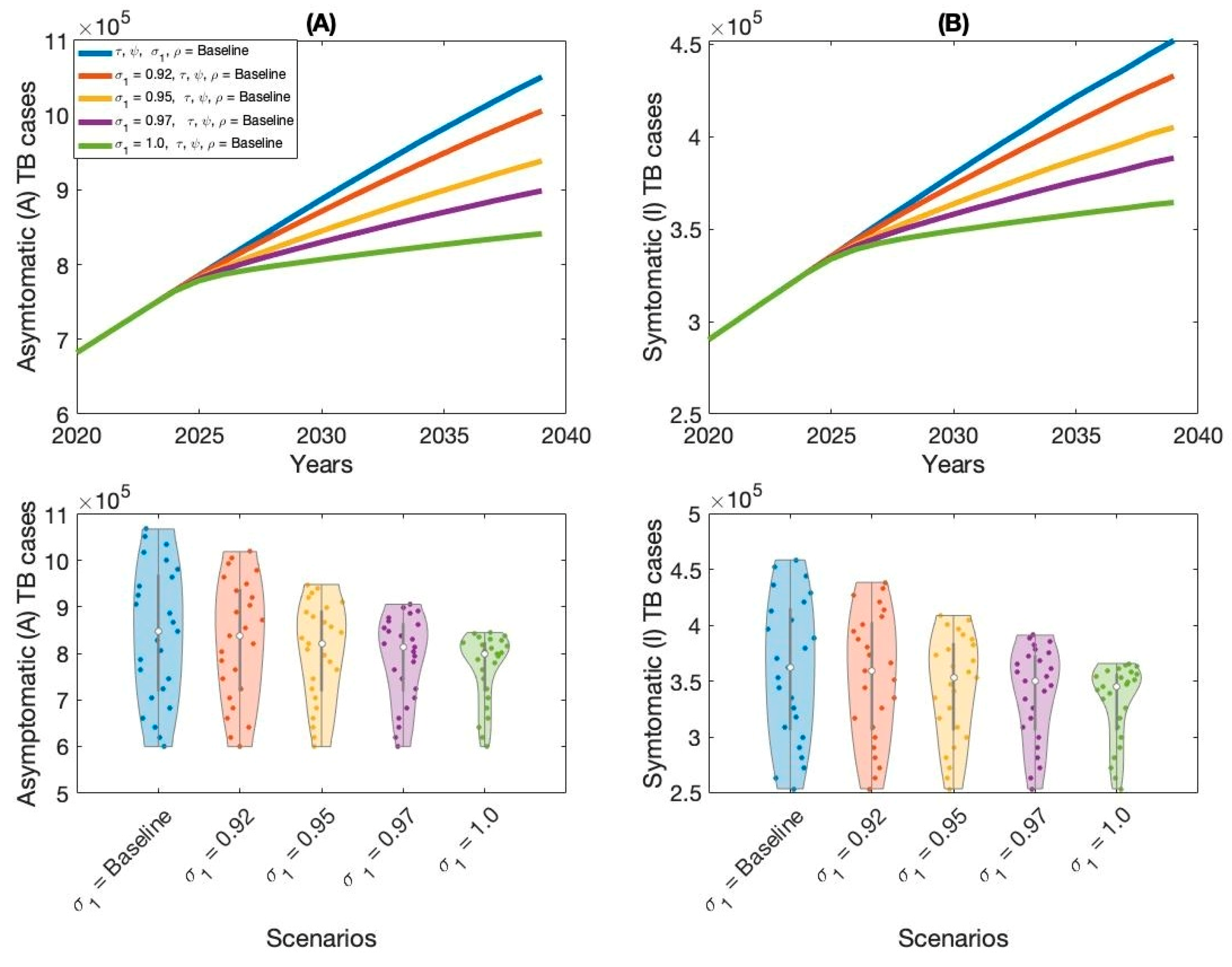
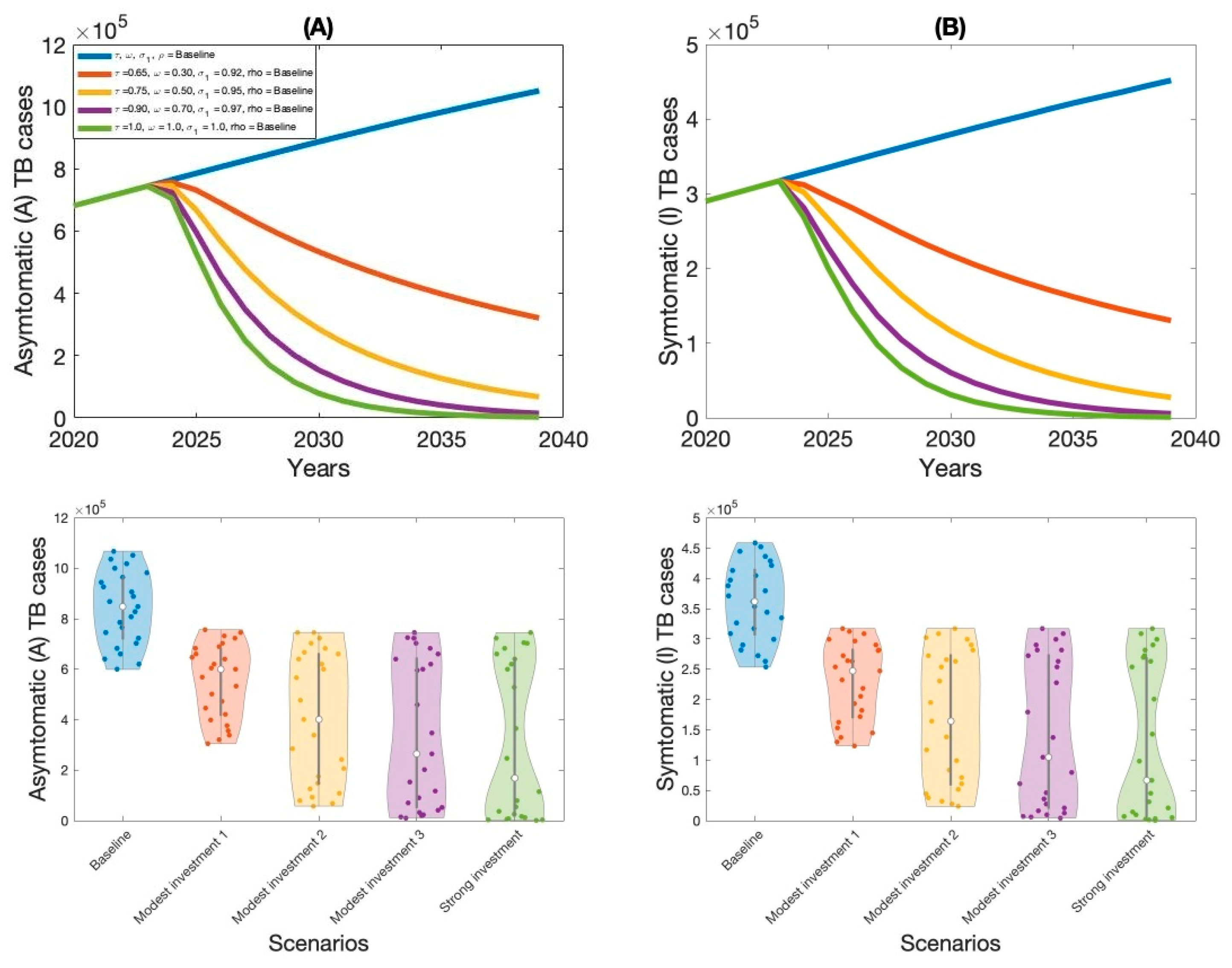
Moreover, this study examined context-specific TB intervention strategies in Thailand by evaluating the projected impact of three key approaches: expanding treatment coverage for latent TB infections (LTBI), enhancing treatment rates for active TB cases, and increasing overall vaccination coverage.
These interventions were selected based on their operational relevance and their capacity to address persistent challenges in the Thai TB program. Using our calibrated compartmental TB model—previously validated with national TB incidence data—we simulated the implementation of these strategies over a 15-year horizon. Our results indicate that increasing treatment coverage for latent tuberculosis (TB) infections had the greatest impact on reducing both asymptomatic and symptomatic TB cases in Thailand. This finding aligns with previous modeling studies and empirical analyses in similar epidemiological contexts [
16], which highlight the substantial role of latent TB reservoirs in sustaining long-term transmission.
Latent TB infection, which represents a large pool of individuals harboring Mycobacterium tuberculosis (MTB) without clinical symptoms, presents a critical challenge for TB control. Without intervention, a proportion of these individuals will progress to active TB over time, particularly under conditions of immunosuppression, poor living conditions, or comorbidities. Identifying and treating latent infections is therefore essential for interrupting future transmission. By preventing the reactivation of latent infections, this approach substantially reduces the incidence of new symptomatic TB cases and curbs overall transmission within the population.
The second most effective intervention was expanding treatment access and adherence among individuals with active TB. While this strategy directly targets the most infectious individuals, reducing their capacity to transmit the disease to others, it is comparatively less effective than preventive therapy in curbing long-term transmission. This is primarily because it does not address the large, often undiagnosed, pool of latent infections that continue to fuel the epidemic over time. Nonetheless, prompt and effective treatment of active TB is vital for reducing immediate incidence and mortality, as well as limiting the development of drug resistance.
Improving vaccination coverage, particularly with the BCG vaccine, was the third most effective strategy. While BCG offers moderate protection against severe TB in children, its efficacy against adult pulmonary TB is variable and declines over time. Therefore, its standalone impact on transmission is limited compared to treatment-based interventions. However, when combined with expanded case detection and treatment, improved vaccination coverage can strengthen population-level immunity and support long-term TB control.
Overall, our findings highlight the importance of a comprehensive TB control strategy that integrates treatment for both latent and active cases, and continued investment in vaccination efforts. These results provide actionable insights for policymakers and public health officials seeking to allocate resources and maximize the impact of TB control programs.
Our analysis demonstrates the substantial epidemiological benefits of combining multiple TB interventions. We compared two strategies: a modest investment approach (Strategy 2) and a comprehensive, high-investment strategy. Strategy 2—with 75% coverage for latent TB treatment, 50% for active TB treatment, and 95% vaccination—significantly reduced both asymptomatic and symptomatic cases. However, full eradication required the high-investment approach, characterized by 100% coverage across all interventions, which under model assumptions eliminated transmission entirely. These findings align with prior research emphasizing that TB elimination in high-burden settings demands extensive coverage and long-term health system commitment.
Importantly, our results highlight the superior effectiveness of combined, multi-component interventions compared to single-policy approaches. Models integrating latent and active TB treatment with robust vaccination consistently outperformed those implementing individual measures alone. This aligns with findings from other infectious disease modeling studies, which show that synergistic interventions can produce nonlinear reductions in disease burden, improv [
15,
16,
18].
In conclusion, this study contributes to the TB modeling literature in Thailand by integrating intervention-specific parameters with sensitivity analysis to inform strategic decision-making. This approach helps identify high-impact interventions, capture nonlinear or delayed responses, and assess the sensitivity of outcomes to changes in key parameters. Additionally, our analysis supports long-term planning and policy decision-making by projecting future trends and illustrating trade-offs between competing strategies. Overall, it enhances the robustness and practical relevance of TB modeling for public health decision-making. The framework can be adapted to other high-burden settings and highlights the importance of combining improved data systems with targeted public health interventions to accelerate TB control efforts.
Several limitations of this study should be acknowledged. First, the model assumes a homogeneously mixed population, overlooking heterogeneity in age, health status, behavior, and socio-economic conditions that influence TB transmission and intervention outcomes. Incorporating population structure in future models could enhance predictive accuracy and policy relevance. Second, the model does not account for acquired or partial immunity. While this may be reasonable in settings with moderate TB transmission, it limits applicability in high-burden areas where immunity significantly affects disease dynamics. Third, several parameters were estimated from reported TB incidence data, which may be affected by under-reporting and misclassification due to limited diagnostic capacity and access to care in Thailand. These data limitations may bias parameter estimates and model outputs. Improved surveillance and diagnostic systems are critical for strengthening model reliability. Finally, the model assumes a constant total population, disregarding demographic changes, migration, and TB-related mortality. This simplification may limit the model’s ability to reflect long-term trends. Despite these limitations, our findings provide useful insights into potential intervention impact and can support evidence-based decision-making in TB control efforts. Future work should incorporate dynamic population structures to better inform public health strategies in Thailand.