Impact of Vaccination and Prior Infection on SARS-CoV-2 Viral Load in Preschool Children During the Omicron Pandemic
Abstract
1. Introduction
2. Methods
2.1. Cycle Threshold (Ct)-Based Validation of Visual Ag Test Grading
2.2. Study Design and Patient Enrollment
2.3. Ethical Considerations
2.4. Statistical Analysis
3. Results
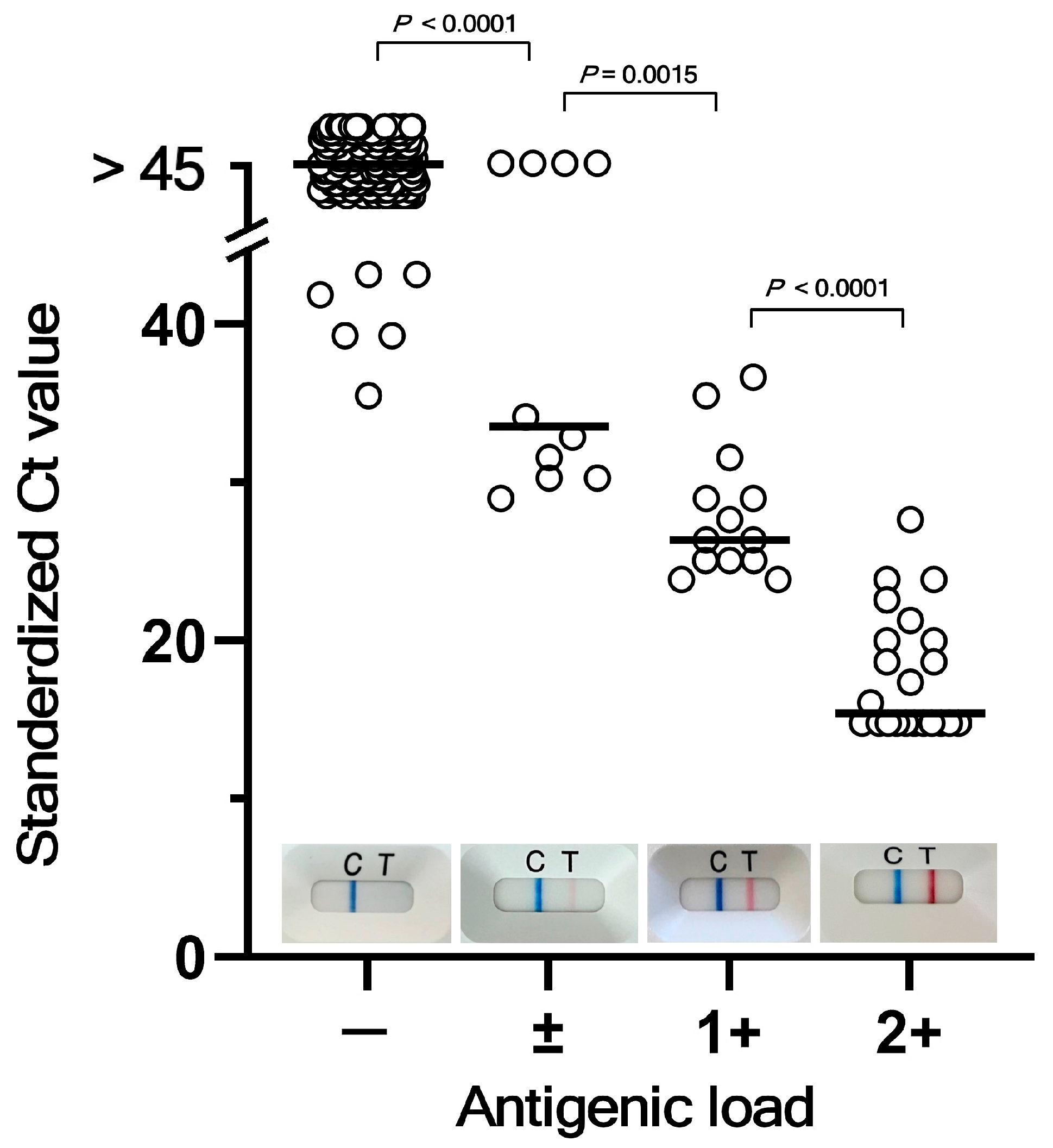
3.1. Correlation Between Quasi-Quantitative Ag Load Analyses and RT-qPCR for SARS-CoV-2
3.2. Analysis of Factors Associated with Viral Load Mitigation
3.2.1. Demographic and Immunization Characteristics of the Study Cohort
3.2.2. SARS-CoV-2 Variant Analysis
3.2.3. Factors Influencing Viral Load at the End of the Isolation Period
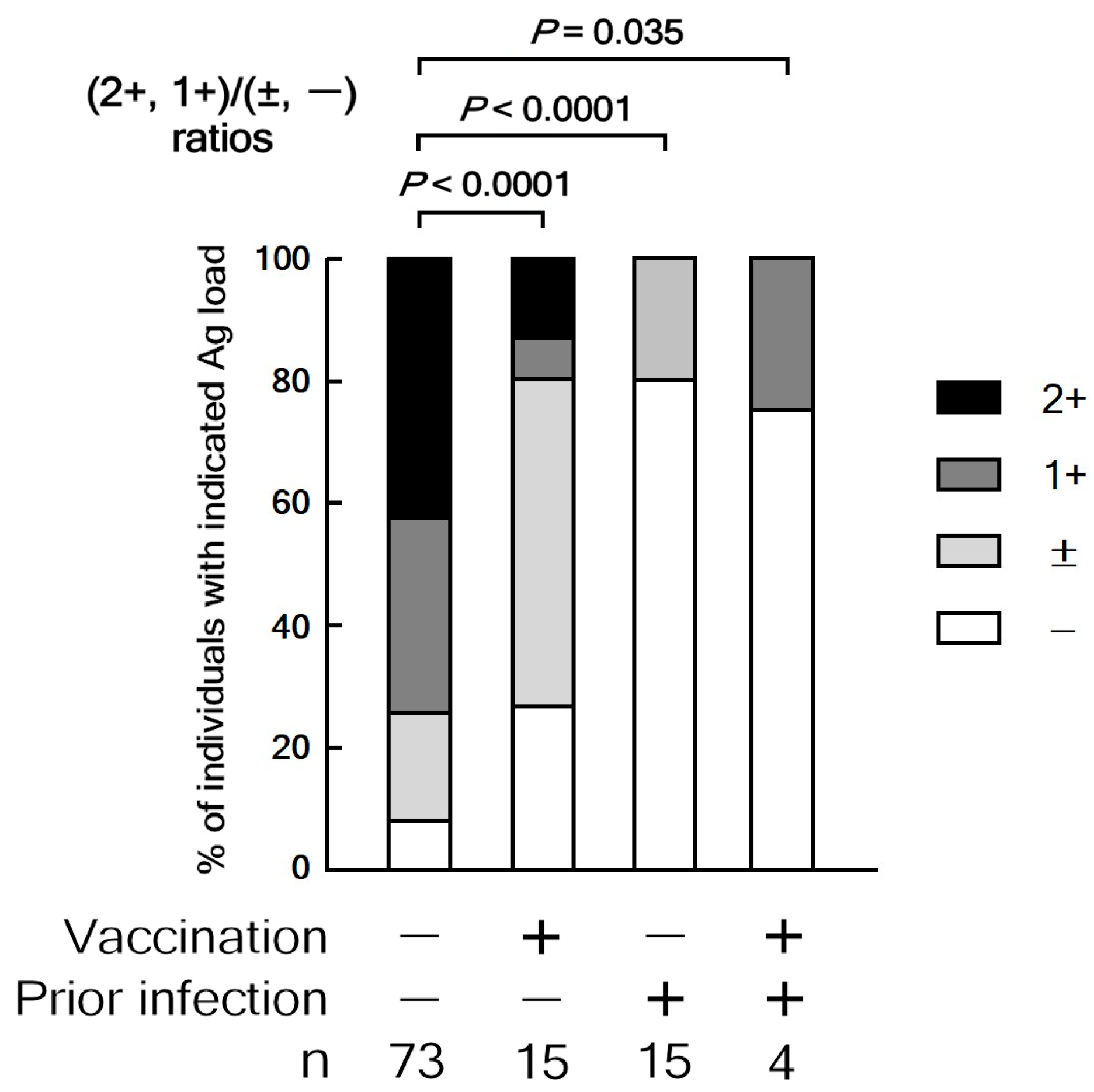
3.2.4. Relationship Between Ag Load, Vaccination, and Prior Infection
3.2.5. Multivariate Analysis of Factors Associated with High Antigen Load
3.2.6. Impact of Prior Infection and Vaccination on Clinical Severity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.M.; Wang, W.; Song, Z.G.; Hu, Y.; Tao, Z.W.; Tian, J.H.; Pei, Y.Y.; et al. A new coronavirus associated with human respiratory disease in China. Nature 2020, 579, 265–269. [Google Scholar] [CrossRef]
- Ludvigsson, J.F. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020, 109, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Lyngse, F.P.; Mortensen, L.H.; Denwood, M.J.; Christiansen, L.E.; Moller, C.H.; Skov, R.L.; Spiess, K.; Fomsgaard, A.; Lassauniere, R.; Rasmussen, M.; et al. Household transmission of the SARS-CoV-2 Omicron variant in Denmark. Nat. Commun. 2022, 13, 5573. [Google Scholar] [CrossRef] [PubMed]
- Murray, T.S.; Malik, A.A.; Shafiq, M.; Lee, A.; Harris, C.; Klotz, M.; Humphries, J.E.; Patel, K.M.; Wilkinson, D.; Yildirim, I.; et al. Association of Child Masking With COVID-19-Related Closures in US Childcare Programs. JAMA Netw. Open 2022, 5, e2141227. [Google Scholar] [CrossRef]
- Braga, P.P.; Souza, M.S.; Oliveira, P.P.; Romano, M.C.C.; Rocha, G.M.; Gesteira, E.C.R. Children wearing face masks to prevent communicable diseases: Scoping review. Rev. Paul. Pediatr. 2022, 41, e2021164. [Google Scholar] [CrossRef]
- World Health Organization. COVID-19 Infection Prevention and Control Living Guideline: Mask Use in Community Settings, 22 December 2021; World Health Organization: Geneva, Switzerland, 2021; Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-IPC_masks-2021.1 (accessed on 7 June 2025).
- Rotulo, G.A.; Palma, P. Understanding COVID-19 in children: Immune determinants and post-infection conditions. Pediatr. Res. 2023, 94, 434–442. [Google Scholar] [CrossRef]
- Constantin, T.; Pek, T.; Horvath, Z.; Garan, D.; Szabo, A.J. Multisystem inflammatory syndrome in children (MIS-C): Implications for long COVID. Inflammopharmacology 2023, 31, 2221–2236. [Google Scholar] [CrossRef]
- Okada, Y.; Nishiura, H. Vaccine-induced reduction of COVID-19 clusters in school settings in Japan during the epidemic wave caused by B.1.1.529 (Omicron) BA.2, 2022. Math. Biosci. Eng. 2024, 21, 7087–7101. [Google Scholar] [CrossRef]
- Thompson, M.G.; Stenehjem, E.; Grannis, S.; Ball, S.W.; Naleway, A.L.; Ong, T.C.; DeSilva, M.B.; Natarajan, K.; Bozio, C.H.; Lewis, N.; et al. Effectiveness of Covid-19 Vaccines in Ambulatory and Inpatient Care Settings. N. Engl. J. Med. 2021, 385, 1355–1371. [Google Scholar] [CrossRef]
- Molteni, E.; Canas, L.S.; Klaser, K.; Deng, J.; Bhopal, S.S.; Hughes, R.C.; Chen, L.; Murray, B.; Kerfoot, E.; Antonelli, M.; et al. Post-vaccination infection rates and modification of COVID-19 symptoms in vaccinated UK school-aged children and adolescents: A prospective longitudinal cohort study. Lancet Reg. Health Eur. 2022, 19, 100429. [Google Scholar] [CrossRef] [PubMed]
- Kandel, C.; Lee, Y.; Taylor, M.; Llanes, A.; McCready, J.; Crowl, G.; Powis, J.; Li, A.X.; Shigayeva, A.; Yip, L.; et al. Viral dynamics of the SARS-CoV-2 Omicron Variant among household contacts with 2 or 3 COVID-19 vaccine doses. J. Infect. 2022, 85, 666–670. [Google Scholar] [CrossRef]
- Smith, R.L.; Gibson, L.L.; Martinez, P.P.; Ke, R.; Mirza, A.; Conte, M.; Gallagher, N.; Conte, A.; Wang, L.; Fredrickson, R.; et al. Longitudinal Assessment of Diagnostic Test Performance Over the Course of Acute SARS-CoV-2 Infection. J. Infect. Dis. 2021, 224, 976–982. [Google Scholar] [CrossRef]
- Puhach, O.; Meyer, B.; Eckerle, I. SARS-CoV-2 viral load and shedding kinetics. Nat. Rev. Microbiol. 2023, 21, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Hiroi, S.; Kubota-Koketsu, R.; Sasaki, T.; Morikawa, S.; Motomura, K.; Nakayama, E.E.; Okuno, Y.; Shioda, T. Infectivity assay for detection of SARS-CoV-2 in samples from patients with COVID-19. J. Med. Virol. 2021, 93, 5917–5923. [Google Scholar] [CrossRef]
- Suzuki, M.; Tokita, A. Effective use of rapid antigen tests and RT-PCR for the detection of SARS-CoV-2 in children. Jap J. Pediatr. 2021, 74, 1309–1312. [Google Scholar]
- Takeuchi, Y.; Akashi, Y.; Kato, D.; Kuwahara, M.; Muramatsu, S.; Ueda, A.; Notake, S.; Nakamura, K.; Ishikawa, H.; Suzuki, H. The evaluation of a newly developed antigen test (QuickNavi-COVID19 Ag) for SARS-CoV-2: A prospective observational study in Japan. J. Infect. Chemother. 2021, 27, 890–894. [Google Scholar] [CrossRef]
- Kiyasu, Y.; Owaku, M.; Akashi, Y.; Takeuchi, Y.; Narahara, K.; Mori, S.; Nagano, T.; Notake, S.; Ueda, A.; Nakamura, K.; et al. Clinical evaluation of the rapid nucleic acid amplification point-of-care test (Smart Gene SARS-CoV-2) in the analysis of nasopharyngeal and anterior nasal samples. J. Infect. Chemother. 2022, 28, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Uraki, R.; Kiso, M.; Iida, S.; Imai, M.; Takashita, E.; Kuroda, M.; Halfmann, P.J.; Loeber, S.; Maemura, T.; Yamayoshi, S.; et al. Characterization and antiviral susceptibility of SARS-CoV-2 Omicron BA.2. Nature 2022, 607, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Fleming-Dutra, K.E.; Britton, A.; Shang, N.; Derado, G.; Link-Gelles, R.; Accorsi, E.K.; Smith, Z.R.; Miller, J.; Verani, J.R.; Schrag, S.J. Association of Prior BNT162b2 COVID-19 Vaccination With Symptomatic SARS-CoV-2 Infection in Children and Adolescents During Omicron Predominance. JAMA 2022, 327, 2210–2219. [Google Scholar] [CrossRef]
- Tenforde, M.W.; Self, W.H.; Naioti, E.A.; Ginde, A.A.; Douin, D.J.; Olson, S.M.; Talbot, H.K.; Casey, J.D.; Mohr, N.M.; Zepeski, A.; et al. Sustained Effectiveness of Pfizer-BioNTech and Moderna Vaccines Against COVID-19 Associated Hospitalizations Among Adults - United States, March-July 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1156–1162. [Google Scholar] [CrossRef]
- Yonker, L.M.; Neilan, A.M.; Bartsch, Y.; Patel, A.B.; Regan, J.; Arya, P.; Gootkind, E.; Park, G.; Hardcastle, M.; St John, A.; et al. Pediatric Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): Clinical Presentation, Infectivity, and Immune Responses. J. Pediatr. 2020, 227, 45–52 e45. [Google Scholar] [CrossRef]
- Sadarangani, M.; Marchant, A.; Kollmann, T.R. Immunological mechanisms of vaccine-induced protection against COVID-19 in humans. Nat. Rev. Immunol. 2021, 21, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Tokuyama, K.; Kitamura, T.; Maruyama, K.; Toriumi, S.; Murano, Y.; Yoneoka, D.; Nakazawa, T.; Shimizu, T. High number of seizures and unconsciousness in patients with SARS-CoV-2 omicron variants: A retrospective study. Front. Pediatr. 2023, 11, 1273464. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.Y.; Wang, H.L.; Yin, G.Q.; Xu, Y.; Tan, J.H.; Liang, X.H.; Wu, H.Y.; Yin, X.T.; Fang, C.X.; Peng, J.Z.; et al. Specific convulsions and brain damage in children hospitalized for Omicron BA.5 infection: An observational study using two cohorts. World J. Pediatr. 2024, 20, 1079–1089. [Google Scholar] [CrossRef] [PubMed]
- Kasai, M.; Sakuma, H.; Abe, Y.; Kuki, I.; Maegaki, Y.; Murayama, K.; Murofushi, Y.; Nagase, H.; Nishiyama, M.; Okumura, A.; et al. Clinical characteristics of SARS-CoV-2-associated encephalopathy in children: Nationwide epidemiological study. J. Neurol. Sci. 2024, 457, 122867. [Google Scholar] [CrossRef]
- Szczygiol, P.; Lukianowski, B.; Koscielska-Kasprzak, K.; Jakuszko, K.; Bartoszek, D.; Krajewska, M.; Krolak-Olejnik, B. Antibodies in the breastmilk of COVID-19 recovered women. BMC Pregnancy Childbirth 2022, 22, 635. [Google Scholar] [CrossRef]

| Date of Specimen Collection | Age (Mon) | Sex | Clade | Nextclade Pango Lineage | Alias Designation |
|---|---|---|---|---|---|
| 2023-05 | 11 | M | 22F (Omicron) | XBB.1.4 | XBB.1.4 |
| 2023-05 | 49 | F | 22D (Omicron) | BN.1.2 | BA.2.75.5.1.2 |
| 2023-07 | 22 | F | 23A (XBB.1.5) | XBB.1.5 | XBB.1.5 |
| 2023-07 | 5 | F | 22F (XBB) | XBB.1.33 | XBB.1.33 |
| 2023-07 | 1 | M | 22F (XBB) | XBB.1.33 | XBB.1.33 |
| 2023-08 | 54 | M | 23F (EG.5.1) | EG.5.1 | XBB.1.9.2.5.1 |
| 2023-08 | 9 | F | 23D (XBB.1.9) | XBB.1.9.2 | XBB.1.9.2 |
| 2023-08 | 19 | M | 23F (EG.5.1) | EG.5.1.1 | XBB.1.9.2.5.1.1 |
| 2023-08 | 41 | M | 23F (EG.5.1) | EG.5.1. | XBB.1.9.2.5.1 |
| 2023-08 | 16 | M | 23A (XBB.1.5) | GK.1.1 | XBB.1.5.70.1.1 |
| 2023-08 | 37 | F | 23C (CH.1.1) | JL.1 | BA.2.75.3.4.1.1.1.1.17.1.3.2.1 |
| 2023-08 | 68 | F | 23C (CH.1.1) | JL.1 | BA.2.75.3.4.1.1.1.1.17.1.3.2.1 |
| 2023-08 | 26 | F | 23F (EG.5.1) | EG.5.1 | XBB.1.9.2.5.1 |
| 2023-08 | 5 | F | 23D (XBB.1.9) | XBB.1.9.2 | XBB.1.9.2 |
| 2023-08 | 1 | F | 23E (XBB.2.3) | XBB.2.3.5 | XBB.2.3.5 |
| Variable | High Ag Load n = 58 | Low Ag Load n = 49 | p-Value |
|---|---|---|---|
| Female, n (%) | 26 (44.8%) | 25 (51.0%) | 0.59 c |
| Age (months), median (IQR) | 30 (11–47) | 45 (28–60) | <0.001 a |
| Infant nutrition method | |||
| Breastfed, n (%) | 22 (37.9%) | 18 (36.7%) | 0.75 c |
| Vaccination status | |||
| ≥2 doses, n (%) | 3 (5.2%) | 13 (27.1%) | 0.005 b |
| History of prior infection, n (%) | 1 (1.7%) | 18 (37.5%) | <0.001 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suzuki, M.; Tokita, A.; Inaba, M.; Tada, Y.; Shuri, K.; Miura, A.; Fukazawa, M.; Fujioka, M.; Sakai-Tagawa, Y.; Yamayoshi, S.; et al. Impact of Vaccination and Prior Infection on SARS-CoV-2 Viral Load in Preschool Children During the Omicron Pandemic. Vaccines 2025, 13, 850. https://doi.org/10.3390/vaccines13080850
Suzuki M, Tokita A, Inaba M, Tada Y, Shuri K, Miura A, Fukazawa M, Fujioka M, Sakai-Tagawa Y, Yamayoshi S, et al. Impact of Vaccination and Prior Infection on SARS-CoV-2 Viral Load in Preschool Children During the Omicron Pandemic. Vaccines. 2025; 13(8):850. https://doi.org/10.3390/vaccines13080850
Chicago/Turabian StyleSuzuki, Mitsuyoshi, Akifumi Tokita, Mariko Inaba, Yoshimi Tada, Kyoko Shuri, Asako Miura, Mitsuharu Fukazawa, Masashi Fujioka, Yuko Sakai-Tagawa, Seiya Yamayoshi, and et al. 2025. "Impact of Vaccination and Prior Infection on SARS-CoV-2 Viral Load in Preschool Children During the Omicron Pandemic" Vaccines 13, no. 8: 850. https://doi.org/10.3390/vaccines13080850
APA StyleSuzuki, M., Tokita, A., Inaba, M., Tada, Y., Shuri, K., Miura, A., Fukazawa, M., Fujioka, M., Sakai-Tagawa, Y., Yamayoshi, S., Iwatsuki-Horimoto, K., Kawaoka, Y., & Miyazawa, M. (2025). Impact of Vaccination and Prior Infection on SARS-CoV-2 Viral Load in Preschool Children During the Omicron Pandemic. Vaccines, 13(8), 850. https://doi.org/10.3390/vaccines13080850






