Understanding Attitudes Toward Zoster Vaccination in the Hospital Setting: A Multidisciplinary Model to Contrast Vaccine Hesitancy in Fragile Patients—A Prospective Longitudinal Observational Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Endpoints of the Study
2.3. Timing of the Study
2.4. Description of the Assessment Tool
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HZ | Herpes Zoster |
| PHN | Postherpetic Neuropathy |
| VZV | Zostavax |
| RZV | Recombinant vaccine adjuvanted with the glycoprotein E subunit of VZV Shingrix |
| PNPV | National Vaccine Prevention Plan |
| VH | Vaccine Hesitancy |
| FPG | Fondazione Policlinico Universitario Agostino Gemelli |
| IRCCS | Istituto di Ricovero e Cura a Carattere Scientifico |
| A.S.L. | Azienda Sanitaria Locale |
| HUs | Hospital Units |
| VAX | Vaccination Attitudes Examination |
| SD | Standard Deviations |
| IQR | Interquartile Ranges |
References
- Oleszko, M.; Zapolnik, P.; Kmiecik, W.; Czajka, H. Herpes Zoster: Risk Factors for Occurrence, Complications, and Recurrence with a Focus on Immunocompromised Patients. Diseases 2025, 13, 71. [Google Scholar] [CrossRef]
- van Oorschot, D.; Vroling, H.; Bunge, E.; Diaz-Decaro, J.; Curran, D.; Yawn, B. A systematic literature review of herpes zoster incidence worldwide. Hum. Vaccines Immunother. 2021, 17, 1714–1732. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.-X.; Yeh, F.-Y.; Shen, Y.-J.; Tai, Y.-H.; Huang, N.; Chang, Y.-T.; Chen, T.-J.; Li, C.-P.; Wu, C.-Y. Cigarette smoking and risk of herpes zoster: A population-based cohort study in Taiwan. Clin. Exp. Dermatol. 2021, 46, 1293–1298. [Google Scholar] [CrossRef] [PubMed]
- Patil, A.; Goldust, M.; Wollina, U. Herpes zoster: A Review of Clinical Manifestations and Management. Viruses 2022, 14, 192. [Google Scholar] [CrossRef] [PubMed]
- CDC. Clinical Considerations for Shingrix Use in Immunocompromised Adults Aged ≥19 Years. 2024. Available online: https://www.cdc.gov/shingles/hcp/vaccine-considerations/immunocompromised-adults.html (accessed on 16 November 2024).
- Lee, C.; Jiang, N.; Tang, H.; Ye, C.; Yuan, Y.; Curran, D. Potential public health impact of the adjuvanted recombinant zoster vaccine among people aged 50 years and older in Beijing. Hum. Vaccines Immunother. 2021, 17, 3735–3746. [Google Scholar] [CrossRef]
- Sun, Y.; Kim, E.; Kong, C.L.; Arnold, B.F.; Porco, T.C.; Acharya, N.R. Effectiveness of the Recombinant Zoster Vaccine in Adults Aged 50 and Older in the United States: A Claims-Based Cohort Study. Clin. Infect. Dis. 2021, 73, 949–956. [Google Scholar] [CrossRef]
- Harbecke, R.; Cohen, J.I.; Oxman, M.N. Herpes Zoster Vaccines. J. Infect. Dis. 2021, 224, S429–S442. [Google Scholar] [CrossRef]
- Gupta, S.; Arasaratnam, R.J.; Solow, E.B.; Bajaj, P. A Medical Records Review Study Assessing Safety of Zoster Vaccine Recombinant, Adjuvanted in Patients With Rheumatic Disease. J. Clin. Rheumatol. 2022, 28, e528–e531. [Google Scholar] [CrossRef]
- WHO. The European Immunization Agenda 2030 (EIA2030). 2021. Available online: https://www.who.int/europe/publications/i/item/9789289056052 (accessed on 16 November 2024).
- Calabrò, G.E.; Tognetto, A.; Carini, E.; Mancinelli, S.; Sarnari, L.; Colamesta, V.; Ricciardi, W.; de Waure, C. Strategies to Improve Vaccination among At-Risk Adults and the Elderly in Italy. Vaccines 2020, 8, 358. [Google Scholar] [CrossRef]
- Martire, B.; Azzari, C.; Badolato, R.; Canessa, C.; Cirillo, E.; Gallo, V.; Graziani, S.; Lorenzini, T.; Milito, C.; Panza, R.; et al. Vaccination in immunocompromised host: Recommendations of Italian Primary Immunodeficiency Network Centers (IPINET). Vaccine 2018, 36, 3541–3554. [Google Scholar] [CrossRef]
- Martin, L.R.; Petrie, K.J. Understanding the Dimensions of Anti-Vaccination Attitudes: The Vaccination Attitudes Examination (VAX) Scale. Ann. Behav. Med. 2017, 51, 652–660. [Google Scholar] [CrossRef]
- Taylor, S.; Landry, C.A.; Paluszek, M.M.; Groenewoud, R.; Rachor, G.S.; Asmundson, G.J.G. A Proactive Approach for Managing COVID-19: The Importance of Understanding the Motivational Roots of Vaccination Hesitancy for SARS-CoV-2. Front. Psychol. 2020, 11, 575950. [Google Scholar] [CrossRef]
- Kolobova, I.; Nyaku, M.K.; Karakusevic, A.; Bridge, D.; Fotheringham, I.; O’Brien, M. Vaccine uptake and barriers to vaccination among at-risk adult populations in the US. Hum. Vaccines Immunother. 2022, 18, 2055422. [Google Scholar] [CrossRef]
- Bechini, A.; Boccalini, S.; Del Riccio, M.; Pattyn, J.; Hendrickx, G.; Wyndham-Thomas, C.; Gabutti, G.; Maggi, S.; Ricciardi, W.; Rizzo, C.; et al. Overview of adult immunization in Italy: Successes, lessons learned and the way forward. Hum. Vaccines Immunother. 2024, 20, 2411821. [Google Scholar] [CrossRef] [PubMed]
- Cesaroni, G.; Calandrini, E.; Balducci, M.; Cappai, G.; Di Martino, M.; Sorge, C.; Nicastri, E.; Agabiti, N.; Davoli, M. Educational Inequalities in COVID-19 Vaccination: A Cross-Sectional Study of the Adult Population in the Lazio Region, Italy. Vaccines 2022, 10, 364. [Google Scholar] [CrossRef] [PubMed]
- Salussolia, A.; Capodici, A.; Scognamiglio, F.; La Fauci, G.; Soldà, G.; Montalti, M.; Di Valerio, Z.; Pia Fantini, M.; Odone, A.; Costantino, C.; et al. Herpes zoster (HZ) vaccine coverage and confidence in Italy: A Nationwide cross-sectional study, the OBVIOUS project. BMC Infect. Dis. 2024, 24, 438. [Google Scholar] [CrossRef] [PubMed]
- Diedenhofen, G.; Colaiocco, G.; Adamo, G.; Amoriello Lamberti, P.; Gherardi, S.M.; Trani, F.; Zuccaro, O.; Isidori, C.; De Angelis, G.; Fano, V. The role of sociodemographic factors on herpes zoster vaccine uptake among hard-to-reach populations. Eur. J. Public Health 2024, 34, ckae144.1257. [Google Scholar] [CrossRef]
- Gazzetta Ufficiale. Ministero della Salute Piano Nazionale Prevenzione Vaccinale 2023–2025. Available online: https://www.gazzettaufficiale.it/eli/id/2023/08/21/23A04685/sg (accessed on 2 February 2024).
- Czajka, H.; Czajka, S.; Biłas, P.; Pałka, P.; Jędrusik, S.; Czapkiewicz, A. Who or What Influences the Individuals’ Decision-Making Process Regarding Vaccinations? Int. J. Environ. Res. Public Health 2020, 17, 4461. [Google Scholar] [CrossRef]
- Regione Lazio. Available online: https://www.regione.lazio.it (accessed on 16 November 2024).
- EMA. Shingrix: Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/shingrix-epar-product-information_en.pdf (accessed on 31 July 2025).
- Regione Lazio. Programma di Immunizzazione Contro Herpes Zoster. Revisione della Strategia Generale. 2024. Available online: https://www.vaccinarsinlazio.org/assets/uploads/files/215/20-revisione-strategia-regionale-anti-hz-1536968-13dic24.pdf (accessed on 1 July 2025).
- Bilancio di Missione Fondazione Policlinico Universitario Agostino Gemelli IRCCS—Università Cattolica del Sacro Cuore. Available online: https://www.policlinicogemelli.it/informazioni/bilancio-missione/ (accessed on 31 July 2025).
- Bruno, F.; Laganà, V.; Pistininzi, R.; Tarantino, F.; Martin, L.; Servidio, R. Validation and psychometric properties of the Italian Vaccination Attitudes Examination (VAX-I) scale. Curr. Psychol. 2022, 42, 21287–21297. [Google Scholar] [CrossRef]
- Cepeda-Perez, A.S.; Tello Winniczuk, N.; Diaz-Borjon, A. Adherence to Current Vaccination Recommendations for Patients With Rheumatoid Arthritis in Mexico. Reumatol. Clin. Engl. Ed. 2021, 17, 155–159. [Google Scholar] [CrossRef]
- Al-Omar, H.A.; Sherif, H.M.; Mayet, A.Y. Vaccination status of patients using anti-TNF therapy and the physicians’ behavior shaping the phenomenon: Mixed-methods approach. PLoS ONE 2019, 14, e0223594. [Google Scholar] [CrossRef]
- Adams, S.H.; Schaub, J.P.; Nagata, J.M.; Park, M.J.; Brindis, C.D.; Irwin, C.E. Young Adult Perspectives on COVID-19 Vaccinations. J. Adolesc. Health 2021, 69, 511–514. [Google Scholar] [CrossRef]
- Zhang, P.; Li, Y.; Wang, H.; Luo, L.; Wang, P.; Wang, H.; Li, Q.; Meng, Z.; Yang, H.; Liu, Y.; et al. COVID-19 Vaccine Hesitancy Among Older Adolescents and Young Adults: A National Cross-Sectional Study in China. Front. Public Health 2022, 10, 877668. [Google Scholar] [CrossRef]
- Artna, E.; Abi-Jaoudé, A.; Sockalingam, S.; Perry, C.; Johnson, A.; Wun, C.; Kozloff, N.; Henderson, J.; Levinson, A.; Buchman, D.Z. Understanding attitudes and beliefs regarding COVID-19 vaccines among transitional-aged youth with mental health concerns: A youth-led qualitative study. BMJ Open 2024, 14, e080707. [Google Scholar] [CrossRef]
- Tartof, S.Y.; Slezak, J.M.; Puzniak, L.; Hong, V.; Frankland, T.B.; Xie, F.; Ackerson, B.K.; Takhar, H.; Ogun, O.A.; Simmons, S.; et al. Analysis of mRNA COVID-19 Vaccine Uptake Among Immunocompromised Individuals in a Large US Health System. JAMA Netw. Open 2023, 6, e2251833. [Google Scholar] [CrossRef] [PubMed]
- Cummings, P.E.; Lakoh, S.; Yendewa, S.A.; Massaquoi, S.P.E.; James, P.B.; Sahr, F.; Deen, G.F.; Salata, R.A.; Gevao, P.; Yendewa, G.A. Understanding COVID-19 Vaccine Uptake and Hesitancy among People with HIV in Freetown, Sierra Leone: A Cross-Sectional Study. Vaccines 2023, 11, 1685. [Google Scholar] [CrossRef] [PubMed]
- Huynh, H.P. Examining four types of anti-vaccination attitudes prior to and during the COVID-19 pandemic. Curr. Psychol. 2022, 42, 28812–28819. [Google Scholar] [CrossRef] [PubMed]
- Ibenyenwa, N.C.; Onyekwere, O.K.; Ugwu, N.F.; Adams, A.B.; Ajewole, P.I.; Makinde, V.I.; Onyekachi, B.N.; Anibueze, A.U.; Opele, J.K.; Nwogu, O.F. Bolstering the willingness to uptake COVID-19 vaccination through multidisciplinary health communication intervention: A cue for reaching herd immunity in Nigeria. Afr. Health Sci. 2023, 23, 168–176. [Google Scholar] [CrossRef]
- David, S.S.B.; Shamai-Lubovitz, O.; Mourad, V.; Goren, I.; Iunger, E.C.; Alcalay, T.; Irony, A.; Greenfeld, S.; Adler, L.; Cahan, A. A Nationwide Digital Multidisciplinary Intervention Aimed at Promoting Pneumococcal Vaccination in Immunocompromised Patients. Vaccines 2023, 11, 1355. [Google Scholar] [CrossRef]
- Moosa, A.S.; Wee, Y.M.S.; Jaw, M.H.; Tan, Q.F.; Tse, W.L.D.; Loke, C.Y.; Ee, G.L.A.; Ng, C.C.D.; Aau, W.K.; Koh, Y.L.E.; et al. A multidisciplinary effort to increase COVID-19 vaccination among the older adults. Front. Public Health 2022, 10, 904161. [Google Scholar] [CrossRef]
- Baumann, N.; Chen, S.; McDonald, J.R.; Davis, M.H.; Petroff, C.; McKelvy, P. Mitigating COVID-19 Vaccine Waste Through a Multidisciplinary Inpatient Vaccination Initiative. J. Healthc. Qual. 2022, 44, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Chau, J.P.C.; Lo, S.H.S.; Choi, K.C.; Lee, V.W.Y.; Lui, G.C.Y.; Chan, K.M.; Lau, A.Y.L. Effects of a multidisciplinary team-led school-based human papillomavirus vaccination health-promotion programme on improving vaccine acceptance and uptake among female adolescents: A cluster randomized controlled trial. Medicine 2020, 99, e22072. [Google Scholar] [CrossRef] [PubMed]
- Zerbo, O.; Bartlett, J.; Fireman, B.; Lewis, N.; Goddard, K.; Dooling, K.; Duffy, J.; Glanz, J.; Naleway, A.; Donahue, J.G.; et al. Effectiveness of Recombinant Zoster Vaccine Against Herpes Zoster in a Real-World Setting. Ann. Intern. Med. 2024, 177, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Larson, H.; Leask, J.; Aggett, S.; Sevdalis, N.; Thomson, A. A Multidisciplinary Research Agenda for Understanding Vaccine-Related Decisions. Vaccines 2013, 1, 293. [Google Scholar] [CrossRef]
- Hastall, M.R.; Koinig, I.; Kunze, U.; Meixner, O.; Sachse, K.; Würzner, R. Multidisciplinary expert group: Communication measures to increase vaccine compliance in adults. Wien. Med. Wochenschr. 2024, 174, 149–152. [Google Scholar] [CrossRef]
- Sansone, V.; Giudice, G.M.d; Polla, G.D.; Angelillo, I.F. Knowledge, Attitudes, and Coverage of Recommended Vaccinations in Individuals with Chronic Medical Conditions: A Cross-Sectional Telephone Survey in Italy. Vaccines 2024, 12, 336. [Google Scholar] [CrossRef]
- Napolitano, F.; Polla, G.D.; Capano, M.S.; Augimeri, M.; Angelillo, I.F. Vaccinations and Chronic Diseases: Knowledge, Attitudes, and Self-Reported Adherence among Patients in Italy. Vaccines 2020, 8, 560. [Google Scholar] [CrossRef]
- Istituto Superiore di Sanità. Guida alle Controindicazioni alle Vaccinazioni. 2018. Available online: https://www.epicentro.iss.it/vaccini/pdf/19_3_web.pdf (accessed on 31 July 2025).
- Regione Piemonte. Direzione Sanità e Welfare SPeV-AcdLFSAA, Franco Giovanetti (SISP–ASL CN2). La Vaccinazione dei Soggetti Che Pre-Sentano un Rischio Aumentato di Infezione Invasiva da Batteri Capsulati (Streptococcus Pneumoniae, Neisseria Meningi-Tidis, Haemophilus Influenzae). Available online: https://www.regione.piemonte.it/web/temi/cultura-turismo-sport/cultura/archivi-biblioteche-istituti-culturali/archivi (accessed on 16 November 2024).
- Kroger, A.; Bahta, L.; Long, S.; Sanchez, P. General Best Practice Guidelines for Immunization. Vaccine Recommendations and Guidelines of the ACIP. CDC. Available online: https://www.cdc.gov/vaccines/hcp/acip-recs/general-recs/downloads/general-recs.pdf (accessed on 16 November 2024).
| All Patients (N:178) | Recalled Patients (N:90) | Patients Lost to Follow-Up (N:88) | Recalled Patients vs. Patients Lost to Follow-Up | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | n. (%) | Mean ± SD | Median (IQR) | n. (%) | Mean ± SD | Median (IQR) | n. (%) | Mean ± SD | Median (IQR) | p-Value |
| Demographic Characteristics | ||||||||||
| Age (years) | 61.91 ± 15.39 | 63.5 (19) | 61.16 ± 13.16 | 62 (16.75) | 62.68 ± 17.41 | 66 (17) | 0.5111 † | |||
| Age groups | 0.1529 ‡ | |||||||||
| 18–44 | 22 (12.36%) | 12 (13.33%) | 7 (7.95%) | |||||||
| 45–64 | 72 (40.45%) | 41 (45.56%) | 31 (35.23%) | |||||||
| 65+ | 84 (47.19%) | 37 (41.11%) | 47 (53.41%) | |||||||
| Sex | 0.0711 ‡ | |||||||||
| Male | 86 (48.31%) | 50 (55.56%) | 52 (59.09%) | |||||||
| Female | 92 (51.69%) | 40 (44.44%) | 36 (40.91%) | |||||||
| Clinical Characteristics | ||||||||||
| Previous zoster | 0.5546 ‡ | |||||||||
| No | 126 (70.79%) | 66 (73.33%) | 60 (68.18%) | |||||||
| Yes | 52 (29.21%) | 24 (26.67%) | 28 (31.82%) | |||||||
| Primary indication for vaccination | 0.3183 ‡ | |||||||||
| Acquired immunodeficiency syndrome | 61 (34.27%) | 35 (38.89%) | 26 (29.55%) | |||||||
| Rheumatological diseases under treatment | 38 (21.35%) | 18 (20.00%) | 20 (22.73%) | |||||||
| Subjects with recurrences or particular forms HZ | 41 (23.03%) | 19 (21.11%) | 22 (25.00%) | |||||||
| Oncohematologic diseases under treatment | 14 (7.87%) | 8 (8.89%) | 6 (6.82%) | |||||||
| Other indications | 24 (13.48%) | 10 (11.11%) | 14 (15.90%) | |||||||
| Hospital department | 0.5182 ‡ | |||||||||
| Infectious Diseases | 62 (43.36%) | 38 (42.70%) | 34 (38.64%) | |||||||
| Rheumatology | 26 (18.18%) | 19 (21.11%) | 26 (29.55%) | |||||||
| Geriatrics Medicine | 25 (17.48%) | 14 (15.56%) | 13 (14.77%) | |||||||
| Nephrology | 7 (3.93%) | 2 (2.22%) | 5 (5.68%) | |||||||
| Hematology | 13 (7.30%) | 8 (8.89%) | 5 (5.68%) | |||||||
| Other departments | 30 (20.98%) | 9 (10%) | 5 (5.68%) | |||||||
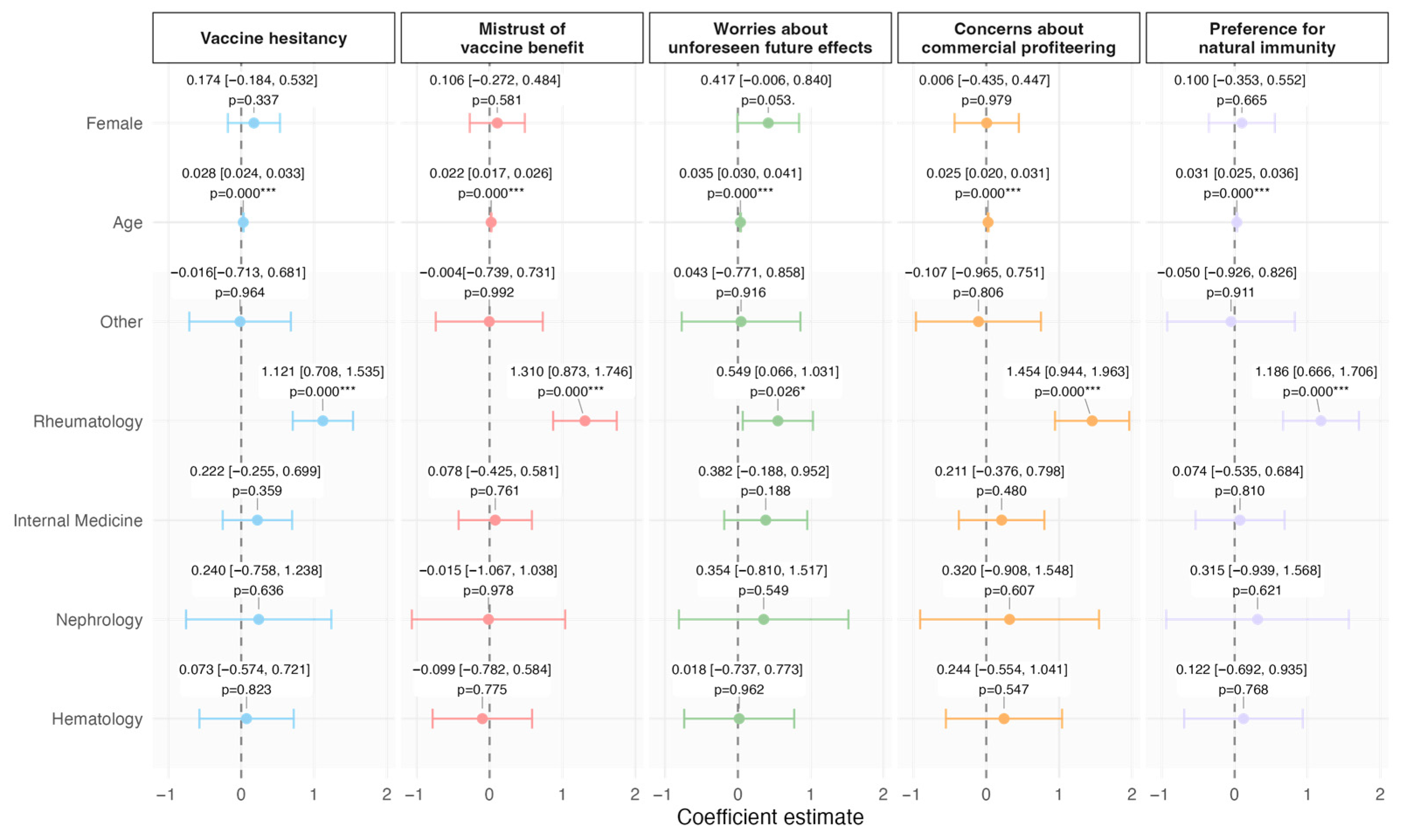
| Vaccine Hesitancy | ||||||||||
| Mistrust of vaccine benefit | 1.85 ± 1.09 | 1.33 (1.17) | 1.73 ± 0.88 | 1.50 (1.00) | 2 ± 1.28 | 1.33 (1.33) | 0.1258 † | |||
| Worries about unforeseen future effects | 2.77 ± 1.06 | 2.67 (1.67) | 2.65 ± 1.07 | 2.67 (1.33) | 2.91 ± 1.02 | 3 (1.34) | 0.1017 † | |||
| Concerns about commercial profiteering | 2.09 ± 1.29 | 1.67 (1.67) | 1.95 ± 1.17 | 1.67 (1.67) | 2.26 ± 1.4 | 1.67 (2) | 0.1215 † | |||
| Preference for natural immunity | 2.45 ± 1.18 | 2.33 (1.67) | 2.24 ± 1.10 | 2.33 (1.67) | 2.7 ± 1.22 | 2.67 (1.66) | 0.0123 †* | |||
| Overall hesitancy score | 2.29 ± 0.94 | 2.08 (1.37) | 2.14 ± 0.85 | 1.83 (1.41) | 2.46 ± 1.01 | 2.17 (1.25) | 0.0311 †* | |||
| Vaccination Interval | ||||||||||
| Days between doses | 74.46 ± 42.58 | 64 (16) | 67.62 ± 22.17 | 64 (13.75) | 81.45 ± 55.57 | 64 (27.25) | 0.0319 †* | |||
| Summary Statistics: Sex | Hesitancy Tot * | Mistrust of Vaccine Benefit * | Worries About Unforeseen Future Effects * | Concerns About Commercial Profiteering * | Preference for Natural Immunity * |
|---|---|---|---|---|---|
| Female | 2.39 (0.91) | 1.93 (1.14) | 2.93 (1.02) | 2.18 (1.29) | 2.51 (1.13) |
| Male | 2.18 (0.95) | 1.75 (1.01) | 2.59 (1.06) | 2 (1.28) | 2.38 (1.23) |
| Summary statistics: age class | |||||
| 18–44 | 2.53 (1.08) | 2.10 (1.21) | 2.96 (1.23) | 2.20 (1.42) | 2.87 (1.25) |
| 45–64 | 2.44 (0.98) | 2.09 (1.13) | 2.86 (1.06) | 2.28 (1.39) | 2.55 (1.22) |
| 65+ | 2.08 (0.80) | 1.55 (0.94) | 2.63 (0.99) | 1.89 (1.12) | 2.24 (1.08) |
| Summary statistics: units | |||||
| Others | 1.79 (0.73) | 1.39 (0.66) | 2.33 (1.06) | 1.51 (0.93) | 1.93 (0.85) |
| Rheumatology | 2.54 (1.04) | 2.14 (1.28) | 2.82 (1.14) | 2.43 (1.31) | 2.76 (1.33) |
| Geriatrics Medicine | 2.05 (0.74) | 1.48 (0.71) | 2.84 (1.01) | 1.79 (0.99) | 2.02 (0.96) |
| Infectious Diseases | 2.16 (0.78) | 1.72 (0.77) | 2.76 (1.11) | 1.83 (1.12) | 2.36 (1.13) |
| Nephrology | 2.28 (0.74) | 1.53 (0.51) | 3.13 (1.21) | 2 (1.22) | 2.46 (1.26) |
| Hematology | 2.12 (0.67) | 1.46 (0.61) | 2.74 (0.81) | 1.97 (1.05) | 2.31 (0.91) |
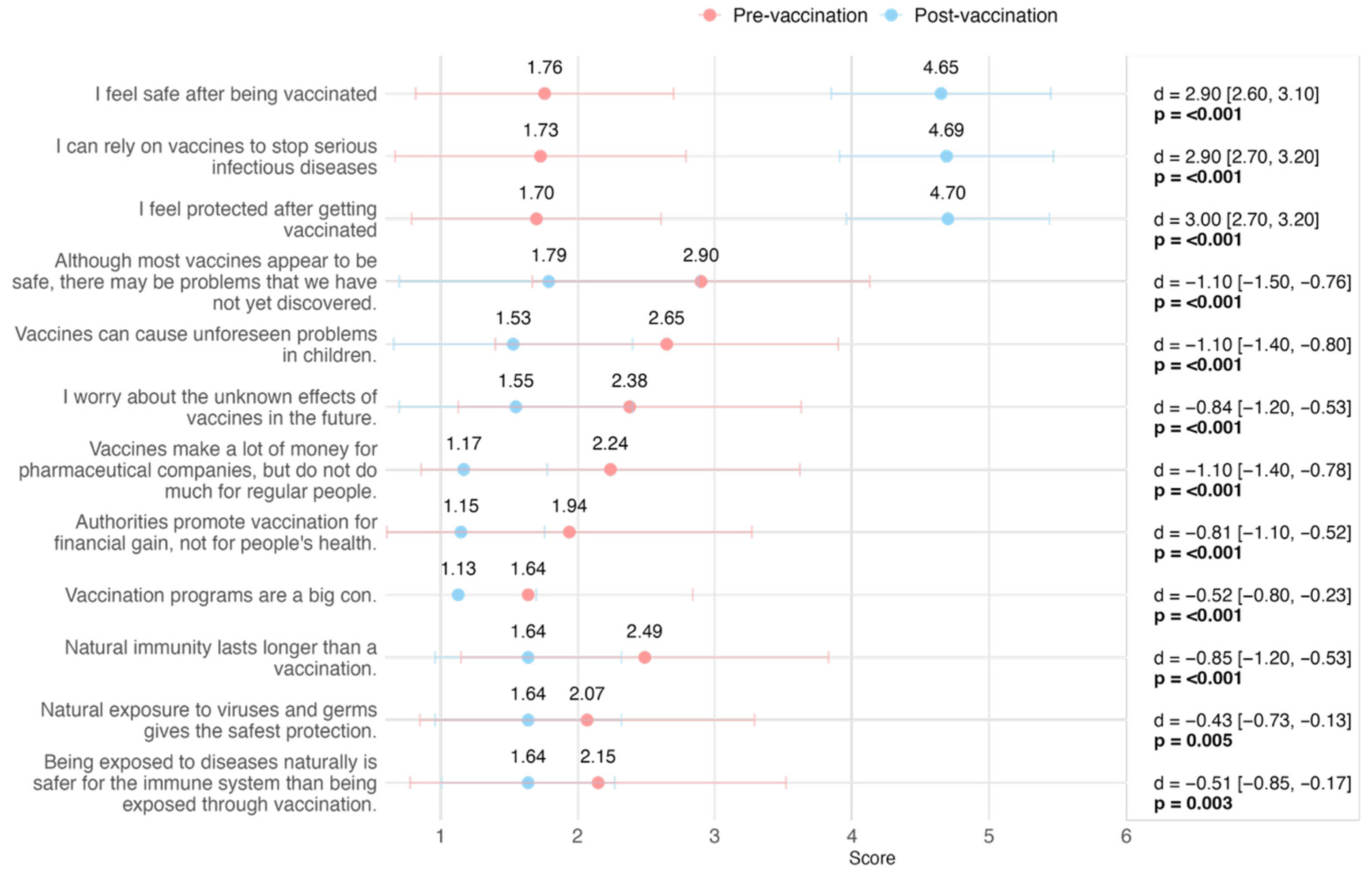
| Item | Pre (n = 90) | Post (n = 90) | Difference * | 95% CI ** | p-Value *** |
|---|---|---|---|---|---|
| I feel safe after being vaccinated | 2.9 | 2.6, 3.1 | <0.001 | ||
| Mean (SD) | 1.76 (0.94) | 4.65 (0.80) | |||
| Median [IQR] | 1.00 [1.00, 2.00] | 5.00 [5.00, 5.00] | |||
| Missing (N) | 0 | 1 | |||
| I can rely on vaccines to stop serious infectious diseases | 2.9 | 2.7, 3.2 | <0.001 | ||
| Mean (SD) | 1.73 (1.06) | 4.69 (0.78) | |||
| Median [IQR] | 2.00 [1.00, 2.00] | 5.00 [5.00, 5.00] | |||
| Missing (N) | 0 | 1 | |||
| I feel protected after getting vaccinated | 3.0 | 2.7, 3.2 | <0.001 | ||
| Mean (SD) | 1.70 (0.91) | 4.70 (0.74) | |||
| Median [IQR] | 1.00 [1.00, 2.00] | 5.00 [5.00, 5.00] | |||
| Missing (N) | 0 | 1 | |||
| Although most vaccines appear to be safe, there may be problems that we have not yet discovered | −1.1 | −1.5, −0.76 | <0.001 | ||
| Mean (SD) | 2.90 (1.23) | 1.79 (1.09) | |||
| Median [IQR] | 3.00 [2.00, 4.00] | 1.00 [1.00, 2.00] | |||
| Missing (N) | 1 | 1 | |||
| Vaccines can cause unforeseen problems in children | −1.1 | −1.4, −0.80 | <0.001 | ||
| Mean (SD) | 2.65 (1.25) | 1.53 (0.87) | |||
| Median [IQR] | 3.00 [2.00, 3.00] | 1.00 [1.00, 2.00] | |||
| Missing (N) | 2 | 1 | |||
| I worry about the unknown effects of vaccines in the future | −0.84 | −1.2, −0.53 | <0.001 | ||
| Mean (SD) | 2.38 (1.25) | 1.55 (0.85) | |||
| Median [IQR] | 2.00 [1.00, 3.00] | 1.00 [1.00, 2.00] | |||
| Missing (N) | 0 | 1 | |||
| Vaccines make a lot of money for pharmaceutical companies, but do not do much for regular people | −1.1 | −1.4, −0.78 | <0.001 | ||
| Mean (SD) | 2.24 (1.38) | 1.17 (0.61) | |||
| Median [IQR] | 2.00 [1.00, 3.00] | 1.00 [1.00, 1.00] | |||
| Missing (N) | 0 | 1 | |||
| Authorities promote vaccination for financial gain, not for people’s health | −0.81 | −1.1, −0.52 | <0.001 | ||
| Mean (SD) | 1.94 (1.33) | 1.15 (0.61) | |||
| Median [IQR] | 1.00 [1.00, 3.00] | 1.00 [1.00, 1.00] | |||
| Missing (N) | 1 | 1 | |||
| Vaccination programs are a big con | −0.52 | −0.80, −0.23 | <0.001 | ||
| Mean (SD) | 1.64 (1.20) | 1.13 (0.57) | |||
| Median [IQR] | 1.00 [1.00, 2.00] | 1.00 [1.00, 1.00] | |||
| Missing (N) | 0 | 1 | |||
| Natural immunity lasts longer than a vaccination | −0.85 | −1.2, −0.53 | <0.001 | ||
| Mean (SD) | 2.49 (1.34) | 1.64 (0.68) | |||
| Median [IQR] | 3.00 [1.00, 3.00] | 2.00 [1.00, 2.00] | |||
| Missing (N) | 2 | 1 | |||
| Natural exposure to viruses and germs gives the safest protection | −0.43 | −0.73, −0.13 | 0.005 | ||
| Mean (SD) | 2.07 (1.22) | 1.64 (0.68) | |||
| Median [IQR] | 2.00 [1.00, 3.00] | 2.00 [1.00, 2.00] | |||
| Missing (N) | 1 | 1 | |||
| Being exposed to diseases naturally is safer for the immune system than being exposed through vaccination | −0.51 | −0.85, −0.17 | 0.003 | ||
| Mean (SD) | 2.15 (1.37) | 1.64 (0.63) | |||
| Median [IQR] | 2.00 [1.00, 3.00] | 2.00 [1.00, 2.00] | |||
| Missing (N) | 1 | 1 |
| Num. Patients | % | |
|---|---|---|
| Side Effects: 1st dose | ||
| No | 72 | 80.00 |
| Fatigue, chills, fever | 11 | 12.22 |
| Pain, redness or swelling at the injection site | 5 | 5.56 |
| General discomfort | 5 | 5.56 |
| Doesn’t know/Can’t remember | 2 | 2.22 |
| Stomach and digestive disorders (including nausea, vomiting, diarrhea, abdominal pain) | 1 | 1.11 |
| Myalgia | 1 | 1.11 |
| Headache | 1 | 1.11 |
| Tingling at the injection site | 1 | 1.11 |
| Lymphadenopathy | 0 | 0 |
| Side Effects: 2nd dose | ||
| No | 67 | 74.44 |
| Fatigue, chills, fever | 16 | 17.78 |
| Pain, redness or swelling at the injection site | 7 | 7.78 |
| General discomfort | 7 | 7.78 |
| Stomach and digestive disorders (including nausea, vomiting, diarrhea, abdominal pain) | 2 | 2.22 |
| Tingling at the injection site | 2 | 2.22 |
| Arthralgia | 1 | 1.11 |
| Myalgia | 1 | 1.11 |
| Headache | 1 | 1.11 |
| Arthralgia | 0 | 0 |
| Lymphadenopathy | 0 | 0 |
| Doesn’t know/Can’t remember | 0 | 0 |
| Breakthrough Infections | ||
| No | 87 | 96.67 |
| Yes, after 2nd dose—of which | 3 | 3.33 |
| Oral | 2 | 66.67 * |
| Lumbar | 1 | 33.33 * |
| Ophthalmic | 1 | 33.33 * |
| Yes, after 1st dose | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Regazzi, L.; Martinelli, S.; Rizzo, F.; Tamburrini, E.; Salvo, P.F.; Bosello, S.L.; Landi, F.; Sica, S.; Spadea, A.; Pascucci, D.; et al. Understanding Attitudes Toward Zoster Vaccination in the Hospital Setting: A Multidisciplinary Model to Contrast Vaccine Hesitancy in Fragile Patients—A Prospective Longitudinal Observational Study. Vaccines 2025, 13, 843. https://doi.org/10.3390/vaccines13080843
Regazzi L, Martinelli S, Rizzo F, Tamburrini E, Salvo PF, Bosello SL, Landi F, Sica S, Spadea A, Pascucci D, et al. Understanding Attitudes Toward Zoster Vaccination in the Hospital Setting: A Multidisciplinary Model to Contrast Vaccine Hesitancy in Fragile Patients—A Prospective Longitudinal Observational Study. Vaccines. 2025; 13(8):843. https://doi.org/10.3390/vaccines13080843
Chicago/Turabian StyleRegazzi, Luca, Silvia Martinelli, Federica Rizzo, Enrica Tamburrini, Pierluigi Francesco Salvo, Silvia Laura Bosello, Francesco Landi, Simona Sica, Antonietta Spadea, Domenico Pascucci, and et al. 2025. "Understanding Attitudes Toward Zoster Vaccination in the Hospital Setting: A Multidisciplinary Model to Contrast Vaccine Hesitancy in Fragile Patients—A Prospective Longitudinal Observational Study" Vaccines 13, no. 8: 843. https://doi.org/10.3390/vaccines13080843
APA StyleRegazzi, L., Martinelli, S., Rizzo, F., Tamburrini, E., Salvo, P. F., Bosello, S. L., Landi, F., Sica, S., Spadea, A., Pascucci, D., & Laurenti, P. (2025). Understanding Attitudes Toward Zoster Vaccination in the Hospital Setting: A Multidisciplinary Model to Contrast Vaccine Hesitancy in Fragile Patients—A Prospective Longitudinal Observational Study. Vaccines, 13(8), 843. https://doi.org/10.3390/vaccines13080843





